Sex Differences in the Oxygenation of the Left and Right Prefrontal Cortex during Moderate-Intensity Exercise
Abstract
1. Introduction
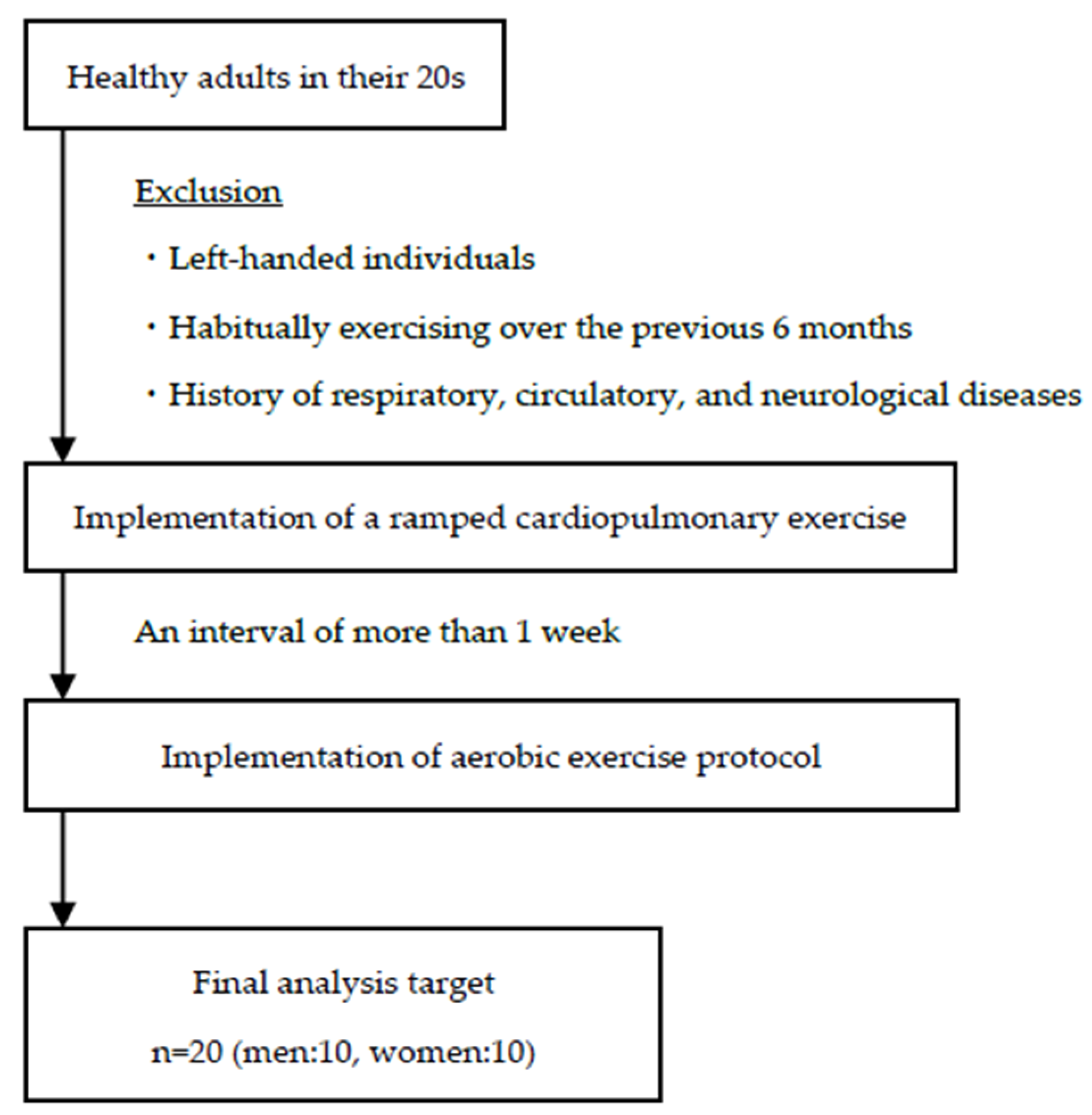
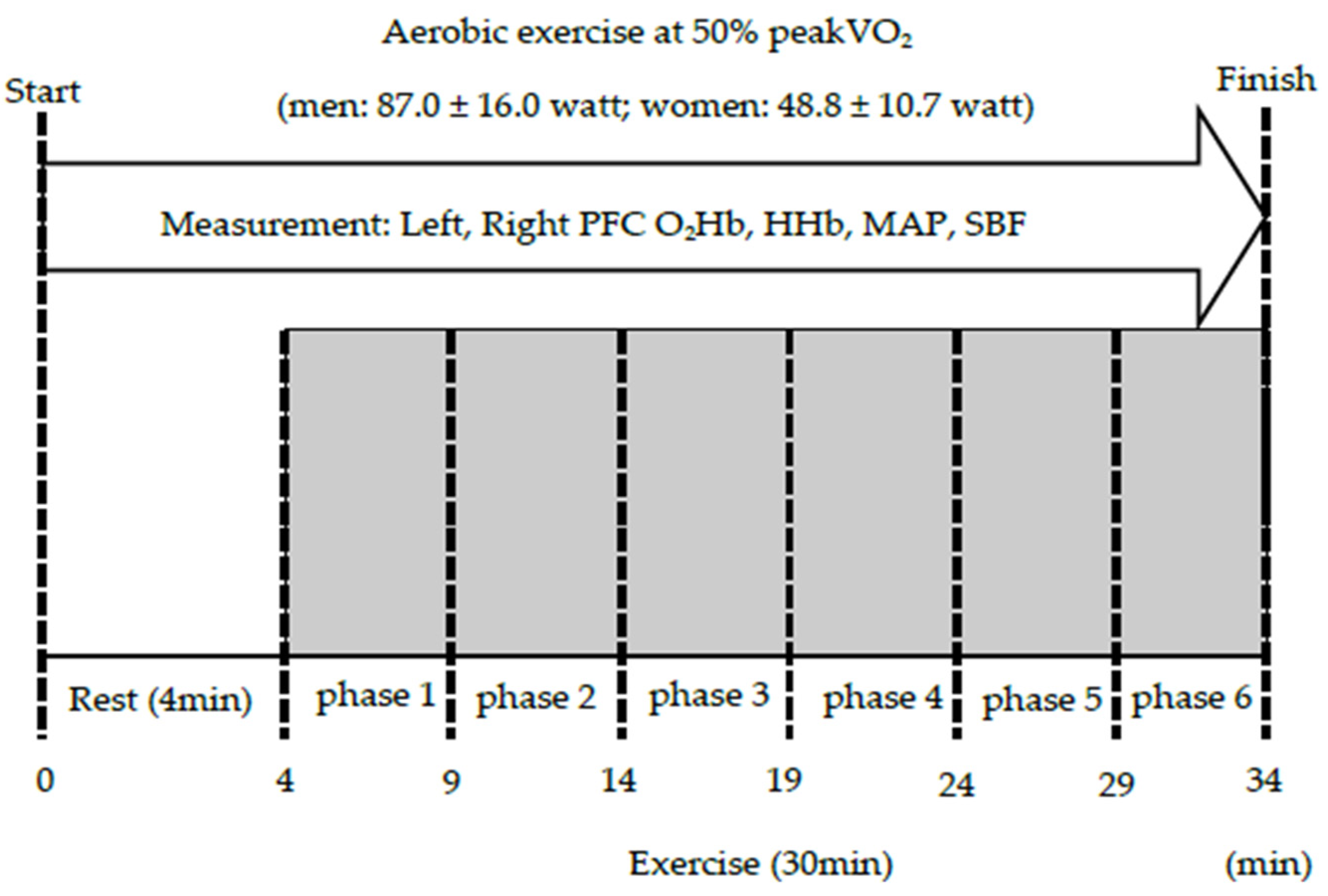
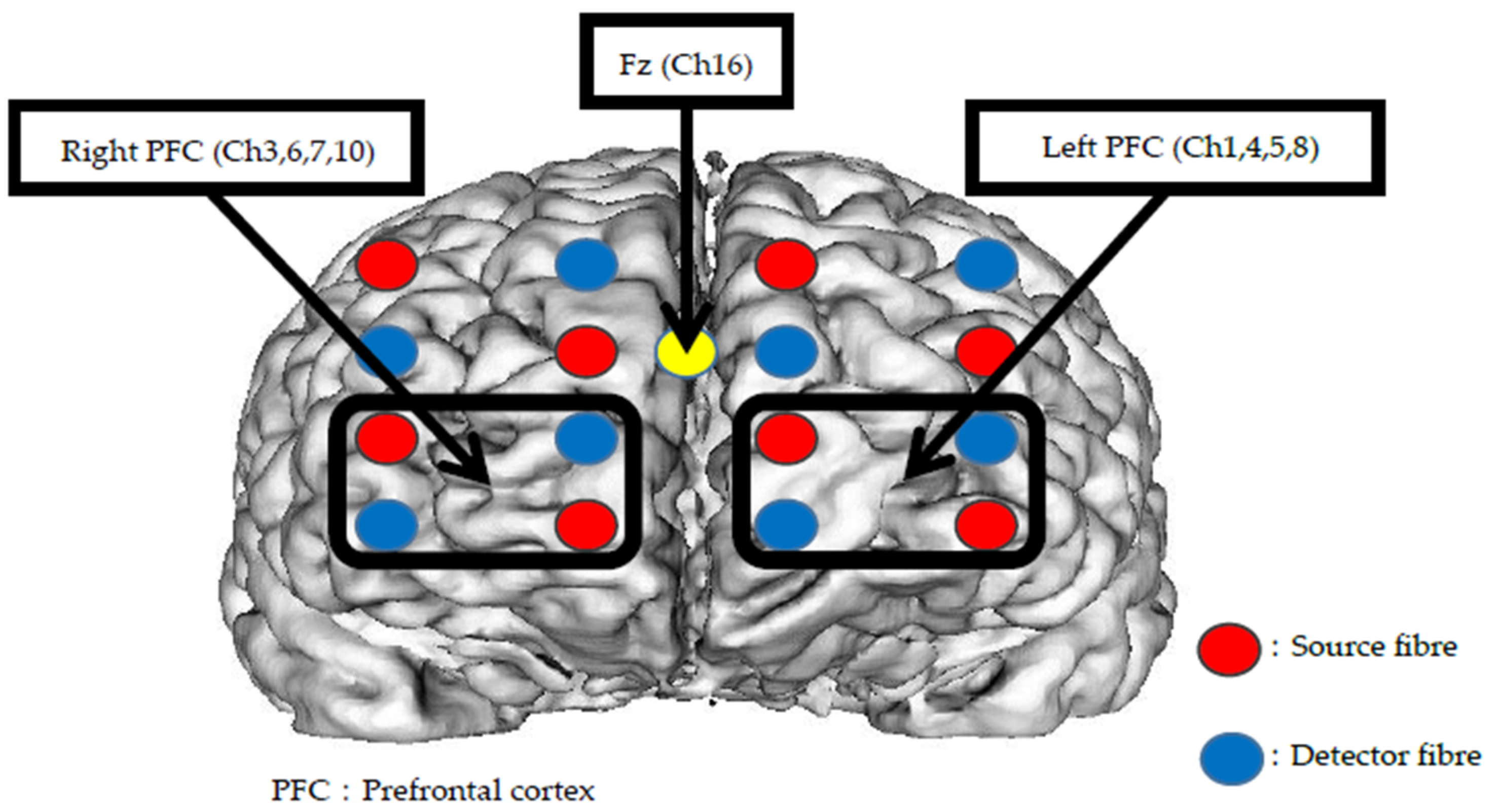
2. Materials and Methods
3. Results
3.1. Changes in the Oxygenation Dynamics of the PFC during Exercise
3.1.1. Left and Right PFC Oxygen Dynamics
3.1.2. Changes in the MAP and SBF during Exercise
3.2. Comparison of the Peak Time and Peak Value for Each Measure during Exercise
4. Discussion
4.1. Change in Each Measure during Exercise, Compared to the Resting Mean
4.2. Peak Time and Peak Value of Each Measure during Exercise
4.3. Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bähr, R.; Høstmark, A.T.; Newsholme, E.A.; Grønnerød, O.; Sejersted, O.M. Effect of exercise on recovery changes in plasma levels of FFA, glycerol, glucose and catecholamines. Acta Physiol. Scand. 1991, 143, 105–115. [Google Scholar] [CrossRef]
- Al Saif, A.; Alsenany, S. Aerobic and anaerobic exercise training in obese adults. J. Phys. Ther. Sci. 2015, 27, 1697–1700. [Google Scholar] [CrossRef]
- Jakicic, J.; Davis, K. Obesity and physical activity. Psychiatr. Clin. N. Am. 2011, 34, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Bennie, J.A.; De Cocker, K.; Pavey, T.; Stamatakis, E.; Biddle, S.J.H.; Ding, D. Muscle strengthening, aerobic exercise, and obesity: A pooled analysis of 1.7 million US adults. Obesity 2020, 28, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Ohsugi, H.; Ohgi, S.; Kodama, T. Physical activity affects cognitive function in view of prefrontal cortex activation. J. Allied Health Sci. 2014, 5, 69–77. [Google Scholar] [CrossRef][Green Version]
- León-Carrion, J.; Damas-López, J.; Martín-Rodríguez, J.F.; Domínguez-Roldán, J.M.; Murillo-Cabezas, F.; Martin, J.M.B.Y.; Domínguez-Morales, M.R. The hemodynamics of cognitive control: The level of concentration of oxygenated hemoglobin in the superior prefrontal cortex varies as a function of performance in a modified Stroop task. Behav. Brain Res. 2008, 193, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Yokogawa, M.; Kojima, H.; Tanaka, S.; Nakagawa, T. Effect of moderate exercise intensities on the cortical activity in young adults. J. Phys. Ther. Sci. 2018, 30, 1257–1261. [Google Scholar] [CrossRef][Green Version]
