Prevalence of Type 2 Diabetes in South Africa: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Outcome Measures
2.3.1. Primary Outcome
2.3.2. Secondary Outcomes
2.4. Study Selection, Quality Assessment and Data Extraction
2.5. Data Synthesis and Analysis
2.6. Confidence in Cumulative Evidence
2.7. Patient and Public Involvement
3. Results
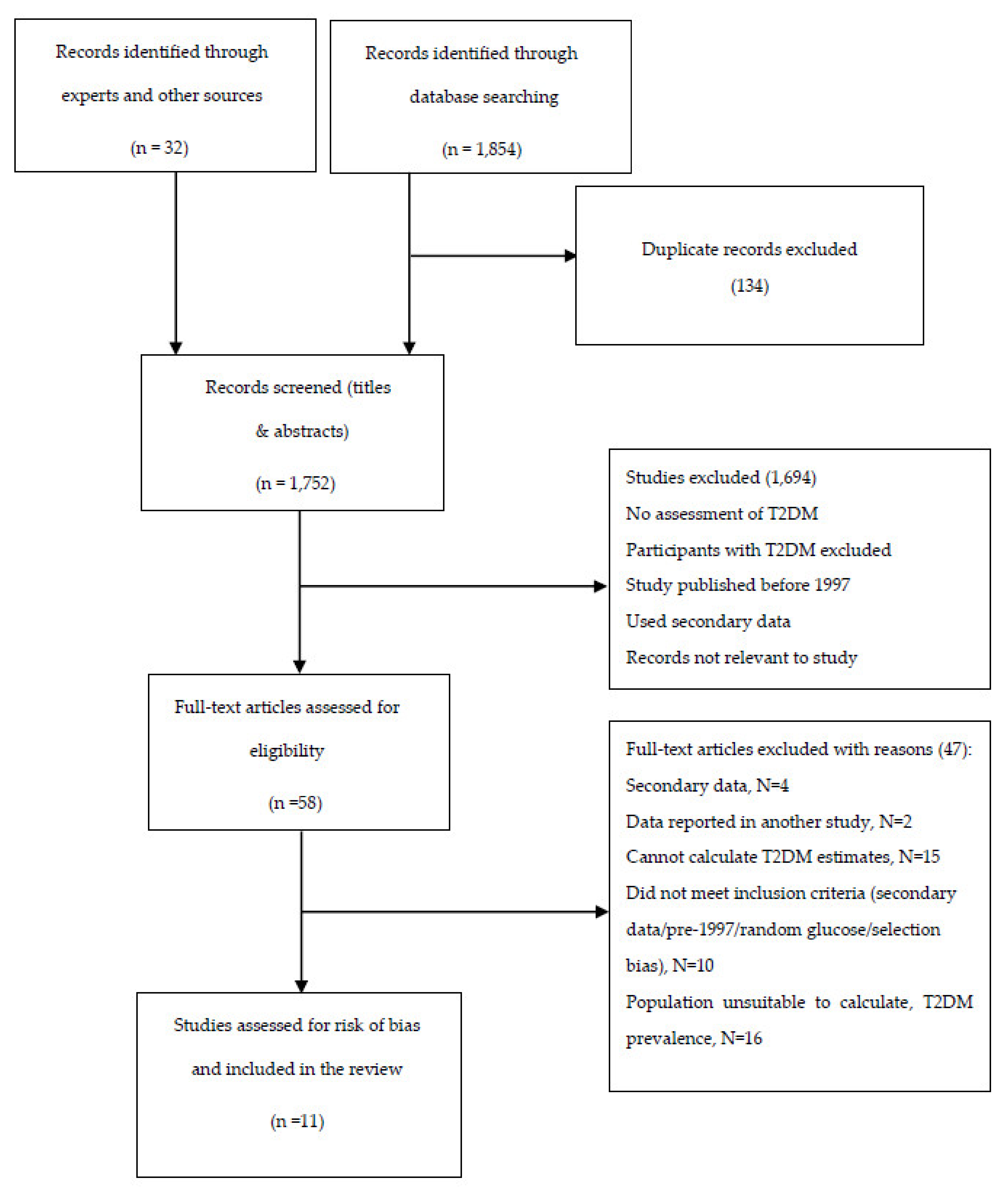
3.1. Results of Study Search
