A Single Session of Whole-Body Electromyostimulation Increases Muscle Strength, Endurance and proNGF in Early Parkinson Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Experimental Procedures
2.4. Experimental Set Up
2.5. Baseline Assessment
2.5.1. Physical Fitness Assessment
2.5.2. Neurotrophic Factors Serum Levels
2.6. Statistical Analysis
3. Results
3.1. Physical Assessment
3.2. Neurotrophic Factors
3.3. Scales for the Assessment of the Pathology
4. Discussion
Limitations
- Small number of enrolled participants, with different characteristics and PD symptoms;
- Data collected are not generalizable to all clinical diagnosis of PD patients since participants included in this study were in the stage from mild (1) to moderate (3) assessed by the Hoehn and Yahr scale;
- The acute effects of physical activity with superimposed WB-EMS were evaluated. Further studies will assess long-term effects of the WB-EMS protocol on PD patients;
- Further studies will confirm these outcomes by enrolling a higher number of PD patients, in order to obtain a more reliable statistical result.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Dorsey, E.R.; Elbaz, A.; Nichols, E.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.Y.J.; Collado-Mateo, D.; et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef]
- Gronek, P.; Haas, A.N.; Czarny, W.; Podstawski, R.; do Santos Delabary, M.; Clark, C.C.; Boraczyński, M.; Tarnas, M.; Wycichowska, P.; Pawlaczyk, M.; et al. The mechanism of physical activity-induced amelioration of Parkinson’s disease: A narrative review. Aging Dis. 2021, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease: MDS-PD clinical diagnostic criteria. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Pluck, G.C. Apathy in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2002, 73, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.H.; Brown, R.G.; Comella, C.; Garber, C.E.; Krupp, L.B.; Lou, J.S.; Marsh, L.; Nail, L.; Shulman, L.; Taylor, C.B. Working group on fatigue in Parkinson’s disease. Fatigue in Parkinson’s disease: A review. Mov. Disord. 2007, 22, 297–308. [Google Scholar] [CrossRef]
- Calabresi, P.; Picconi, B.; Parnetti, L.; Filippo, M.D. A Convergent Model for Cognitive Dysfunctions in Parkinson’s Disease: The Critical Dopamine–Acetylcholine Synaptic Balance. Lancet Neurol. 2006, 5, 974–983. [Google Scholar] [CrossRef]
- Garcia-Ruiz, P.J.; Espay, A.J. Parkinson disease: An evolutionary perspective. Front. Neurol. 2017, 8, 5. [Google Scholar] [CrossRef]
- Aarsland, D.; Larsen, J.P.; Tandberg, E.; Laake, K. Predictors of nursing home placement in Parkinson’s disease: A populationbased, prospective study. J. Am. Geriatr. Soc. 2000, 48, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Lauzé, M.; Daneault, J.F.; Duval, C. The effects of physical activity in Parkinson’s disease: A review. J. Parkinsons Dis. 2016, 6, 685–698. [Google Scholar] [CrossRef]