- Giles, G.E.; Brunyé, T.T.; Eddy, M.D.; Mahoney, C.R.; Gagnon, S.A.; Taylor, H.A.; Kanarek, R.B. Acute exercise increases oxygenated and deoxygenated hemoglobin in the prefrontal cortex. Neuroreport 2014, 25, 1320–1325. [Google Scholar] [CrossRef]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2017, 52, 154–160. [Google Scholar] [CrossRef]
- Falck, R.S.; Davis, J.C.; Best, J.R.; Crockett, R.A.; Liu-Ambrose, T. Impact of exercise training on physical and cognitive function among older adults: A systematic review and meta-analysis. Neurobiol. Aging 2019, 79, 119–130. [Google Scholar] [CrossRef]
- El-Sayes, J.; Turco, C.V.; Skelly, L.E.; Nicolini, C.; Fahnestock, M.; Gibala, M.J.; Nelson, A.J. The Effects of Biological Sex and Ovarian Hormones on Exercise-Induced Neuroplasticity. Neuroscience 2019, 410, 29–40. [Google Scholar] [CrossRef]
- Hagmar, M.; Berglund, B.; Brismar, K.; Hirschberg, A.L. Body Composition and Endocrine Profile of Male Olympic Athletes Striving for Leanness. Clin. J. Sport Med. 2013, 23, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Di Luigi, L.; Romanelli, F.; Sgrò, P.; Lenzi, A. Andrological aspects of physical exercise and sport medicine. Endocrine 2012, 42, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Woodhouse, L.; Casaburi, R.; Singh, A.B.; Bhasin, D.; Berman, N.; Chen, X.; Yarasheski, K.E.; Magliano, L.; Dzekov, C.; et al. Testosterone dose-response relationships in healthy young men. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1172–E1181. [Google Scholar] [CrossRef] [PubMed]
- Sinha-Hikim, I.; Cornford, M.; Gaytan, H.; Lee, M.L.; Bhasin, S. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J. Clin. Endocrinol. Metab. 2006, 91, 3024–3033. [Google Scholar] [CrossRef] [PubMed]