3.2. Characteristics of Included Studies
3.3. Type 2 Diabetes Prevalence
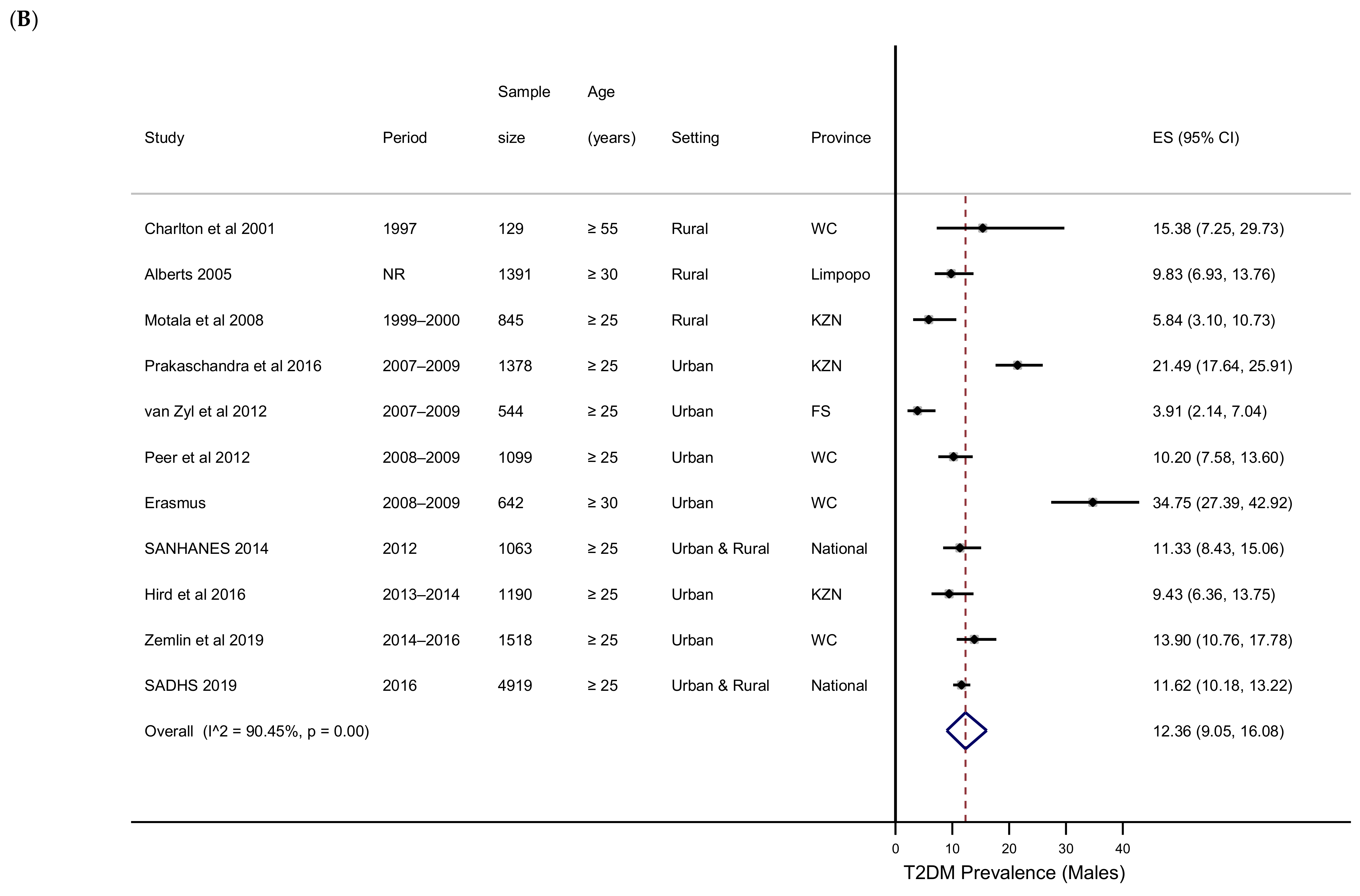
3.4. T2DM Prevalence by Subgroups
3.5. Impaired Glucose Tolerance, Impaired Fasting Glucose and Newly Diagnosed Diabetes
3.6. Publication Bias
3.7. GRADE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. The Top 10 Causes of Death. Available online: http://www.who.int/mediacentre/factsheets/fs310/en/ (accessed on 9 January 2019).