- Portugal, E.M.M.; Cevada, T.; Monteiro-Junior, R.S.; Guimarães, T.T.; Blois, C.; Deslandes, A.C. Neuroscience of exercise: From neurobiology mechanisms to mental health. Neuropsychobiology 2013, 68, 1–14. [Google Scholar] [CrossRef]
- Voss, M.; Prakash, R.S.; Erickson, K.I.; Basak, C.; Chaddock, L.; Alves, H.; Heo, S.; Szabo, A.N.; White, S.M.; Wójcicki, T.R.; et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front. Aging Neurosci. 2010, 2, 32. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, E.; Fiorilli, G.; Aquino, G.; Di Costanzo, A.; Calcagno, G.; di Cagno, A. Twelve-week exercise influences memory complaint but not memory performance in older adults: A randomized controlled study. J. Aging Phys. Act. 2017, 25, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Schlenstedt, C.; Paschen, S.; Kruse, A.; Raethjen, J.; Weisser, B.; Deuschl, G. Resistance versus balance training to improve postural control in Parkinson’s disease: A randomized rater blinded controlled study. PLoS ONE 2015, 17, e0140584. [Google Scholar] [CrossRef]
- David, F.J.; Robichaud, J.A.; Leurgans, S.E.; Poon, C.; Kohrt, W.M.; Goldman, J.G.; Comella, C.L.; Vaillancourt, D.E.; Corcos, D.M. Exercise improves cognition in Parkinson’s disease: The PRET-PD randomized, clinical trial. Mov. Disord. 2015, 30, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.H.; Moore, K.; Browner, N.; Sklerov, M.; Dayan, E. Physical Activity mediates the association between striatal dopamine transporter availability and cognition in Parkinson’s disease. Parkinsonism Relat. Disord. 2019, 62, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Stengel, S.V.; Schwarz, J.; Mayhew, J.L. Effect of whole-body electromyostimulation on energy expenditure during exercise. J. Strength Cond. Res. 2012, 26, 240–245. [Google Scholar] [CrossRef]
- Ramazzina, I.; Bernazzoli, B.; Costantino, C. Systematic review on strength training in Parkinson’s disease: An unsolved question. Clin. Interv. Aging 2017, 12, 619. [Google Scholar] [CrossRef]
- Tidman, M.; Skotzke, E. Effects of a community-based exercise program on mobility, balance, cognition, sleep, activities of daily living, and quality of life in PD: A pilot study. Neurodegener. Dis. Man. 2017, 10, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Van Nimwegen, M.; Speelman, A.D.; Overeem, S.; Deeg, D.J.H.; Borm, G.F.; Bloem, B.R.; Munneke, M. Physical inactivity in Parkinson’s disease. J. Neurol. 2011, 258, 2214–2221. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthi, N.; Fleury, J.; Belyea, M.; Shill, H.A.; Abbas, J.J. ReadySteady intervention to promote physical activity in older adults with Parkinson’s disease: Study design and methods. Contemp. Clin. Trials Commun. 2020, 17, 100513. [Google Scholar] [CrossRef]
- LaHue, S.C.; Comella, C.L.; Tanner, C.M. The Best Medicine? The influence of physical activity and inactivity on Parkinson’s disease. Mov. Disord. 2016, 31, 1444–1454. [Google Scholar] [CrossRef]
- McGill, A.; Houston, S.; Lee, R.Y. Dance for Parkinson’s: A new framework for research on its physical, mental, emotional, and social benefits. Complement. Ther. Med. 2014, 22, 426–432. [Google Scholar] [CrossRef]
