The Rare, Unexpected Condition of a Twisted Leiomyoma in Mayer-Rokitansky-Küster-Hauser (MRKH) Syndrome: Etiopathogenesis, Diagnosis and Management. Our Experience and Narrative Review of the Literature
Abstract
1. Introduction
2. Case
3. Materials and Methods
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choussein, S.; Nasioudis, D.; Schizas, D.; Economopoulos, K.P. Mullerian dysgenesis: A critical review of the literature. Arch. Gynecol. Obstet. 2017, 295, 1369–1381. [Google Scholar] [CrossRef]
- Salem Wehbe, G.; Bitar, R.; Zreik, T.; Samaha, M.; Walter, C.; Sleiman, Z. Intra-peritoneal leiomyoma a of the round ligament in a patient with Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome. Facts Views Vis. Obgyn 2016, 8, 233–2352. [Google Scholar] [PubMed]
- Amaratunga, T.; Kirkpatrick, L.; Yan, Y.; Karlicki, F. Ectopic Pelvic Fibroid in a Woman with Uterine Agenesis and Mayer-Rokitansky-Küster-Hauser Syndrome. Ultrasound Q. 2017, 33, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Papa, G.; Andreotti, M.; Giannubilo, S.R.; Cesari, R.; Ceré, I.; Tranquilli, A.L. Case report and surgical solution for a voluminous uterine leiomyoma in a woman with complicated Mayer-Rokitansky-Küster-Hauser syndrome. Fertil. Steril. 2008, 90, 2014.e5–2014.e6. [Google Scholar] [CrossRef]
- Fletcher, H.M.; Campbell-Simpson, K.; Walcott, D.; Harriott, J. Müllerian remnant leiomyomas in women with Mayer-Rokitansky-Küster-Hauser syndrome. Obstet Gynecol. 2012, 119 Pt 2, 483–485. [Google Scholar] [CrossRef]
- Yan, C.M.; Mok, K.M. Uterine fibroids and adenomyosis in a woman with Rokitansky-Kuster-Hauser syndrome. J. Obstet. Gynaecol. 2002, 22, 561–574. [Google Scholar] [CrossRef]
- Taylor, E.; Gomel, V. The uterus and fertility. Fertil. Steril. 2008, 89, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, T.; Dueholm, M.; Leone, F.P.; Valentin, L.; Rasmussen, C.K.; Votino, A.; Van Schoubroeck, D.; Landolfo, C.; Installé, A.J.; Guerriero, S.; et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: A consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet. Gynecol. 2015, 46, 284–298. [Google Scholar] [CrossRef]