- Rogerson, S.; Weatherby, R.P.; Deakin, G.B.; Meir, R.A.; Coutts, R.A.; Zhou, S.; Marchall-Gradisnik, M. The effect of short-term use of testosterone enanthate on muscular strength and power in healthy young men. J. Strength Cond. Res. 2007, 21, 354–361. [Google Scholar]
- Grandys, M.; Majerczak, J.; Duda, K.; Zapart-Bukowska, J.; Kulpa, J.; Zoladz, J. Endurance Training of Moderate Intensity Increases Testosterone Concentration in Young, Healthy Men. Int. J. Sports Med. 2009, 30, 489–495. [Google Scholar] [CrossRef]
- Fitzgerald, L.Z.; Robbins, W.A.; Kesner, J.S.; Xun, L. Reproductive hormones and interleukin-6 in serious leisure male athletes. Eur. J. Appl. Physiol. 2012, 112, 3765–3773. [Google Scholar] [CrossRef]
- Ding, E.L.; Song, Y.; Malik, V.S.; Liu, S. Sex Differences of Endogenous Sex Hormones and Risk of Type 2 Diabetes. JAMA 2006, 295, 1288–1299. [Google Scholar] [CrossRef]
- Hak, A.E.; Witteman, J.C.M.; De Jong, F.H.; Geerlings, M.I.; Hofman, A.; Pols, H.A.P. Low Levels of Endogenous Androgens Increase the Risk of Atherosclerosis in Elderly Men: The Rotterdam Study. J. Clin. Endocrinol. Metab. 2002, 87, 3632–3639. [Google Scholar] [CrossRef]
- Rosano, G.M.C.; Sheiban, I.; Massaro, R.; Pagnotta, P.; Marazzi, G.; Vitale, C.; Mercuro, G.; Volterrani, M.; Aversa, A.; Fini, M. Low testosterone levels are associated with coronary artery disease in male patients with angina. Int. J. Impot. Res. 2006, 19, 176–182. [Google Scholar] [CrossRef]
- Ohlsson, C.; Barrett-Connor, E.; Bhasin, S.; Orwoll, E.; Labrie, F.; Karlsson, M.K.; Ljunggren, Ö.; Vandenput, L.; Mellström, D.; Tivesten, Å. High serum testosterone is associated with reduced risk of cardiovascular events in elderly men. The MrOS (Osteoporotic Fractures in Men) study in Sweden. J. Am. Coll. Cardiol. 2011, 58, 1674–1681. [Google Scholar] [CrossRef]
- Behan, F.P.; Maden-Wilkinson, T.M.; Pain, M.T.G.; Folland, J.P. Sex differences in muscle morphology of the knee flexors and knee extensors. PLoS ONE 2018, 13, e0190903. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, R.R.; Heleniak, R.J.; Tryniecki, J.L.; Kraemer, G.R.; Okazaki, N.J.; Castracane, V.D. Follicular and luteal phase hormonal responses to low-volume resistive exercise. Med. Sci. Sports Exerc. 1995, 27, 809–817. [Google Scholar] [CrossRef]