- Schenkman, M.; Moore, C.G.; Kohrt, W.M.; Hall, D.A.; Delitto, A.; Comella, C.L.; Josbeno, D.A.; Christiansen, C.L.; Berman, B.D.; Kluger, B.M.; et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: A phase 2 randomized clinical trial. JAMA Neurol. 2018, 75, 219–226. [Google Scholar] [CrossRef]
- Pistone, E.M.; Laudani, L.; Camillieri, G.; di Cagno, A.; Tomassi, G.; Macaluso, A.; Giombini, A. Effects of early whole-body vibration treatment on knee neuromuscular function and postural control after anterior cruciate ligament reconstruction: A randomized controlled trial. J. Rehab. Med. 2016, 48, 880–886. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.M.D.; Felício, D.C.; Filho, J.E.; Durigan, J.L.Q.; Fonseca, D.S.; José, A.; Oliveira, C.C.; Malaguti, C. Effects of whole-body electromyostimulation on function, muscle mass, strength, social participation, and falls-efficacy in older people: A randomized trial protocol. PLoS ONE 2021, 16, e0245809. [Google Scholar] [CrossRef]
- Kemmler, W.; Teschler, M.; Weissenfels, A.; Bebenek, M.; Von Stengel, S.; Kohl, M.; Freiberger, E.; Goisser, S.; Jakob, F.; Sieber, C.; et al. Whole-body electromyostimulation to fight sarcopenic obesity in community-dwelling older women at risk. Results of the randomized controlled FORMOsA-sarcopenic obesity study. Osteo. Int. 2016, 27, 3261–3270. [Google Scholar] [CrossRef]
- Pano-Rodriguez, A.; Beltran-Garrido, J.V.; Hernandez-Gonzalez, V.; Reverter-Masia, J. Effects of Whole body electromyostimulation on physical fitness and health in postmenopausal women: A study protocol for a randomized controlled trial. Front. Public Health 2020, 8, 12. [Google Scholar] [CrossRef]
- Watanabe, K.; Yoshida, T.; Ishikawa, T.; Kawade, T.; Moritani, T. Effect of the combination of whole-body neuromuscular electrical stimulation and voluntary exercise on metabolic responses in human. Front. Physiol. 2019, 10, 8. [Google Scholar] [CrossRef]
- Wahl, P.; Hein, M.; Achtzehn, S.; Bloch, W.; Mester, J. Acute effects of superimposed electromyostimulation during cycling on myokines and markers of muscle damage. J. Musculoskelet. Neuronal. Interact. 2015, 15, 53–59. [Google Scholar] [PubMed]
- Huang, Y.; Yun, W.; Zhang, M.; Luo, W.; Zhou, X. Serum concentration and clinical significance of brain-derived neurotrophic factor in patients with Parkinson’s disease or essential tremor. J. Int. Med. Res. 2018, 46, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Tosserams, A.; de Vries, N.M.; Bloem, B.R.; Nonnekes, J. Multidisciplinary care to optimize functional mobility in Parkinson disease. Clin. Geriatr. Med. 2020, 36, 159–172. [Google Scholar] [CrossRef]
- Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C.; Stebbins, G.T.; Counsell, C.; Giladi, N.; Holloway, R.G.; Moore, C.G.; Wenning, G.K.; et al. Movement disorder society task force report on the Hoehn and Yahr staging scale: Status and recommendations the movement disorder society task force on rating scales for Parkinson’s disease. Mov. Disord. 2004, 19, 1020–1028. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression, and mortality. Neurology 1998, 50, 318. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; Mchugh, R. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Enright, P.L. The six-minute walk test. Respir. Care. 2003, 48, 3. [Google Scholar]
- Pano-Rodriguez, A.; Beltran-Garrido, J.V.; Hernández-González, V.; Reverter-Masia, J. Effects of whole-body electromyostimulation on health and performance: A systematic review. Complement. Altern. Med. 2019, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Weissenfels, A.; Willert, S.; Shojaa, M.; von Stengel, S.; Filipovic, A.; Kleinöder, H.; Berger, J.; Fröhlich, M. Efficacy and safety of low frequency whole-body electromyostimulation (WB-EMS) to improve health-related outcomes in non-athletic adults. A systematic review. Front. Physiol. 2018, 9, 19. [Google Scholar] [CrossRef]
- Borg, G.A.; Noble, B.J. Perceived exertion. Exerc. Sport Sci. Rev. 1974, 2, 131–154. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sport Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Washburn, R.A.; McAuley, E.; Katula, J.; Mihalko, S.L.; Boileau, R.A. The physical activity scale for the elderly (PASE): Evidence for validity. J Clin Epidemiol. 1999, 52, 643–651. [Google Scholar] [CrossRef]
- Logan, S.L.; Gottlieb, B.H.; Maitland, S.B.; Meegan, D. The physical activity scale for the elderly (PASE) questionnaire; Does it predict physical health? Int. J. Env. Res. Public Health 2013, 10, 3967. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Marie, D.; Fredrick, A.; Bertram, J.; Utley, K.; Fess, E.E. Predicting hand function in older adults: Evaluations of grip strength, arm curl strength, and manual dexterity. Aging Clin. Exp. Res. 2017, 29, 753–760. [Google Scholar] [CrossRef]
- Massy-Westropp, N.M.; Gill, T.K.; Taylor, A.W.; Bohannon, R.W.; Hill, C.L. Hand grip strength: Age and gender stratified normative data in a population-based study. BMC Res. Notes 2011, 4, 127. [Google Scholar] [CrossRef]
- Jones, C.J.; Rikli, R.E.; Beam, W.C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Quart. Exerc. Sport 1999, 70, 113–119. [Google Scholar] [CrossRef]
- Cancela, J.M.; Varela, S.; Ayán, C. The “8-Foot Up and Go” Test as a physical performance measurement in Parkinson’s disease: A pilot study. Rev. Ecuat. Neurol. 2013, 22, 20–23. [Google Scholar]
- Jones, C.J.; Rikli, R.E.; Max, J.; Noffal, G. The Reliability and validity of a chair sit-and-reach test as a measure of hamstring flexibility in older adults. Res. Quart. Exerc. Sport 1998, 69, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.A. Tests for fitness in older adults: AAHPERD fitness task force. J. Physical Educ. Recreat. Dance 1989, 60, 66–71. [Google Scholar] [CrossRef]