- Jokimaa, V.; Virtanen, J.; Kujari, H.; Ala-Nissilä, S.; Rantanen, V. A Mayer-Rokitansky-Kuster-Hauser patient with leiomyoma and dysplasia of neovagina: A case report. BMC Womens Health 2020, 20, 157. [Google Scholar] [CrossRef]
- Sharma, R.; Guleria, K.; Suneja, A.; Bhaskaran, S.; Tanveer, N. Giant leiomyoma with extensive myxoid degeneration in Mayer–Rokitansky–Küster–Hauser syndrome. Int. J. Gynecol. Obstet. 2017, 138, 125–127. [Google Scholar] [CrossRef]
- Narayanan, R.; Mariappan, S.; Paulraj, S.; Shankar, B. Imaging of leiomyomas arising from Müllerian remnants in a case of Mayer-Rokitansky-Küster-Hauser syndrome. BMJ Case Rep. 2015. [Google Scholar] [CrossRef]
- Park, J.W.; Hwang, D.W.; Jang, J.A.; Choi, Y.J.; Chun, K.C.; Kim, Y.A. Leiomyoma mimicking a pelvic tumour in Mayer-Rokitansky-Küster-Hauser syndrome: A case report. J. Obstet. Gynaecol. 2016, 36, 279–280. [Google Scholar] [CrossRef]
- Hasegawa, A.; Igarashi, H.; Ohta, T.; Kurachi, H.; Takahashi, K. Three-dimensional computed tomography of pelvic masses in Mayer-Rokitansky-Küster-Hauser syndrome. Obstet Gynecol. 2015, 125, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.; Kim, Y.M.; Ji, Y.I. Uterine adenomyosis which developed from hypoplastic uterus in postmenopausal woman with mayer-rokitansky-kuster-hauser syndrome: A case report. J. Menopausal. Med. 2013, 19, 135–138. [Google Scholar] [CrossRef][Green Version]
- Jadoul, P.; Pirard, C.; Squifflet, J.; Smets, M.; Donnez, J. Pelvic mass in a woman with Mayer-Rokitansky-Kuster-Hauser syndrome. Fertil. Steril. 2004, 81, 203–204. [Google Scholar] [CrossRef]
- Páez-López, G.; los Ríos-Posada, D.; Fernando, J.; Arango-Martínez, A.M.; Castañeda-Roldán, J.D.; Serna-Agudelo, E.; Vásquez-Ruiz, R.; Almanza-Payares, L.A.; Calle-Gómez, G.A. Laparoscopic management of uterine myomatosis in a patient with rokitansky syndrome. case report and review of the literature. Rev. Colomb. Obs. Ginecol. 2013, 64, 469–474. [Google Scholar] [CrossRef]