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017. [Google Scholar]
- Mayosi, B.M.; Flisher, A.J.; Lalloo, U.G.; Sitas, F.; Tollman, S.M.; Bradshaw, D. The Burden of Non-Communicable Diseases in South Africa. Lancet 2009, 374, 934–947. [Google Scholar] [CrossRef]
- United Nations Sustainable Develoment Goal 3: Ensure Healthy Lives and Promote Well-Being for All at All Ages. Available online: https://www.un.org/sustainabledevelopment/health/ (accessed on 7 August 2019).
- World Bank. Upper Middle Income; World Bank: Washington, DC, USA, 2014. [Google Scholar]
- Coovadia, H.; Jewkes, R.; Barron, P.; Sanders, D.; McIntyre, D. The Health and Health System of South Africa: Historical Roots of Current Public Health Challenges. Lancet 2009, 374, 817–834. [Google Scholar] [CrossRef]
- Bradshaw, D.; Norman, R.; Pieterse, D.; Levitt, N.S. South African Comparative Risk Assessment Collaborating Group Estimating the Burden of Disease Attributable to Diabetes in South Africa in 2000. South Afr. Med. J. 2007, 97, 700–706. [Google Scholar]
- Bertram, M.Y.; Jaswal, A.V.S.; Van Wyk, V.P.; Levitt, N.S.; Hofman, K.J. The Non-Fatal Disease Burden Caused by Type 2 Diabetes in South Africa, 2009. Glob. Health Action 2013, 6, 19244. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, Regional, and National Prevalence of Overweight and Obesity in Children and Adults during 1980–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Joubert, J.; Norman, R.; Bradshaw, D.; Goedecke, J.H.; Steyn, N.P.; Puoane, T. South African Comparative Risk Assessment Collaborating Group Estimating the Burden of Disease Attributable to Excess Body Weight in South Africa in 2000. South Afr. Med. J. 2007, 97, 683–690. [Google Scholar]
- Peer, N.; Steyn, K.; Lombard, C.; Lambert, E.V.; Vythilingum, B.; Levitt, N.S. Rising Diabetes Prevalence among Urban-Dwelling Black South Africans. PLoS ONE 2012, 7, e43336. [Google Scholar] [CrossRef]
- Erasmus, R.T.; Soita, D.J.; Hassan, M.S.; Blanco-Blanco, E.; Vergotine, Z.; Kegne, A.P.; Matsha, T.E. High Prevalence of Diabetes Mellitus and Metabolic Syndrome in a South African Coloured Population: Baseline Data of a Study in Bellville, Cape Town. South Afr. Med. J. 2012, 102, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Santaguida, P.; Raina, P.; Morrison, K.M.; Balion, C.; Hunt, D.; Yazdi, H.; Booker, L. Annual Incidence and Relative Risk of Diabetes in People with Various Categories of Dysglycemia: A Systematic Overview and Meta-Analysis of Prospective Studies. Diabetes Res. Clin. Pract. 2007, 78, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Levitt, N.S.; Katzenellenbogen, J.M.; Bradshaw, D.; Hoffman, M.N.; Bonnici, F. The Prevalence and Identification of Risk Factors for NIDDM in Urban Africans in Cape Town, South Africa. Diabetes Care 1993, 16, 601–607. [Google Scholar] [CrossRef]
- Omar, M.A.; Seedat, M.A.; Motala, A.A.; Dyer, R.B.; Becker, P. The Prevalence of Diabetes Mellitus and Impaired Glucose Tolerance in a Group of Urban South African Blacks. South Afr. Med. J. 1993, 83, 641–643. [Google Scholar]
- Erasmus, R.T.; Blanco Blanco, E.; Okesina, A.B.; Matsha, T.; Gqweta, Z.; Mesa, J.A. Prevalence of Diabetes Mellitus and Impaired Glucose Tolerance in Factory Workers from Transkei, South Africa. South Afr. Med. J. 2001, 91, 157–160. [Google Scholar]
- Groenewald, A.; Van Wyk, H.J.; Walsh, C.M.; Van Zyl, S.; Van der Merwe, L.J. Prevalence of Diabetes Mellitus in the Rural Southern Free State. South Afr. Fam. Pract. 2009, 51, 502–505. [Google Scholar] [CrossRef]