- Tinetti, E.; Williams, T.F.; Mayewski, R. Fall risk index for elderly patients based on number of chronic disabilities. Am. J. Med. 1986, 80, 6. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, C.; Zhang, Q.; Wu, W.; Sun, J. Serum BDNF discriminates Parkinson’s disease patients with depression from without depression and reflect motor severity and gender differences. J. Neurol. 2021, 268, 1411–1418. [Google Scholar] [CrossRef]
- Davis, R.L.; Wong, S.L.; Carling, P.J.; Payne, T.; Sue, C.M.; Bandmann, O. Serum FGF-21, GDF-15, and blood MtDNA copy number are not biomarkers of Parkinson disease. Neurol. Clin. Pract. 2019, 10, 40–46. [Google Scholar] [CrossRef]
- Soligo, M.; Protto, V.; Florenzano, F.; Bracci-Laudiero, L.; De Benedetti, F.; Chiaretti, A.; Manni, L. The mature/pro nerve growth factor ratio is decreased in the brain of diabetic rats_ Analysis by Elisa methods. Brain Res. 2015, 1624, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Rawson, K.S.; Cavanaugh, J.T.; Colon-Semenza, C.; DeAngelis, T.; Duncan, R.P.; Fulford, D.; LaValley, M.P.; Mazzoni, P.; Nordahl, T.; Quintiliani, L.M.; et al. Design of the WHIP-PD study: A phase II, twelve-month, dual-site, randomized controlled trial evaluating the effects of a cognitive-behavioral approach for promoting enhanced walking activity using mobile health technology in people with Parkinson-disease. BMC Neurol. 2020, 20, 146. [Google Scholar] [CrossRef]
- Ellis, T.; Latham, N.K.; DeAngelis, T.R.; Thomas, C.A.; Saint-Hilaire, M.; Bickmore, T.W. Feasibility of a virtual exercise coach to promote walking in community-dwelling persons with Parkinson disease. Am. J. Phys. Med. Rehabil. 2013, 92, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Miwa, H.; Miwa, T. Fatigue in patients with Parkinson’s disease: Impact on quality of life. Intern. Med. 2011, 50, 1553–1558. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Herlofson, K.; Kluger, B.M. Fatigue in Parkinson’s disease. J. Neurol. Sci. 2017, 374, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.; Edison, B.; Mantri, S.; Albert, S.; Daeschler, M.; Kopil, C.; Marras, C.; Chahine, L.M. Triggers and alleviating factors for fatigue in Parkinson’s disease. PLoS ONE 2021, 16, e0245285. [Google Scholar] [CrossRef] [PubMed]
- Winward, C.; Sackley, C.; Meek, C.; Izadi, H.; Barker, K.; Wade, D.; Dawes, H. Weekly exercise does not improve fatigue levels in Parkinson’s disease. Mov. Disord. 2012, 27, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, B.; Hagell, P.; Hansson, O.; Nilsson, M.H. Factors associated with fear of falling in people with Parkinson’s disease. BMC Neurol. 2014, 14, 1–7. [Google Scholar] [CrossRef]
- Fiorilli, G.; Iuliano, E.; Aquino, G.; Campanella, E.; Tsopani, D.; Di Costanzo, A.; Calcagno, G.; Di Cagno, A. Different consecutive training protocols to design an intervention program for overweight youth: A controlled study. Diabetes Metab. Syndr. Obes. 2017, 10, 37. [Google Scholar] [CrossRef]