- Edmonds, D.K. Multiple fibroids in a postmenopausal woman with Mayer Rokitansky Kuster Hauser syndrome. J. Pediatr. Adolesc. Gynecol. 2003, 16, 65–66. [Google Scholar] [CrossRef]
- Lamarca, M.; Navarro, R.; Ballesteros, M.E.; García-Aguirre, S.; Conte, M.P.; Duque, J.A. Leiomyomas in both uterine remnants in a woman with the Mayer-Rokitansky-Küster-Hauser syndrome. Fertil. Steril. 2009, 91, 931.e13–931.e15. [Google Scholar] [CrossRef]
- Rawat, K.S.; Buxi, T.; Yadav, A.; Ghuman, S.S.; Dhawan, S. Large leiomyoma in a woman with Mayer-Rokitansky-Kuster-Hauser syndrome. J. Radiol. Case Rep. 2013, 7, 39–46. [Google Scholar] [CrossRef]
- Deligeoroglou, E.; Kontoravdis, A.; Makrakis, E.; Christopoulos, P.; Kountouris, A.; Creatsas, G. Development of leiomyomas on the uterine remnants of two women with Mayer-Rokitansky-Küster-Hauser syndrome. Fertil. Steril. 2004, 81, 1385–1387. [Google Scholar] [CrossRef]
- Kulkarni, M.M.; Deshmukh, S.D.; Hol, K.; Nene, N. A rare case of Mayer-Rokitansky-Kuster-Hauser syndrome with multiple leiomyomas in hypoplastic uterus. J. Hum. Reprod. Sci. 2015, 8, 242. [Google Scholar] [CrossRef]
- Girma, W.; Woldeyes, W. Leiomyoma Arising from Mullerian Remnant, Mimicking Ovarian Tumor in a Woman with MRKH Syndrome and Unilateral Renal Agenesis. Ethiop. J. Health Sci. 2015, 25, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, I.; Pagidas, K.; Vaughan, D.; Kim, Y.B. Mitotically Active Leiomyoma in a Woman with Mayer-Rokitansky-Küster-Hauser Syndrome: A Case Report. J. Reprod. Med. 2016, 61, 299–301. [Google Scholar]
- Karthik, S.D.S.; Mahey, R.; Kachhawa, G.; Kriplani, A. Laparoscopic management of leiomyoma arising from mullerian Remnant in Mayer-Rokitansky-Kuster-Hauser syndrome. J. Gynecolocic Surg. 2017, 38, 276–279. [Google Scholar] [CrossRef]
- Harzif, A.K.; Ambalagen, S.; Charilda, F.E.; Mutia, H.D. A rare case of multiple leiomyomas on rudimentary uterus in a woman with Mayer Rokitansky Kuster Hauser (MRKH) syndrome: A challenging diagnosis and laparoscopic approach. Int. J. Surg. Case Rep. 2021, 81, 105711. [Google Scholar] [CrossRef]