- Walberg-Rankin, J.; Franke, W.D.; Gwazdauskas, F.C. Response of beta-endorphin and estradiol to resistance exercise in females during energy balance and energy restriction. Int. J. Sports Med. 1992, 13, 542–547. [Google Scholar] [CrossRef]
- Bar, P.; Amelink, G.; Oldenburg, B.; Blankenstein, M. Prevention of exercise-induced muscle membrane damage by oestradiol. Life Sci. 1988, 42, 2677–2681. [Google Scholar] [CrossRef]
- Kochanowicz, J.; Krejza, J.; Koroza, O.; Mariak, Z. Influence of estrogens on the impedance of cerebral blood vessels. Neurol. Neurochir. Pol. 2005, 39, 175–180. [Google Scholar]
- Robison, L.S.; Gannon, O.J.; Salinero, A.E.; Zuloaga, K.L. Contributions of sex to cerebrovascular function and pathology. Brain Res. 2019, 1710, 43–60. [Google Scholar] [CrossRef]
- Acar, M.; Cevrioglu, A.S.; Haktanir, A.; Demirel, R.; Albayrak, R.; Degirmenci, B.; Yucel, A.; Akyol, A.M. Effect of Aerodiol® administration on cerebral blood flow volume in postmenopausal women. Maturitas 2005, 52, 127–133. [Google Scholar] [CrossRef]
- Berent-Spillson, A.; Briceno, E.; Pinsky, A.; Simmen, A.; Persad, C.C.; Zubieta, J.-K.; Smith, Y.R. Distinct cognitive effects of estrogen and progesterone in menopausal women. Psychoneuroendocrinology 2015, 59, 25–36. [Google Scholar] [CrossRef]
- Coltheart, M.; Hull, E.; Slater, D. Sex differences in imagery and reading. Nature 1975, 253, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.L.; Craig, J.C.; Liu, Y.; Vidoni, E.D.; Maletsky, R.; Poole, D.C.; Billinger, S.A. Effect of healthy aging and sex on middle cerebral artery blood velocity dynamics during moderate-intensity exercise. Am. J. Physiol. Circ. Physiol. 2018, 315, H492–H501. [Google Scholar] [CrossRef] [PubMed]