- Rejeski, W.J.; Katula, J.; Rejeski, A.; Rowley, J.; Sipe, M. Strength training in older adults: Does desire determine confidence? J. Gerontol. B. Psychol. Sci. Soc. Sci. 2005, 60, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Warabi, T.; Noda, H.; Yanagisawa, N.; Tashiro, K.; Shindo, R. Changes in sensorimotor function associated with the degree of bradykinesia of Parkinson’s disease. Brain 1986, 109, 1209–1224. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, N.; Fujimoto, S.; Tamaru, F. Bradykinesia in Parkinson’s disease: Disorders of onset and execution of fast movement. Europ. Neurol. 1989, 29, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Gondin, J.; Guette, M.; Ballay, Y.; Martin, A. Neural and muscular changes to detraining after electrostimulation training. Europ. J Appl. Physiol. 2006, 97, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Maffiuletti, N.A.; Cometti, G.; Amiridis, I.G.; Martin, A.; Pousson, M.; Chatard, J. The effects of electromyostimulation training and basketball practice on muscle strength and jumping ability. Int. J. Sports Med. 2000, 21, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Saldıran, T.Ç.; Atıcı, E.; Rezaei, D.A.; Öztürk, Ö.; Uslu, B.; Özcan, B.A.; Okudan, B. The acute effects of different intensity whole-body vibration exposure on muscle tone and strength of the lower legs, and hamstring flexibility: A pilot study. J. Sport Rehab. 2021, 30, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, E.; di Cagno, A.; Cristofano, A.; Angiolillo, A.; D’Aversa, R.; Ciccotelli, S.; Corbi, G.; Fiorilli, G.; Calcagno, G.; Di Costanzo, A. Physical exercise for prevention of dementia (EPD) study: Background, design and methods. BMC Public Health 2019, 19, 659. [Google Scholar] [CrossRef]
- Zetusky, W.J.; Jankovic, J.; Pirozzolo, F.J. The heterogeneity of Parkinson’s disease clinical and prognostic implications. Neurology 1985, 35, 522. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, C.; Yun, W. Peripheral BDNF/TrkB protein expression is decreased in Parkinson’s disease but not in essential tremor. J. Clin. Neurosci. 2019, 63, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Chassot, M.; Dussan-Sarria, J.A.; Sehn, F.C.; Deitos, A.; de Souza, A.; Vercelino, R.; Torres, I.L.; Fregni, F.; Caumo, W. Electroacupuncture analgesia is associated with increased serum brain-derived neurotrophic factor in chronic tension-type headache: A randomized, sham controlled, crossover trial. BMC Complement. Altern. Med. 2015, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Dong, M.X.; Feng, X.; Liu, Y.; Pan, Y.X.; Jia, S.Y.; Cao, D.; Wei, Y.D. Decreased serum ProNGF concentration in patients with Parkinson’s disease. Neurol. Sci. 2018, 39, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Soligo, M.; Nori, S.L.; Protto, V.; Florenzano, F.; Manni, L. Acupuncture and neurotrophin modulation. Int. Rev. Neurobiol. 2013, 111, 91–124. [Google Scholar] [PubMed]
- Aloe, L.; Bracci-Laudiero, L.; Alleva, E.; Lambiase, A.; Micera, A.; Tirassa, P. Emotional stress induced by parachute jumping enhances blood nerve growth factor levels and the distribution of nerve growth factor receptors in lymphocytes. Proc. Natl. Acad. Sci. USA 1994, 91, 10440–10444. [Google Scholar] [CrossRef]