- Lanowska, M.; Favero, G.; Schneider, A.; Kholer, C. Laparoscopy for differential diagnosis of a pelvic mass in a patient with Mayer-Rokitanski-Kuster-Hauser (MRKH) syndrome. Fertil. Steril. 2009, 91, 931.e17–931.e18. [Google Scholar] [CrossRef]
- Rhee, C.S.; Kim, J.S.; Woo, S.K.; Suh, S.J. MRI of Round ligament leiomyoma associated with Mayer-Rokitansky-Kuster-Huser syndrome. Abdom Imaging 1999, 24, 201–204. [Google Scholar] [CrossRef]
- Tsin, D.A.; Waters, T.K.; Granato, R.C. Laparoscopic myomectomy in patient with Mayer-Rokitansky-Kuster-Hauser Syndrome. J. Am. Assoc. Gynecolocic Laparosc. 2000, 7, 411–413. [Google Scholar] [CrossRef]
- Fukuda, J.; Kumazawa, Y.; Fujimoto, T.; Tanaka, T.J. Mayer-Rokitansky-Kustner Hauser syndrome complicated by either uterine leiomyoma or ovarian tumor. Obstet Gynaecol. Res. 2010, 36, 191–194. [Google Scholar] [CrossRef]
- Albahlol, I.A.; Elshamy, M.; El-Hady, H.A.F.; Abd-Elwahab, E.M. Leiomyomas in a case of Mayer–Rokitansky–Kuster–Hauser syndrome: Case report. Eur. J. Obstet. Gynecol. 2019. [Google Scholar] [CrossRef]
- Pati, S.; Mukhopadhyay, A. Leiomyoma in a young woman with Mayer-Rokitansky-Huster-Hauser syndrome. J. Indian Med. Assoc. 2009, 107, 460–461. [Google Scholar]
- Vidyashree, P.G.; Muralidhar, P.V.; Jayaram, N.; Latha, K. Mayer-Rokitansky-Kuster-Hauser syndrome with multiple leiomyomas. Int. J. Gynecol. Obstet. 2015, 128, 270–271. [Google Scholar] [CrossRef]
- Kundu, K.; Cohen, A.W.; Goldberg, J. Acute torsion of uterine remnant leiomyoma with Mayer-Rokitansky-Küster-Hauser syndrome. Fertil. Steril. 2014, 102, 607–609. [Google Scholar] [CrossRef]
- Yi, S.; Xu, B.; Zeng, F.; Xue, M. Acute Torsion of Paraovarian Cyst and Ipsilateral Uterine Remnant Leiomyoma in a Patient with Mayer-Rokitansky-Küster-Hauser Syndrome. J. Minim. Invasive Gynecol. 2017, 24, 6–7. [Google Scholar] [CrossRef]
- Hoo, P.S.; Norhaslinda, A.R.; Shah Reza, J.N. Case Report Rare Case of Leiomyoma and Adenomyosis in Mayer-Rokitansky-Kuster-Hauser Syndrome. Case Rep. Obstet. Gynecol. 2016, 2016, 3725043. [Google Scholar]