- Ashley, J.D.; Shelley, J.H.; Sun, J.; Song, J.; Trent, J.A.; Ambrosio, L.D.; Larson, D.J.; Larson, R.D.; Yabluchanskiy, A.; Kellawan, J.M. Cerebrovascular responses to graded exercise in young healthy males and females. Physiol. Rep. 2020, 8, e14622. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, M.; Fukuda, M.; Uehara, T.; Mikuni, M. Sex and age dependencies of cerebral blood volume changes during cognitive activation: A multichannel near-infrared spectroscopy study. NeuroImage 2004, 22, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Luo, Q.; Gong, H. Gender-specific hemodynamics in prefrontal cortex during a verbal working memory task by near-infrared spectroscopy. Behav. Brain Res. 2010, 209, 148–153. [Google Scholar] [CrossRef]
- Goldstein, J.M.; Jerram, M.; Poldrack, R.; Anagnoson, R.; Breiter, H.C.; Makris, N.; Goodman, J.M.; Tsuang, M.T.; Seidman, L.J. Sex differences in prefrontal cortical brain activity during fMRI of auditory verbal working memory. Neuropsychology 2005, 19, 509–519. [Google Scholar] [CrossRef]
- Hoshi, Y. Functional near-infrared spectroscopy: Current status and future prospects. J. Biomed. Opt. 2007, 12, 062106. [Google Scholar] [CrossRef]
- Fox, P.T.; Raichle, M.E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc. Natl. Acad. Sci. USA 1986, 83, 1140–1144. [Google Scholar] [CrossRef]
- Herrmann, M.; Walter, A.; Fallgatter, A.J. Cerebral oxygenation changes in the prefrontal cortex: Effects of age and sex. Neurobiol. Aging 2006, 27, 888–894. [Google Scholar] [CrossRef]
- Ji, Z.; Feng, T.; Mei, L.; Li, A.; Zhang, C. Influence of acute combined physical and cognitive exercise on cognitive function: An NIRS study. PeerJ 2019, 7, e7418. [Google Scholar] [CrossRef]
- Tsubaki, A.; Takai, H.; Kojima, S.; Miyaguchi, S.; Sugawara, K.; Sato, D.; Tamaki, H.; Onishi, H. Changes in Cortical Oxyhaemoglobin Signal During Low-Intensity Cycle Ergometer Activity: A Near-Infrared Spectroscopy Study. Adv. Exp. Med. Biol. 2016, 876, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Bellin, D. Ramp Exercise Protocols for Clinical and Cardiopulmonary Exercise Testing. Sports Med. 2000, 30, 23–29. [Google Scholar] [CrossRef]