- Stener-Victorin, E.; Lundeberg, T.; Cajander, S.; Aloe, L.; Manni, L.; Waldenstrom, U.; Janson, P.O. Steroid-induced polycystic ovaries in rats: Effect of electro-acupuncture on concentrations of endothelin-1 and nerve growth factor (NGF), and expression of NGF MRNA in the ovaries, the adrenal glands, and the central nervoussystem. Reprod. Biol. Endocrinol. 2003, 1, 33. [Google Scholar] [CrossRef] [PubMed]


| Variables | Mean ± SD |
|---|---|
| Age | 72.60 ± 6.82 |
| Gender (n) | |
| Males | 6 |
| Females | 4 |
| Hoehn and Yahr scale | 1.60 ± 0.52 |
| UPDRS score | 40.20 ± 20.67 |
| MMSE score | 26.60 ± 1.26 |
| PASE score | 69.31 ± 47.86 |
| WB-EMS | No WB-EMS | Intervention | Time | Interaction | |||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | F (1,9) | F (1,9) | F (1,9) | |
| Tremor (Hz) | 48.8 (0.7) | 48.3 (0.6) | 48.8 (0.7) | 48.4 (0.6) | F < 1; p = 0.86 | F = 3.3; p = 0.10 | F < 1; p = 0.86 |
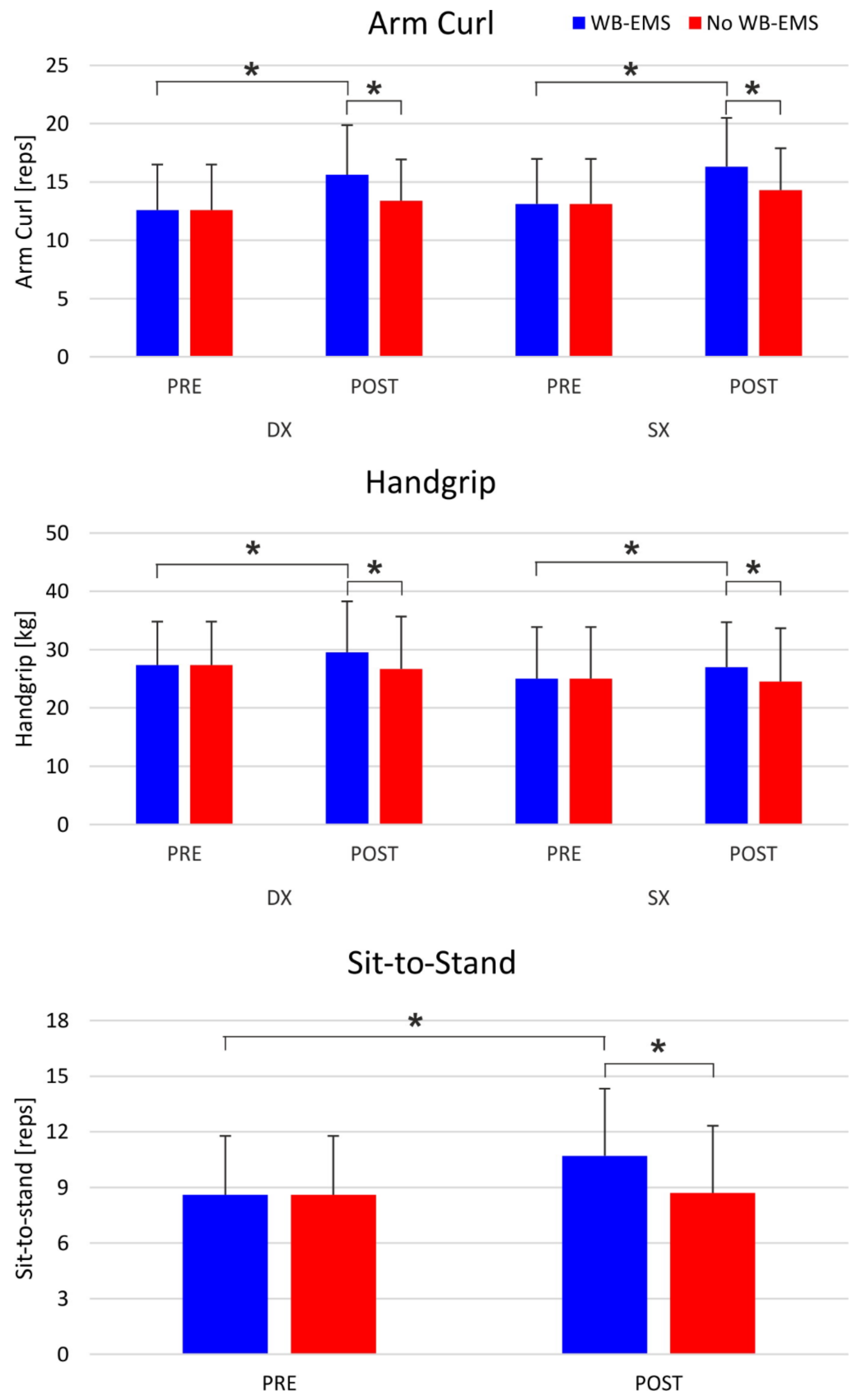
| Sit-To-Stand (reps) *, §, # | 8.6 (3.2) | 10.7 (3.6) | 8.6 (3.2) | 8.7 (3.6) | F = 20.1; p < 0.001 | F = 12.9; p < 0.01 | F = 20.0; p < 0.01 |
| Soda pop (s) *, # | 12.2 (5.4) | 11.5 (6.5) | 12.2 (5.4) | 14.4 (8.5) | F = 10.1; p = 0.011 | F < 1; p = 0.35 | F = 10.1; p = 0.011 |
| 8-feet up & go (s) | 16.5 (12.0) | 14.2 (11.5) | 16.5 (12.0) | 17.5 (14.3) | F = 1.24; p = 0.29 | F = 0.15; p = 0.69 | F = 1.24; p = 0.29 |
| 6-mwt (m) | 308.7 (132.0) | 310.2 (131.8) | 308.7 (132.0) | 295.2 (128.5) | F < 1; p = 0.39 | F < 1; p = 0.66 | F < 1; p = 0.39 |
| Chair sit and Reach (cm) | −6.1 (8.0) | −3.4 (8.6) | −6.1 (8.0) | −4.9 (8.8) | F < 1; p = 0.56 | F = 8.3; p = 0.0181 | F < 1; p = 0.56 |
| Tinetti (score) | 24.0 (4.2) | 25.0 (3.7) | 24.0 (4.2) | 23.8 (4.6) | F = 3.8; p = 0.081 | F = 1.0; p = 0.34 | F =3.8; p = 0.081 |
| Arm Curl (reps) *, §, # | 12.8 (3.8) | 15.9 (4.1) | 12.8 (3.8) | 13.8 (3.5) | F = 9.5; p = 0.0127 | F = 5.4; p = 0.0440 | F = 9.5; p = 0.013 |
| Handgrip (kg) *, # | 26.2 (8.0) | 28.3 (8.1) | 26.2 (8.0) | 25.6 (8.8) | F = 9.0; p = 0.0145 | F = 1.4; p = 0.26 | F = 9.0; p = 0.014 |
| WB-EMS | no WB-EMS | Intervention | Time | Interaction | |||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post15 | Post60 | Pre | Post15 | Post60 | F (1,9) | F (2,18) | F (2,18) | |
| BDNF (pg/mL) | 2243.5 (1050.7) | 2247.7 (962.0) | 2353.8 (1144.0) | 2317.7 (980.1) | 2448.1 (949.1) | 2419.8 (949.1) | F = 1.41; p = 0.264 | F = 2.47; p = 0.112 | F = 1.10; p = 0.352 |
| FGF21 (pg/mL) | 715.6 (477.5) | 663.9 (427.0) | 688.0 (379.7) | 697.6 (383.5) | 972.1 (648.9) | 776.8 (440.2) | F = 2.27; p = 0.165 | F = 1.14; p = 0.339 | F = 3.14; p = 0.067 |
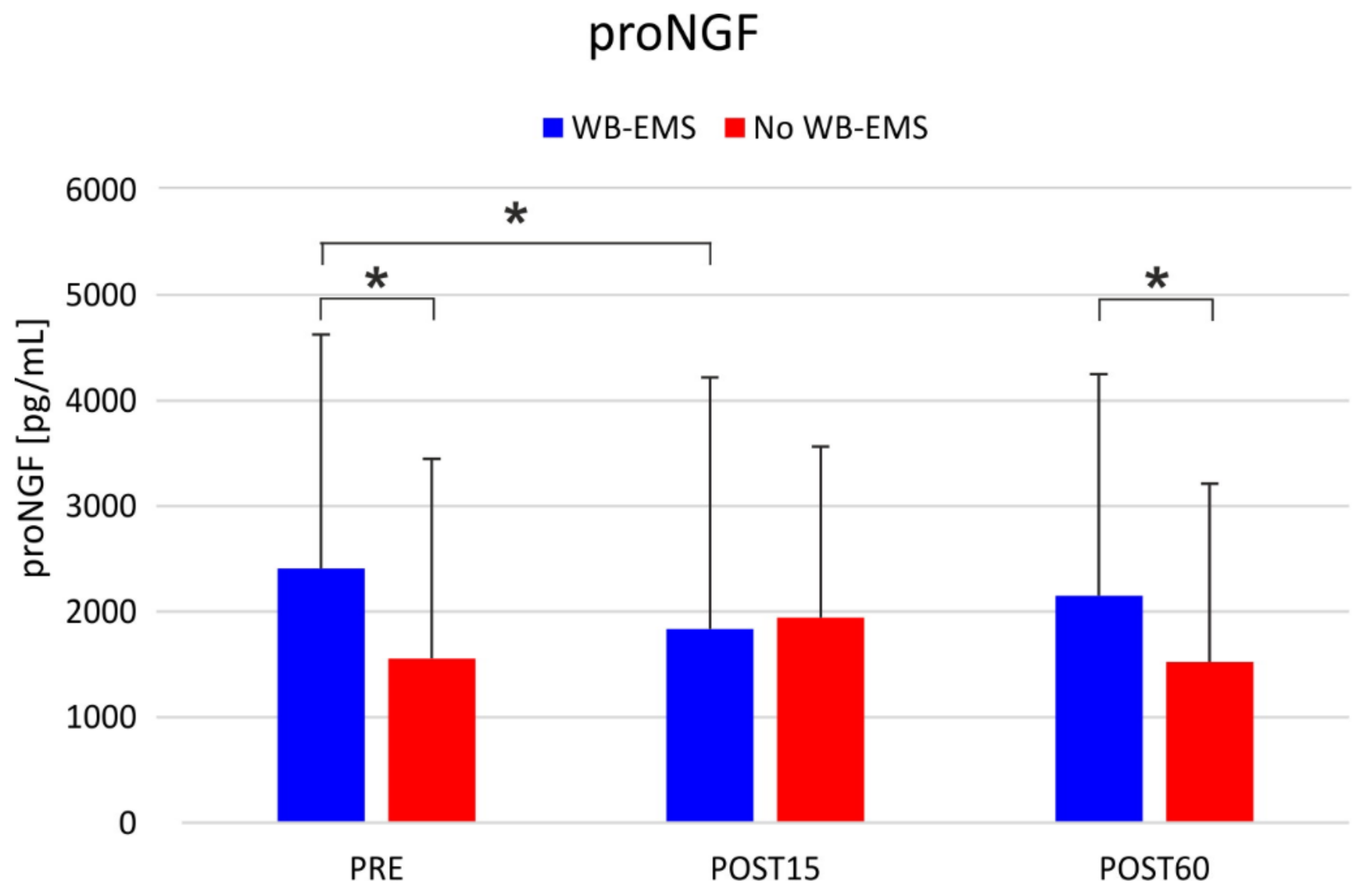
| proNFG (pg/mL) *, # | 2408.2 (2219.2) | 1833.5 (2388.0) | 2151.2 (2099.9) | 1552.6 (1896.2) | 1942.8 (1622.6) | 1522.6 (1689.8) | F = 4.1; p = 0.074 | F < 1; p = 0.503 | F = 8.9; p = 0.002 |