- Petrić, A.; Stefanović, M.; Vukomanović, P.; Zivadinović, R.; Tubić, A.; Janjić, Z. Acute abdomen in a patient with Mayer-Rokitansky-Kuster-Hauser syndrome. Vojn. Pregl. 2008, 65, 706–709. [Google Scholar] [CrossRef]
- Galajdova, L.; Verbeken, K.; Dhont, M. Recurrent multiple leiomyomata in a patient with Mayer-Rokitansky-Küster-Hauser syndrome. J. Obstet. Gynaecol. 2003, 23, 448–449. [Google Scholar] [CrossRef]
- Stabile, G.; Zinicola, G.; Romano, F.; Buonomo, F.; Mangino, F.P.; Ricci, G. Management of Non-Tubal Ectopic Pregnancies: A Single Center Experience. Diagnostics 2020, 10, 652. [Google Scholar] [CrossRef]
- Stewart, E.A.; Laughlin-Tommaso, S.K.; Catherino, W.H.; Lalitkumar, S.; Gupta, D.; Vollenhoven, B. Uterine fibroids. Nat. Rev. Dis. Primers. 2016, 2, 16043. [Google Scholar] [CrossRef]
- Ura, B.; Monasta, L.; Arrigoni, G.; Battisti, I.; Licastro, D.; Di Lorenzo, G.; Romano, F.; Aloisio, M.; Peterlunger, I.; Stabile, G.; et al. Phosphoproteins Involved in the Inhibition of Apoptosis and in Cell Survival in the Leiomyoma. J. Clin. Med. 2019, 8, 691. [Google Scholar] [CrossRef] [PubMed]
- Ura, B.; Monasta, L.; De Spelorzi, Y.C.C.; Arrigoni, G.; Franchin, C.; Biffi, S.; Aloisio, M.; Gaita, B.; Licastro, D.; Athanasakis, E.; et al. Proteins involved in oxidative stress in leiomyoma tissues treated with ulipristal acetate. Mol. Med. Rep. 2021, 23, 1. [Google Scholar] [CrossRef] [PubMed]
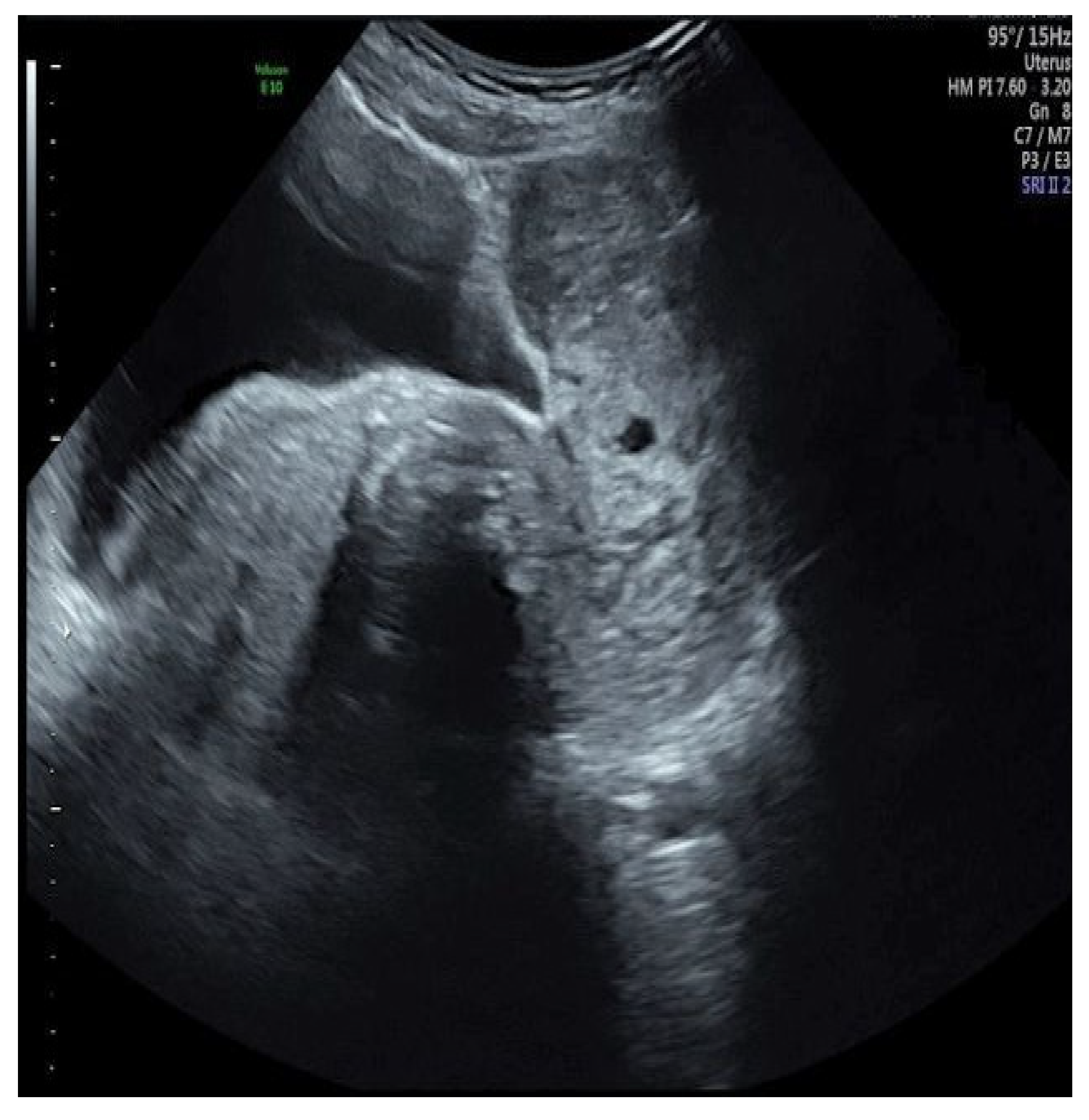
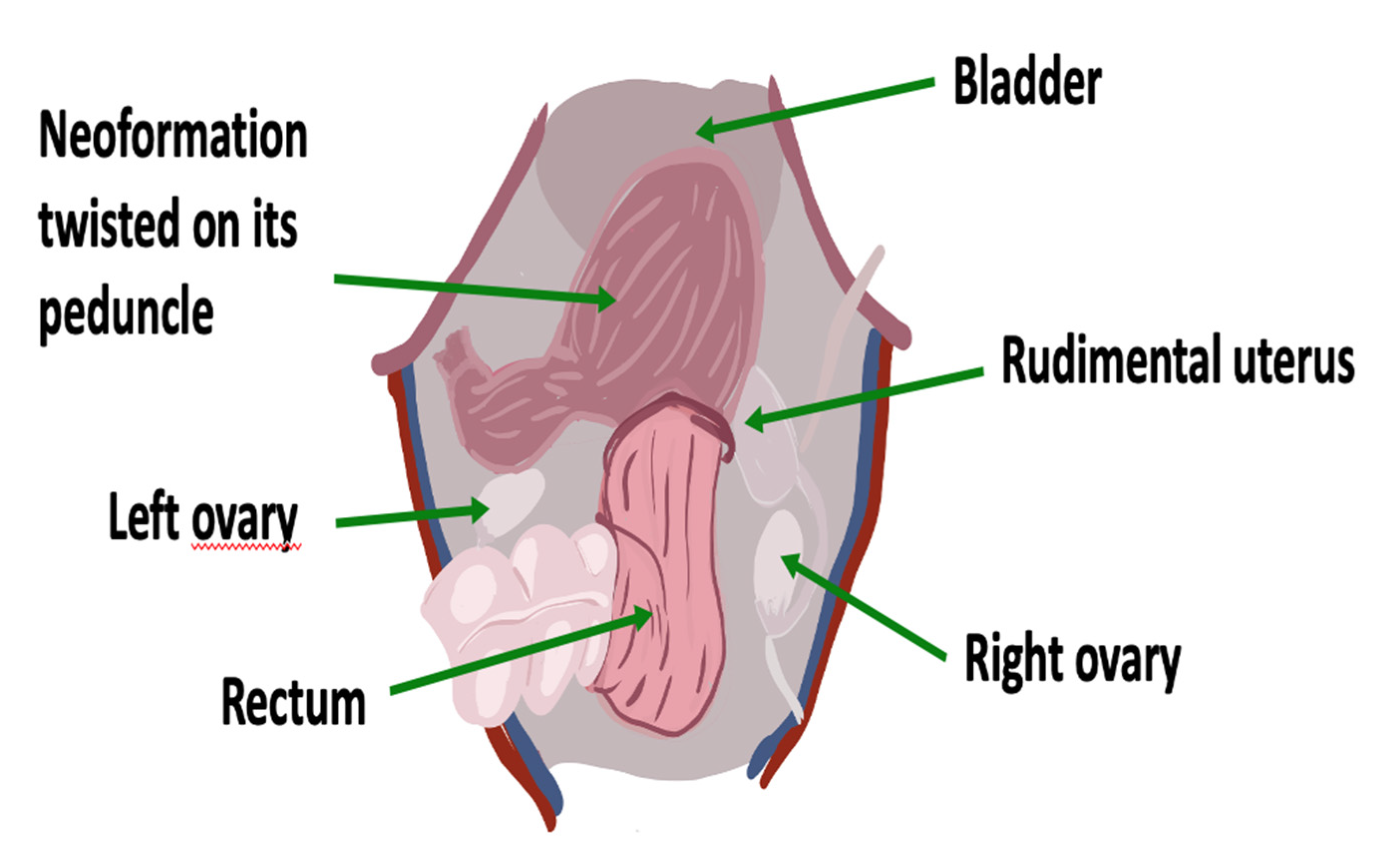

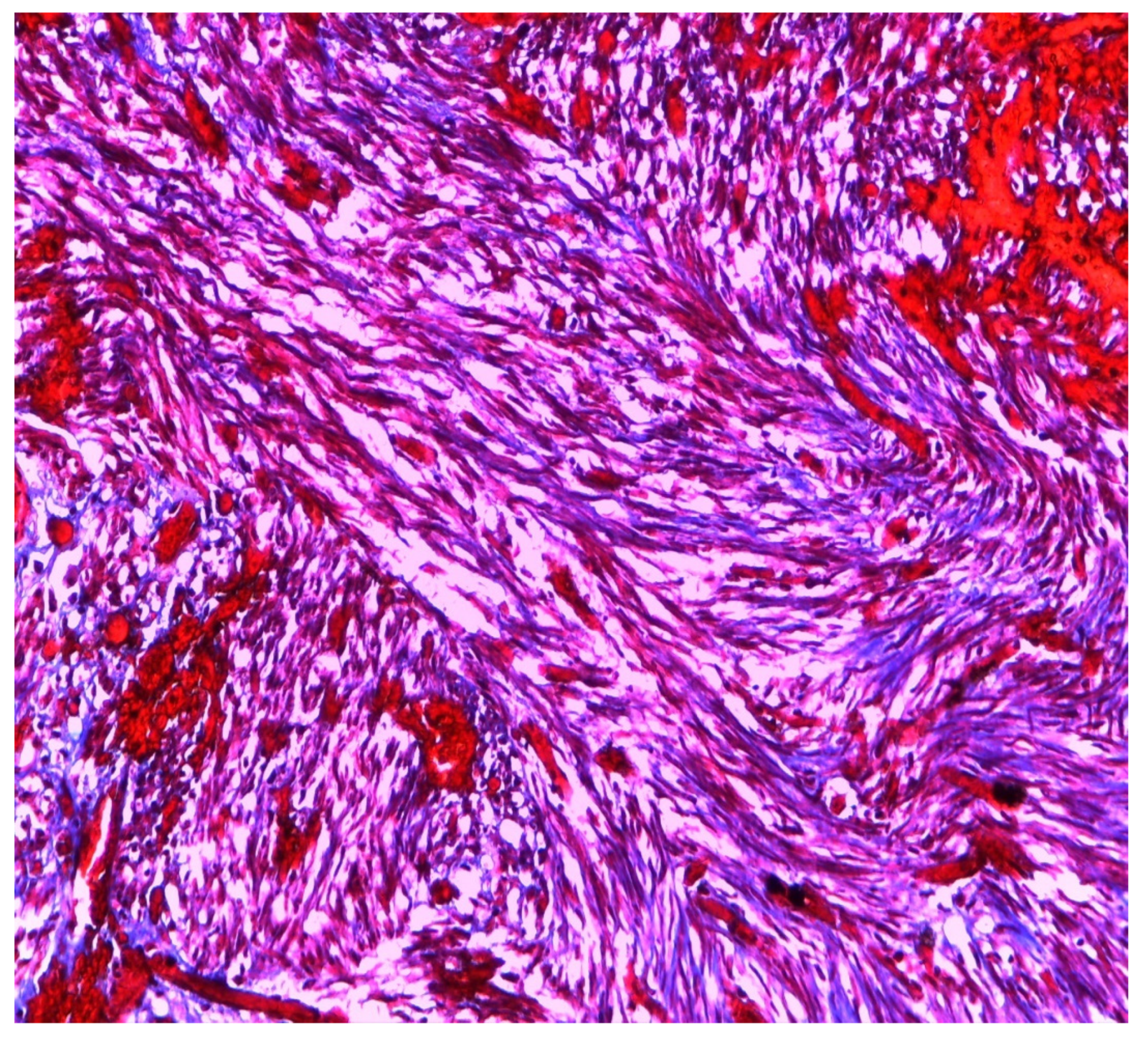
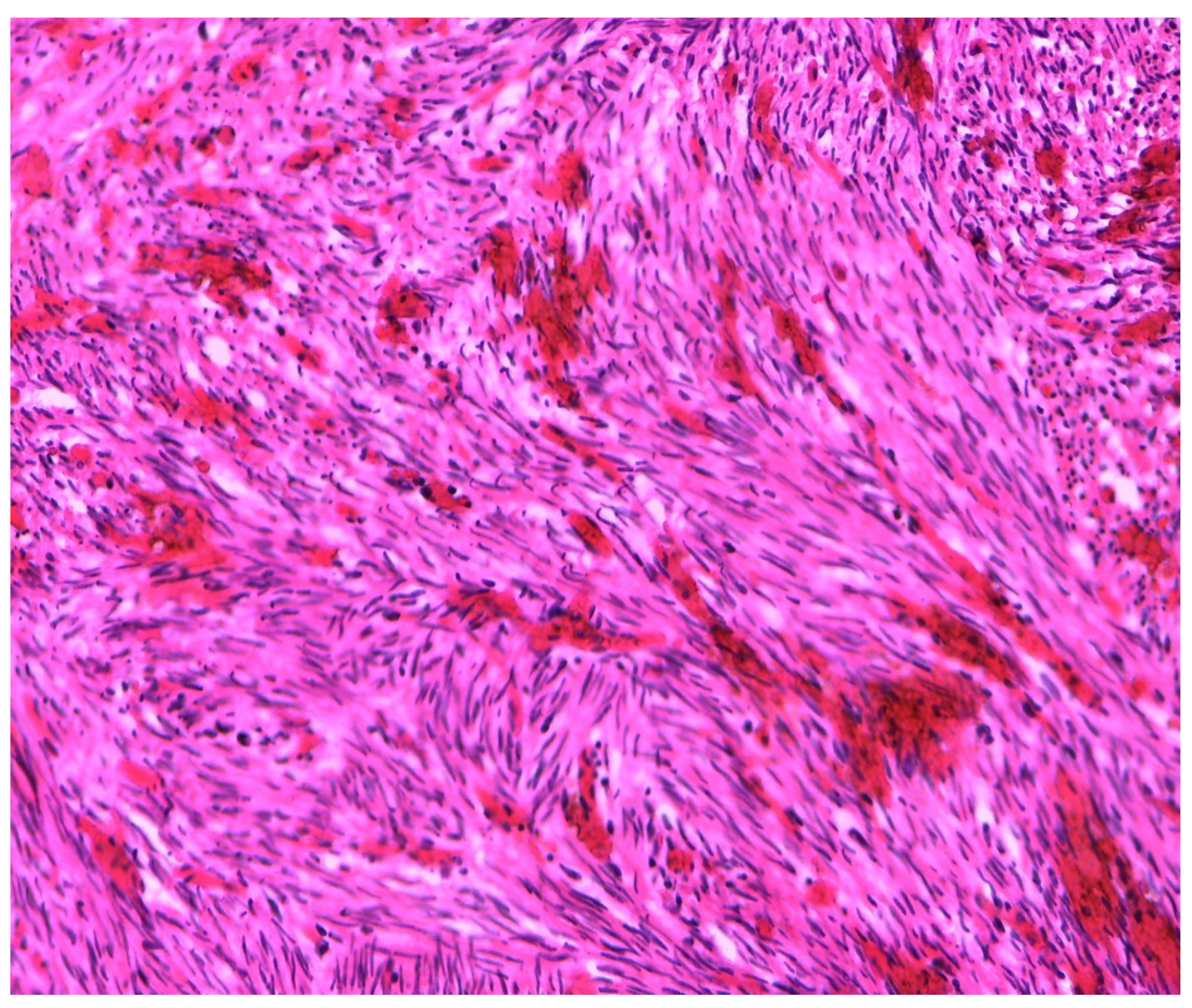
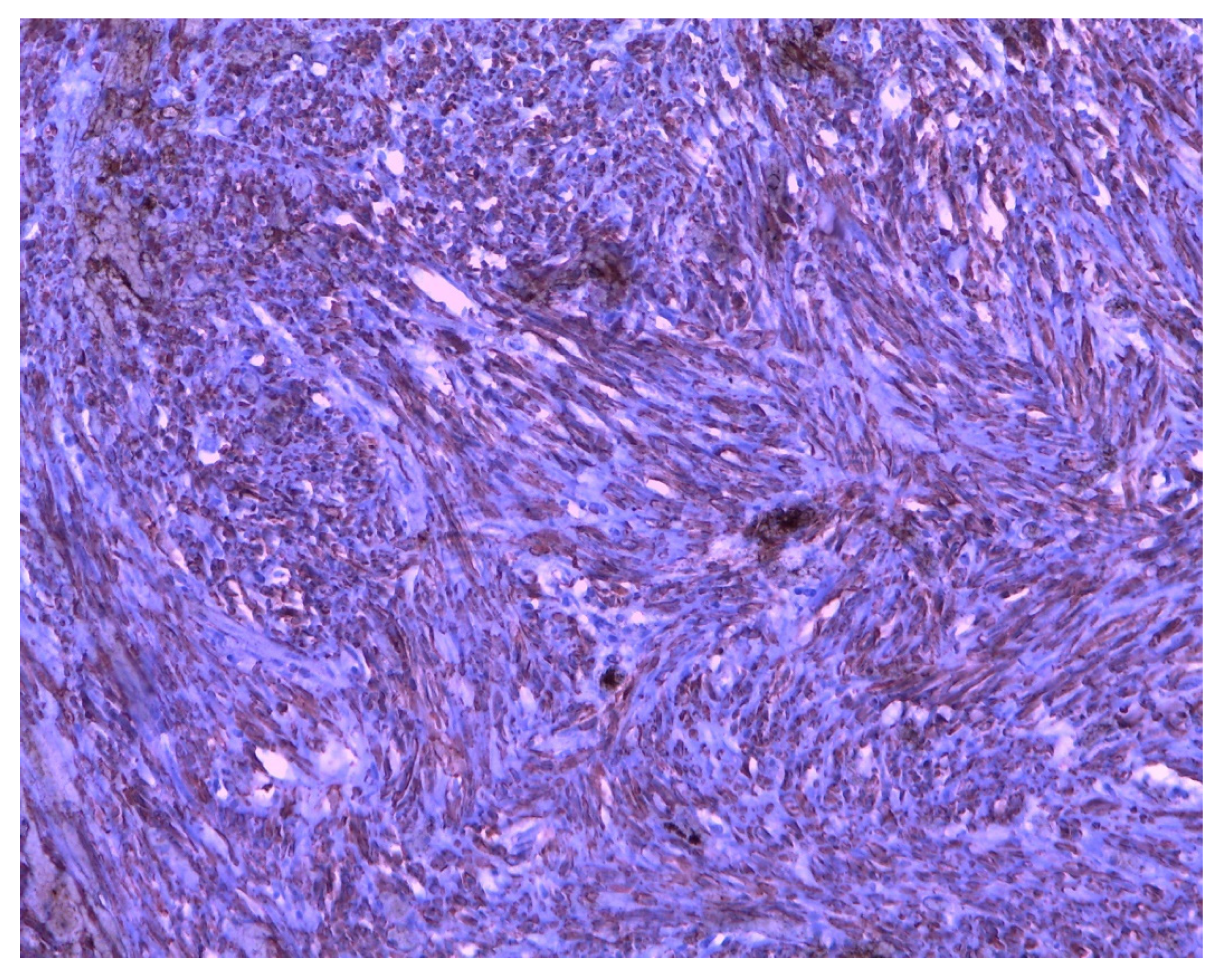
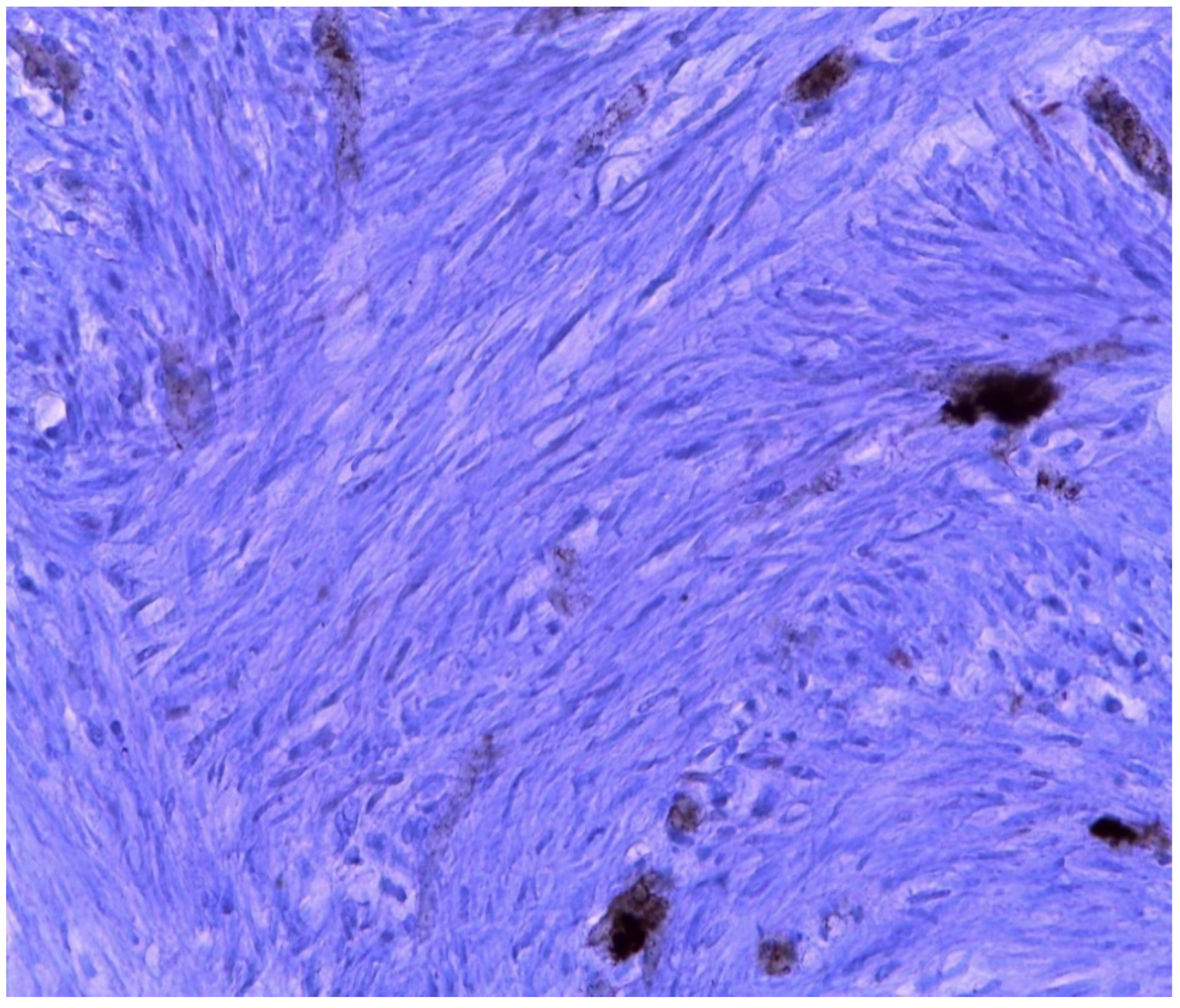
| MRKH Syndrome and Myomatosis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Case | Authors | Nationality | Age of Patients | Symptoms | Imaging | Location | Dimensions | Tipe of Surgery |
| 1 | Rhee Abdom. Imaging 1999 [27] | Korea | 49 | None | MRI | Rudimentary uterus | 10 cm × 10 cm × 6 cm | LPS |
| 2 | Tsin J. Am. Assoc. Gynecol. Laparosc. 2000 [28] | USA | 36 | None | US + CT | - | 8.4 cm | LPS |
| 3 | Endemonds J. Pediatr. Adolesc. Gynecol. 2003 [17] | England | 70 | Abdominal swelling –nocturia | - | - | 10 cm | LPT |