- Motala, A.A.; Esterhuizen, T.; Gouws, E.; Pirie, F.J.; Omar, M.A.K. Diabetes and Other Disorders of Glycemia in a Rural South African Community: Prevalence and Associated Risk Factors. Diabetes Care 2008, 31, 1783–1788. [Google Scholar] [CrossRef]
- Motala, A.A.; Pirie, F.J.; Gouws, E.; Amod, A.; Omar, M.A.K. High Incidence of Type 2 Diabetes Mellitus in South African Indians: A 10-Year Follow-up Study. Diabet. Med. 2003, 20, 23–30. [Google Scholar] [CrossRef]
- National Department of Health; Statistics South Africa; South African Medical Research Council; ICF. South Africa Demographic and Health Survey 2016; NDoH: Pretoria, South Africa, 2019. [Google Scholar]
- Shisana, O.; Labadarios, D.; Rehle, T.; Simbayi, L.; Zuma, K.; Dhansay, A.; Reddy, P.; Parker, W.; Hoosain, E.; Naidoo, P.; et al. South African National Health and Nutrition Examination Survey (SANHANES-1); HSRC Press: Cape Town, South Africa, 2014. [Google Scholar]
- Kengne, A.P.; Echouffo-Tcheugui, J.-B.; Sobngwi, E.; Mbanya, J.-C. New Insights on Diabetes Mellitus and Obesity in Africa-Part 1: Prevalence, Pathogenesis and Comorbidities. Heart 2013, 99, 979–983. [Google Scholar] [CrossRef]
- Borges Migliavaca, C.; Stein, C.; Colpani, V.; Barker, T.H.; Munn, Z.; Falavigna, M.; on behalf of the Prevalence Estimates Reviews—Systematic Review Methodology Group (PERSyst). How Are Systematic Reviews of Prevalence Conducted? A Methodological Study. BMC Med. Res. Methodol. 2020, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Pheiffer, C.; Pillay-van Wyk, V.; Joubert, J.D.; Levitt, N.; Nglazi, M.D.; Bradshaw, D. The Prevalence of Type 2 Diabetes in South Africa: A Systematic Review Protocol. BMJ Open 2018, 8, e021029. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation 2006; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- World Health Organization. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus. Abbreviated Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2011; Volume WHO/NMH/CHP/CPM/11.1. [Google Scholar]
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Bradshaw, D.; Kielkowski, D.; Sitas, F. New Birth and Death Registration Forms--a Foundation for the Future, a Challenge for Health Workers? South Afr. Med. J. 1998, 88, 971–974. [Google Scholar]
- Pillay-van Wyk, V.; Roomaney, R.; Awotiwon, O.; Tuwara, E.; Nglazi, M.; Ebrahim, A.; Glass, T.; Bradshaw, D. Burden of Disease Review Manager for Systematic Review of Observational Studies: Technical Report and User Guide; South African Medical Research Council: Cape Town, South Africa, 2018. [Google Scholar]
- Hoy, D.; Brooks, P.; Woolf, A.; Blyth, F.; March, L.; Bain, C.; Baker, P.; Smith, E.; Buchbinder, R. Assessing Risk of Bias in Prevalence Studies: Modification of an Existing Tool and Evidence of Interrater Agreement. J. Clin. Epidemiol. 2012, 65, 934–939. [Google Scholar] [CrossRef]
- Werfalli, M.; Musekiwa, A.; Engel, M.E.; Ross, I.; Kengne, A.P.; Levitt, N.S. The Prevalence of Type 2 Diabetes Mellitus among Older People in Africa: A Systematic Review Study Protocol. BMJ Open 2014, 4, e004747. [Google Scholar] [CrossRef] [PubMed]
- Werfalli, M.; Engel, M.E.; Musekiwa, A.; Kengne, A.P.; Levitt, N.S. The Prevalence of Type 2 Diabetes among Older People in Africa: A Systematic Review. Lancet Diabetes Endocrinol. 2016, 4, 72–84. [Google Scholar] [CrossRef]
- Nyaga, V.N.; Arbyn, M.; Aerts, M. Metaprop: A Stata Command to Perform Meta-Analysis of Binomial Data. Arch. Public Health 2014, 72, 39. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D. Meta-Analysis. Potentials and Promise. BMJ 1997, 315, 1371–1374. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Evidence Prime, Inc. GRADEpro GDT: GRADEpro Guideline Development Tool [Software], McMaster University: Hamilton, ON, USA, 2015.