| mNFG (pg/mL) | 78.0 (485.3) | 42.8 (439.2) | 33.4 (400.5) | 43.2 (426.5) | −18.3 (239.2) | −2.9 (279.2) | F < 1; p = 0.344 | F = 1.30; p = 0.296 | F < 1; p = 0.828 |
| WB-EMS | no WB-EMS | Intervention | Time | Interaction | |||
|---|---|---|---|---|---|---|---|
| Post15 | Post60 | Post15 | Post60 | F (1,9) | F (1,9) | F (1,9) | |
| ΔBDNF (pg/mL) | 4.1 (220.8) | 110.3 (207.3) | 130.4 (223.2) | 102.1 (209.9) | F < 1; p = 0.466 | F < 1; p = 0.438 | F = 1.44; p = 0.260 |
| ΔFGF21 (pg/mL) | −51.6 (126.2) | −27.5 (261.1) | 274.5 (538.4) | 79.2 (354.9) | F = 3.1; p = 0.111 | F = 3.3; p = 0.100 | F = 3.2; p = 0.107 |
| ΔproNGF (pg/mL) *, # | −574.6 (569.1) | −256.9 (721.1) | 390.2 (559.5) | −30.0 (385.0) | F = 6.8; p = 0.027 | F < 1; p = 0.631 | F = 12.2; p = 0.007 |
| ΔmNGF (pg/mL) | −35.1 (59.8) | −44.5 (102.8) | −61.5 (218.2) | −46.1 (173.1) | F < 1; p = 0.786 | F < 1; p = 0.835 | F < 1; p = 0.504 |
| %BDNF (%) | 2.7 (12.5) | 2.1 (16.9) | 7.9 (10.8) | 5.4 (18.1) | F < 1; p = 0462 | F < 1; p = 0.704 | F < 1; p = 0.759 |
| %FGF21 (%) | −6.7 (21.6) | 6.0 (43.5) | 47.2 (94.4) | 20.2 (54.1) | F = 3.4; p = 0.096 | F < 1; p = 0.389 | F = 3.4; p = 0.098 |
| %proNGF (%) | −39.0 (33.3) | 16.0 (84.1) | 225.2 (532.6) | −16.2 (214.4) | F = 1.1; p = 0.312 | F = 3.4; p = 0.096 | F = 4.1; p = 0.074 |
| %mNGF (%) | −13.5 (32.6) | −23.2 (52.8) | 35.1 (103.4) | 21.0 (165.8) | F = 1.2; p = 0.291 | F < 1; p = 0.623 | F < 1; p = 0.905 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorilli, G.; Quinzi, F.; Buonsenso, A.; Casazza, G.; Manni, L.; Parisi, A.; Di Costanzo, A.; Calcagno, G.; Soligo, M.; di Cagno, A. A Single Session of Whole-Body Electromyostimulation Increases Muscle Strength, Endurance and proNGF in Early Parkinson Patients. Int. J. Environ. Res. Public Health 2021, 18, 5499. https://doi.org/10.3390/ijerph18105499
Fiorilli G, Quinzi F, Buonsenso A, Casazza G, Manni L, Parisi A, Di Costanzo A, Calcagno G, Soligo M, di Cagno A. A Single Session of Whole-Body Electromyostimulation Increases Muscle Strength, Endurance and proNGF in Early Parkinson Patients. International Journal of Environmental Research and Public Health. 2021; 18(10):5499. https://doi.org/10.3390/ijerph18105499
Chicago/Turabian StyleFiorilli, Giovanni, Federico Quinzi, Andrea Buonsenso, Giusy Casazza, Luigi Manni, Attilio Parisi, Alfonso Di Costanzo, Giuseppe Calcagno, Marzia Soligo, and Alessandra di Cagno. 2021. "A Single Session of Whole-Body Electromyostimulation Increases Muscle Strength, Endurance and proNGF in Early Parkinson Patients" International Journal of Environmental Research and Public Health 18, no. 10: 5499. https://doi.org/10.3390/ijerph18105499
APA StyleFiorilli, G., Quinzi, F., Buonsenso, A., Casazza, G., Manni, L., Parisi, A., Di Costanzo, A., Calcagno, G., Soligo, M., & di Cagno, A. (2021). A Single Session of Whole-Body Electromyostimulation Increases Muscle Strength, Endurance and proNGF in Early Parkinson Patients. International Journal of Environmental Research and Public Health, 18(10), 5499. https://doi.org/10.3390/ijerph18105499