| 4 | Jadoul Fertil. Steril. 2004 [15] | Belgium | 42 | Dyspareunia | US-MRI | Left rudimentary uterus | 10 cm | LPS |
| 5 | Deligeoroglu Fertil. Steril. 2004 [20] | Greece | Case I 42 Case II 38 | Case I: chronic pain Case II: None | US | Rudimentary uterus | Case I: 5.9 cm × 5.5 cm Case II: 5.8 cm × 9.6 cm | LPS |
| 6 | Papa Fertil. Steril. 2008 [4] | Italy | 47 | Chronic pain | US + MRI | Retrovescical space | 15 cm | LPS |
| 7 | Lamarca Fertil. Steril. 2009 [18] | Spain | 35 | Infertility | US-MRI | Rudimentary uterus | 3–5 cm | LPT |
| 8 | Lanowska Fertil. Steril. 2009 [26] | Germany | 39 | None | US + MRI | Rudimentary uterus | 8.5 | LPS |
| 9 | Pati J. Indian Med. Assoc. 2009 [31] | India | 20 | Primary amenorrhea Chronic pain | - | Rudimentary uterus | 8 cm × 7 cm | LPT |
| 10 | Fukuda J. Obstet. Gynaecol. Res 2010 [29] | Japan | 50 | Chronic pain | CT + MRI | Rudimentary uterus | 7 cm | LPT |
| 11 | Chun J. Menopausal Med. 2013 [14] | Korea | 55 | None | MRI | Rudimentary uterus | 5.4 cm × 4.8 cm × 4.7 cm | LPT |
| 12 | Pàez Lòpez Rev. Colomb. Obstet. Ginecol. 2013 [16] | Colombia | 32 | Chronic pain | US | Rudimentary uterus | 9 cm | LPS |
| 13 | Rawat J. Radiol. Case Rep. 2013 [19] | India | 35 | None | US-CT-MRI | Rudimentary uterus | 25 cm × 18 cm × 12 cm | LPT |
| 14 | Kulkarni J. Hum. Reprod. Sci. 2015 [21] | India | 25 | Primary amenorrhea | US-MRI | Rudimentary uterus | 5 cm × 4 cm × 4 cm | LPT |
| 15 | Girma Ethiop. J. Health Sci. 2015 [22] | Ethiopia | 40 | Chronic pain | US | Right broad ligament and right Mullerian bulb | 18 cm × 10 cm × 8 cm | LPT |
| 16 | Narayanan BMJ Case Rep. 2015 [11] | India | 43 | Chronic pain | US + MRI | Rudimentary uterus | - | LPT |
| 17 | Hasegawa Obstet. Gynecol. 2015 [13] | Japan | 40 | Abdominal swelling | US + MRI + CT | Rudimentary uterus | 20 cm × 30 cm | LPT |
| 18 | Park J. Obstet. Gynaecol. 2016 [12] | Korea | 36 | None | CT | Rudimentary uterus | 16 cm × 92 cm | LPT |
| 19 | Salem Facts Views Vis. Obgyn. 2016 [2] | Iraq | 40 | None | US | Right-sides intra-peritoneal space of round ligament | 7 cm × 9 cm | LPT |