- Morishita, S.; Tsubaki, A.; Nashimoto, S.; Fu, J.B.; Onishi, H. Face scale rating of perceived exertion during cardiopulmonary exercise test. BMJ Open Sport Exerc. Med. 2018, 4, e000474. [Google Scholar] [CrossRef]
- Thomas, R.; Stephane, P. Prefrontal cortex oxygenation and neuromuscular responses to exhaustive exercise. Eur. J. Appl. Physiol. 2008, 102, 153–163. [Google Scholar] [CrossRef]
- Hopker, J.G.; Jobson, S.A.; Pandit, J.J. Controversies in the physiological basis of the ‘anaerobic threshold’ and their implications for clinical cardiopulmonary exercise testing. Anaesthesia 2011, 66, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Sasai, S.; Homae, F.; Watanabe, H.; Taga, G. Frequency-specific functional connectivity in the brain during resting state revealed by NIRS. NeuroImage 2011, 56, 252–257. [Google Scholar] [CrossRef]
- Tong, Y.; Frederick, B.D. Time lag dependent multimodal processing of concurrent fMRI and near-infrared spectroscopy (NIRS) data suggests a global circulatory origin for low-frequency oscillation signals in human brain. NeuroImage 2010, 53, 553–564. [Google Scholar] [CrossRef]
- Hatakenaka, M.; Miyai, I.; Mihara, M.; Sakoda, S.; Kubota, K. Frontal regions involved in learning of motor skill—A functional NIRS study. NeuroImage 2007, 34, 109–116. [Google Scholar] [CrossRef]
- Hada, Y.; Abo, M.; Kaminaga, T.; Mikami, M. Detection of cerebral blood flow changes during repetitive transcranial magnetic stimulation by recording hemoglobin in the brain cortex, just beneath the stimulation coil, with near-infrared spectroscopy. NeuroImage 2006, 32, 1226–1230. [Google Scholar] [CrossRef]
- Bae, S.; Lee, Y.; Chang, P. There is No test–retest reliability of brain activation induced by robotic passive hand movement: A functional NIRS study. Brain Behav. 2020, 10. [Google Scholar] [CrossRef]
- Maki, A.; Yamashita, Y.; Ito, Y.; Watanabe, E.; Mayanagi, Y.; Koizumi, H. Spatial and temporal analysis of human motor activity using noninvasive NIR topography. Med. Phys. 1995, 22, 1997–2005. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Miyai, I.; Hattori, N.; Hatakenaka, M.; Yagura, H.; Kawano, T.; Okibayashi, M.; Danjo, N.; Ishikawa, A.; Inoue, Y.; et al. Neurofeedback Using Real-Time Near-Infrared Spectroscopy Enhances Motor Imagery Related Cortical Activation. PLoS ONE 2012, 7, e32234. [Google Scholar] [CrossRef]