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schunemann, H.J. GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Murad, M.H.; Mustafa, R.A.; Schünemann, H.J.; Sultan, S.; Santesso, N. Rating the Certainty in Evidence in the Absence of a Single Estimate of Effect. Evid. Based Med. 2017, 22, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Hird, T.R.; Pirie, F.J.; Esterhuizen, T.M.; O’Leary, B.; McCarthy, M.I.; Young, E.H.; Sandhu, M.S.; Motala, A.A. Burden of Diabetes and First Evidence for the Utility of HbA1c for Diagnosis and Detection of Diabetes in Urban Black South Africans: The Durban Diabetes Study. PLoS ONE 2016, 11, e0161966. [Google Scholar] [CrossRef]
- Zemlin, A.E.; Barkhuizen, M.; Kengne, A.P.; Erasmus, R.T.; Matsha, T.E. Performance of Glycated Albumin for Type 2 Diabetes and Prediabetes Diagnosis in a South African Population. Clin. Chim. Acta 2018, 488, 122–128. [Google Scholar] [CrossRef]
- Pillay-van Wyk, V.; Msemburi, W.; Laubscher, R.; Dorrington, R.E.; Groenewald, P.; Glass, T.; Nojilana, B.; Joubert, J.D.; Matzopoulos, R.; Prinsloo, M.; et al. Mortality Trends and Differentials in South Africa from 1997 to 2012: Second National Burden of Disease Study. Lancet Glob. Health 2016, 4, e642–e653. [Google Scholar] [CrossRef]
- Gujral, U.P.; Mohan, V.; Pradeepa, R.; Deepa, M.; Anjana, R.M.; Mehta, N.K.; Gregg, E.W.; Narayan, K. Ethnic Variations in Diabetes and Prediabetes Prevalence and the Roles of Insulin Resistance and β-Cell Function: The CARRS and NHANES Studies. J. Clin. Transl. Endocrinol. 2016, 4, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Goedecke, J.H.; Levitt, N.S.; Evans, J.; Ellman, N.; Hume, D.J.; Kotze, L.; Tootla, M.; Victor, H.; Keswell, D. The Role of Adipose Tissue in Insulin Resistance in Women of African Ancestry. J. Obes. 2013, 2013, 952916. [Google Scholar] [CrossRef] [PubMed]
- Goedecke, J.H.; Mtintsilana, A.; Dlamini, S.N.; Kengne, A.P. Type 2 Diabetes Mellitus in African Women. Diabetes Res. Clin. Pract. 2017, 123, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Chandler-Laney, P.C.; Phadke, R.P.; Granger, W.M.; Fernández, J.R.; Muñoz, J.A.; Man, C.D.; Cobelli, C.; Ovalle, F.; Gower, B.A. Age-Related Changes in Insulin Sensitivity and β-Cell Function among European-American and African-American Women. Obesity 2011, 19, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.L.; Ayles, H.; Beyers, N.; Godfrey-Faussett, P.; Muyoyeta, M.; Du Toit, E.; Yudkin, J.S.; Floyd, S. Diabetes Mellitus in Zambia and the Western Cape Province of South Africa: Prevalence, Risk Factors, Diagnosis and Management. Diabetes Res. Clin. Pract. 