| 20 | Dimitriadis J. Reprod. Med. 2016 [23] | USA | 43 | None | - | - | - | LPS+LPT |
| 21 | Amaratunga Ultrasound Quarterly 2017 [3] | Canada | 66 | Chronic pain | US + MRI | Right annexed area | 4.5 cm × 4 cm | LPS |
| 22 | Sharma Int. J. Gynaecol. Obstet. 2017 [10] | India | 45 | Chronic pain | US + CT | Right and left rudimentary horn | 18 cm × 15 cm; 5 cm × 4 cm | LPT |
| 23 | Karthik J. Gynecol. Surg. 2017 [24] | India | 33 | Primary amenorrhea Dyspareunia | US-MRI | Rudimentary uterus | 7.6 cm × 5.3 cm | LPS |
| 24 | Albahlol Eur. J. Obstet. Gynecol. 2019 [30] | Egypt | 45 | Primary amenorrhea Dyspareunia | US + MRI | - | 15 cm × 12 cm | LPT |
| 25 | Jokimma BMC Women’s Health 2020 [9] | Finland | 47 | None | US-MRI | - | 6 cm | - |
| 26 | Harzif Int. J. Surg. Case Rep. 2021 [25] | Indonesia | 38 | Chronic pain | US + MRI | Rudimentary uterus | 6 cm 5 cm | LPS |
| References | Age of Onset | Acute Abdomen | Surgical Approach | Imaging | Dimension of the Lesion | Mass Localization | Type of Surgery |
|---|---|---|---|---|---|---|---|
| YAN, et al. (2002) [6] | 52 | Yes | LPT | US + CT | 15 cm | Rudimentary uterus | Hysterectomy and bilateral salpingo-oophorectomy |
| Galajova, et al. (2003) [37] | 55 | No | LPT | US | 10 cm × 7.5 cm | Not reported | Mass only |
| Petric, et al. (2008) [36] | 53 | Yes | LPT | Not reported | Date not available | Not clear origin | Mass only |
| FLETCHER, et al. (2012) [5] | 28 | Yes | LPT Pfannenstiel | MRI | 10 cm × 15 cm | Paraovarian mass | Mass only |
| VIDYASHREE, et al. (2014) [32] | 40 | No | LPT | CT + MRI | 6 cm × 7 cm and 5 × 6 cm | Rudimental uterus | Bilateral salpingo-oophorectomy and excision of uterine remnant |
| KUNDU, et al. (2014) [33] | 40 | Yes | Vertical LPT | US + CT | 10 cm | Rudimentary uterus | Right salpingo-oophorectomy, excision of right and left hemiuteri with pedunculated leiomyomas, and left salpingectomy |
| YI, et al. (2016) [34] | 47 | Yes | LPS | MRI | Date not available | Rudimentary uterus | Bilateral salpingo-oophorectomy and excision of uterine remnant |