- Sato, K.; Ogoh, S.; Hirasawa, A.; Oue, A.; Sadamoto, T. The distribution of blood flow in the carotid and vertebral arteries during dynamic exercise in humans. J. Physiol. 2011, 589, 2847–2856. [Google Scholar] [CrossRef]
- Secher, N.H.; Seifert, T.; Van Lieshout, J.J. Cerebral blood flow and metabolism during exercise: Implications for fatigue. J. Appl. Physiol. 2008, 104, 306–314. [Google Scholar] [CrossRef]
- Inoue, Y.; Ichinose-Kuwahara, T.; Tanaka, A.; Tanaka, E.; Ueda, H.; Amano, T.; Tochihara, Y.; Kondo, N. Sex differences in effective and ineffective sweating rates during exercise in hot, humid conditions. In Proceedings of the 15th International Conference on Environmental Ergonomics, Queenstown, New Zealand, 11–15 February 2013; pp. 126–129. [Google Scholar]
- Strangman, G.; Culver, J.P.; Thompson, J.H.; Boas, D.A. A Quantitative Comparison of Simultaneous BOLD fMRI and NIRS Recordings during Functional Brain Activation. NeuroImage 2002, 17, 719–731. [Google Scholar] [CrossRef]
| Men (n = 10) | Women (n = 10) | p-Value | |
|---|---|---|---|
| Age | 21.5 ± 0.5 (0.02) | 21.4 ± 0.5 (0.02) | p = 0.67 |
| Height (cm) | 171.7 ± 4.8 (0.03) | 157.6 ± 4.9 (0.03) | p < 0.001 |
| Weight (kg) | 65.6 ± 5.6 (0.09) | 51.3 ± 6.5 (0.13) | p < 0.001 |
| Peak VO2 (mL/min/kg) | 1859.6 ± 244.5 (0.13) | 1143.9 ± 351.0 (0.31) | p < 0.001 |
| Peak load (watt) | 189.7 ± 28.3 (0.15) | 119.4 ± 19.0 (0.16) | p < 0.001 |
| 50% load (watt) | 87.0 ± 16.0 (0.18) | 48.8 ± 10.7 (0.22) | p < 0.001 |
| Rest | Phase 1 | Phase 2 | Phase 3 | Phase 4 | Phase 5 | Phase 6 | Time | Between Sex | Interaction | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Left PFC O2Hb (Mm·cm) | men | 0.00 ± 0 | 0.00 ± 0.01 | 0.09 ± 0.02 | 0.13 ± 0.02 | 0.14 ± 0.02 | 0.14 ± 0.02 | 0.15 ± 0.02 | p < 0.001 | p < 0.001 | p = 0.097 |