2016, 118, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, S.; Van der Merwe, L.J.; Walsh, C.M.; Groenewald, A.J.; Van Rooyen, F.C. Risk-Factor Profiles for Chronic Diseases of Lifestyle and Metabolic Syndrome in an Urban and Rural Setting in South Africa. Afr. J. Prim. Health Care Fam. Med. 2012, 4, 346. [Google Scholar] [CrossRef]
- Goedecke, J.H.; George, C.; Veras, K.; Peer, N.; Lombard, C.; Victor, H.; Steyn, K.; Levitt, N.S. Sex Differences in Insulin Sensitivity and Insulin Response with Increasing Age in Black South African Men and Women. Diabetes Res. Clin. Pract. 2016, 122, 207–214. [Google Scholar] [CrossRef]
- Crowther, N.J.; Norris, S.A. The Current Waist Circumference Cut Point Used for the Diagnosis of Metabolic Syndrome in Sub-Saharan African Women Is Not Appropriate. PLoS ONE 2012, 7, e48883. [Google Scholar] [CrossRef]
- Dias, S.; Adam, S.; Rheeder, P.; Pheiffer, C. Prevalence of and Risk Factors for Gestational Diabetes Mellitus in South Africa. South Afr. Med. J. 2019, 109, 463–467. [Google Scholar] [CrossRef]
- Danaei, G.; Fahimi, S.; Lu, Y.; Zhou, B.; Hajifathalian, K.; Di Cesare, M.; Lo, W.; Reis-Santos, B.; Cowan, M.; Shaw, J.; et al. Effects of Diabetes Definition on Global Surveillance of Diabetes Prevalence and Diagnosis: A Pooled Analysis of 96 Population-Based Studies with 331 288 Participants. Lancet Diabetes Endocrinol. 2015, 3, 624–637. [Google Scholar] [CrossRef]
- Kengne, A.P.; Erasmus, R.T.; Levitt, N.S.; Matsha, T.E. Alternative Indices of Glucose Homeostasis as Biochemical Diagnostic Tests for Abnormal Glucose Tolerance in an African Setting. Prim. Care Diabetes 2017, 11, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Oni, T.; Berkowitz, N.; Kubjane, M.; Goliath, R.; Levitt, N.S.; Wilkinson, R.J. Trilateral Overlap of Tuberculosis, Diabetes and HIV-1 in a High-Burden African Setting: Implications for TB Control. Eur. Respir. J. 2017, 50, 1700004. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC) Worldwide Trends in Diabetes since 1980: A Pooled Analysis of 751 Population-Based Studies with 4.4 Million Participants. Lancet 2016, 387, 1513–1530. [CrossRef]
- Zemlin, A.E.; Matsha, T.E.; Hassan, M.S.; Erasmus, R.T. HbA1c of 6.5% to Diagnose Diabetes Mellitus—Does It Work for Us?—The Bellville South Africa Study. PLoS ONE 2011, 6, e22558. [Google Scholar] [CrossRef]