| HOO, et al. (2016) [35] | 45 | Yes | LPT | US + MRI | 15 cm × 13 cm × 13 cm | Right adnexa | Right salpingo-oophorectomy and excision of uterine remnant |
| Case Described by This Article | 50 | Yes | LPT Pfannenstiel | US + CT | 10 cm × 9 cm × 7 cm | Paraovarian mass | Mass only |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, F.; Carlucci, S.; Stabile, G.; Mirenda, G.; Mirandola, M.; Mangino, F.P.; Romano, A.; Ricci, G. The Rare, Unexpected Condition of a Twisted Leiomyoma in Mayer-Rokitansky-Küster-Hauser (MRKH) Syndrome: Etiopathogenesis, Diagnosis and Management. Our Experience and Narrative Review of the Literature. Int. J. Environ. Res. Public Health 2021, 18, 5895. https://doi.org/10.3390/ijerph18115895
Romano F, Carlucci S, Stabile G, Mirenda G, Mirandola M, Mangino FP, Romano A, Ricci G. The Rare, Unexpected Condition of a Twisted Leiomyoma in Mayer-Rokitansky-Küster-Hauser (MRKH) Syndrome: Etiopathogenesis, Diagnosis and Management. Our Experience and Narrative Review of the Literature. International Journal of Environmental Research and Public Health. 2021; 18(11):5895. https://doi.org/10.3390/ijerph18115895
Chicago/Turabian StyleRomano, Federico, Stefania Carlucci, Guglielmo Stabile, Giuseppe Mirenda, Mariateresa Mirandola, Francesco Paolo Mangino, Andrea Romano, and Giuseppe Ricci. 2021. "The Rare, Unexpected Condition of a Twisted Leiomyoma in Mayer-Rokitansky-Küster-Hauser (MRKH) Syndrome: Etiopathogenesis, Diagnosis and Management. Our Experience and Narrative Review of the Literature" International Journal of Environmental Research and Public Health 18, no. 11: 5895. https://doi.org/10.3390/ijerph18115895
APA StyleRomano, F., Carlucci, S., Stabile, G., Mirenda, G., Mirandola, M., Mangino, F. P., Romano, A., & Ricci, G. (2021). The Rare, Unexpected Condition of a Twisted Leiomyoma in Mayer-Rokitansky-Küster-Hauser (MRKH) Syndrome: Etiopathogenesis, Diagnosis and Management. Our Experience and Narrative Review of the Literature. International Journal of Environmental Research and Public Health, 18(11), 5895. https://doi.org/10.3390/ijerph18115895