- Levitt, N.S.; Peer, N.; Steyn, K.; Lombard, C.; Maartens, G.; Lambert, E.V.; Dave, J.A. Increased Risk of Dysglycaemia in South Africans with HIV; Especially Those on Protease Inhibitors. Diabetes Res. Clin. Pract. 2016, 119, 41–47. [Google Scholar] [CrossRef]
- Abrahams, Z.; Dave, J.A.; Maartens, G.; Levitt, N.S. Changes in Blood Pressure, Glucose Levels, Insulin Secretion and Anthropometry after Long Term Exposure to Antiretroviral Therapy in South African Women. AIDS Res. Ther. 2015, 12, 24. [Google Scholar] [CrossRef]
- Manne-Goehler, J.; Montana, L.; Gómez-Olivé, F.X.; Rohr, J.; Harling, G.; Wagner, R.G.; Wade, A.; Kabudula, C.W.; Geldsetzer, P.; Kahn, K.; et al. The ART Advantage: Health Care Utilization for Diabetes and Hypertension in Rural South Africa. J. Acquir. Immune Defic. Syndr. 2017, 75, 561–567. [Google Scholar] [CrossRef]
- Schoffelen, A.F.; De Groot, E.; Tempelman, H.A.; Visseren, F.L.J.; Hoepelman, A.I.M.; Barth, R.E. Carotid Intima Media Thickness in Mainly Female HIV-Infected Subjects in Rural South Africa: Association With Cardiovascular but Not HIV-Related Factors. Clin. Infect. Dis. 2015, 61, 1606–1614. [Google Scholar] [CrossRef]
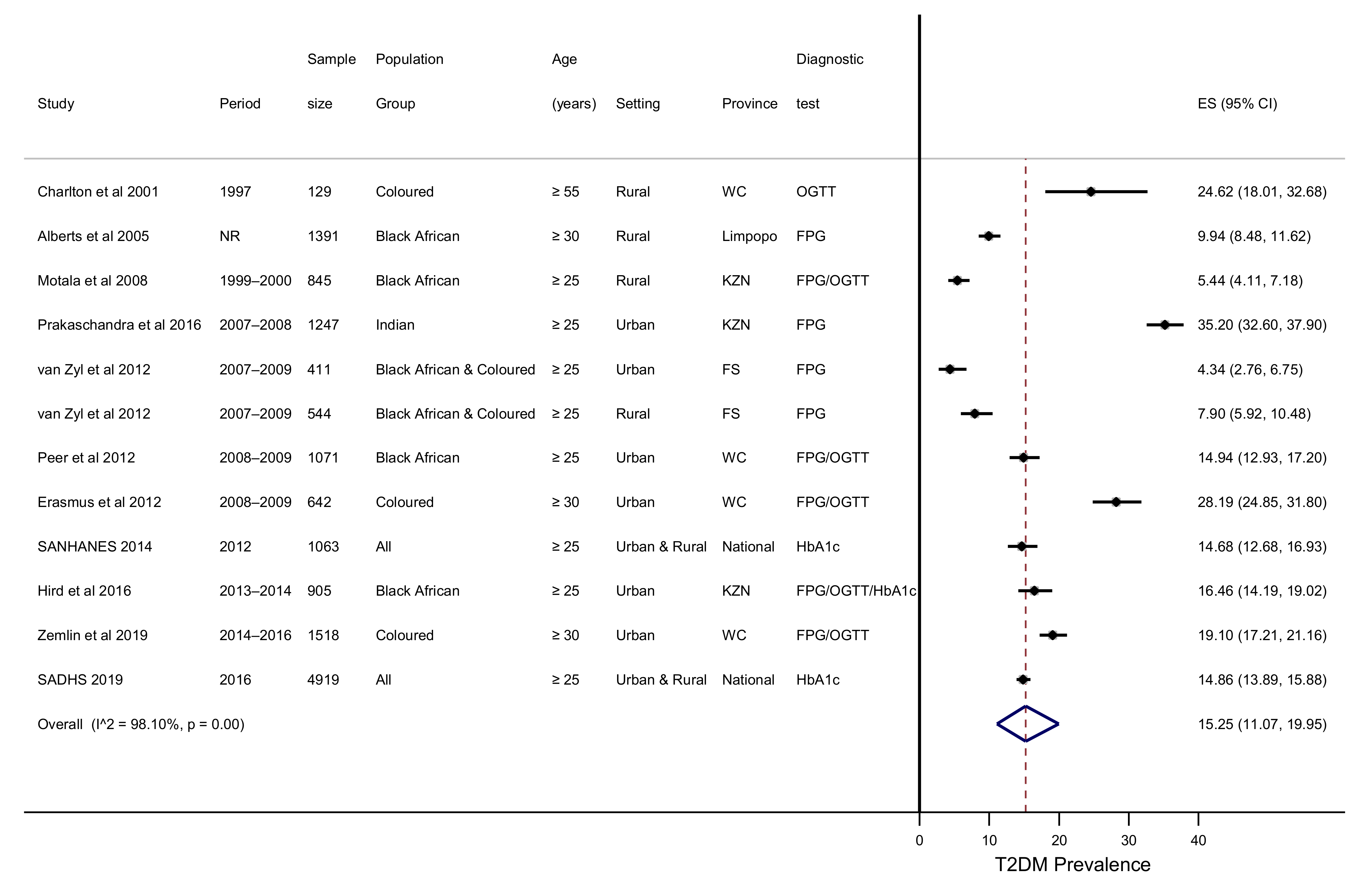


| Condition | Pooled Estimate (95% CI) | I2, p Value |
|---|---|---|
| T2DM (%) | 15.25 (11.07–19.95) | 98.10%, p < 0.001 |
| IGT (%) | 9.59 (5.82–14.17) | 94.18%, p < 0.001 |
| IFG (%) | 3.55 (0.38–9.61) | 98.68%, p < 0.001 |
| Newly detected T2DM (%) | 8.29 (4.97–12.34) | 94.20%, p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pheiffer, C.; Pillay-van Wyk, V.; Turawa, E.; Levitt, N.; Kengne, A.P.; Bradshaw, D. Prevalence of Type 2 Diabetes in South Africa: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 5868. https://doi.org/10.3390/ijerph18115868
Pheiffer C, Pillay-van Wyk V, Turawa E, Levitt N, Kengne AP, Bradshaw D. Prevalence of Type 2 Diabetes in South Africa: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2021; 18(11):5868. https://doi.org/10.3390/ijerph18115868
Chicago/Turabian StylePheiffer, Carmen, Victoria Pillay-van Wyk, Eunice Turawa, Naomi Levitt, Andre P. Kengne, and Debbie Bradshaw. 2021. "Prevalence of Type 2 Diabetes in South Africa: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 18, no. 11: 5868. https://doi.org/10.3390/ijerph18115868
APA StylePheiffer, C., Pillay-van Wyk, V., Turawa, E., Levitt, N., Kengne, A. P., & Bradshaw, D. (2021). Prevalence of Type 2 Diabetes in South Africa: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 18(11), 5868. https://doi.org/10.3390/ijerph18115868