| (0) | (0) | (0.22) | (0.15) | (0.14) | (0.14) | (0.13) | |||||
| women | 0.00 ± 0 | −0.01 ± 0.01 | 0.04 ± 0.01 | 0.06 ± 0.02 | 0.06 ± 0.03 | 0.07 ± 0.03 | 0.07 ± 0.03 | ||||
| (0) | (−1) | (0.25) | (0.33) | (0.5) | (0.43) | (0.43) | |||||
| Right PFC O2Hb (mM·cm) | men | 0.00 ± 0 | 0.00 ± 0.01 | 0.07 ± 0.02 | 0.11 ± 0.02 | 0.11 ± 0.02 | 0.12 ± 0.02 | 0.12 ± 0.02 | p < 0.001 | p < 0.001 | p = 0.21 |
| (0) | (0) | (0.29) | (0.18) | (0.18) | (0.17) | (0.17) | |||||
| women | 0.00 ± 0 | −0.01 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.01 | ||||
| (0) | (−1) | (0.25) | (0.2) | (0.17) | (0.14) | (0.17) |
| Rest | Phase 1 | Phase 2 | Phase 3 | Phase 4 | Phase 5 | Phase 6 | Time | Between Sex | Interaction | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Left PFC HHb (mM·cm) | men | 0.00 ± 0 | 0.00 ± 0 | 0.01 ± 0.01 | 0.03 ± 0.01 † | 0.05 ± 0.01 *,† | 0.07 ± 0.01 **,†† | 0.08 ± 0.01 **,† | p < 0.001 | p < 0.001 | p < 0.001 |
| (0) | (0) | (1) | (0.33) | (0.2) | (0.14) | (0.13) | |||||
| women | 0.00 ± 0 | 0.01 ± 0 | 0.01 ± 0 | 0.00 ± 0 | 0.00 ± 0 | 0.01 ± 0.01 | 0.01 ± 0.01 | ||||
| (0) | (0) | (0) | (0) | (0) | (1) | (1) | |||||
| Right PFC HHb (mM·cm) | men | 0.00 ± 0 | 0.01 ± 0 | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.06 ± 0.01 **,† | 0.07 ± 0.01 **,†† | 0.07 ± 0.02 **,†† | p < 0.001 | p < 0.001 | p = 0.003 |
| (0) | (0) | (0.5) | (0.25) | (0.17) | (0.14) | (0.29) | |||||
| women | 0.00 ± 0 | 0.01 ± 0 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0 | 0.02 ± 0.00 * | 0.03 ± 0.01 ** | ||||
| (0) | (0) | (1) | (0.5) | (0) | (0) | (0.33) |
| Rest | Phase 1 | Phase 2 | Phase 3 | Phase 4 | Phase 5 | Phase 6 | Time | Between Sex | Interaction | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MAP (mmHg) | men | 0.01 ± 0.02 | 25.05 ± 2.51 | 25.49 ± 3.88 | 18.73 ± 4.28 | 18.40 ± 4.22 | 17.13 ± 4.83 | 18.71 ± 3.53 | p < 0.001 | p = 0.033 | p = 0.97 |
| (2.0) | (0.10) | (0.15) | (0.23) | (0.23) | (0.28) | (0.19) | |||||
| women | −0.03 ± 0.06 | 21.68 ± 2.25 | 19.51 ± 1.64 | 14.65 ± 1.92 | 13.78 ± 2.07 | 14.38 ± 2.44 | 15.68 ± 2.84 | ||||
| (−2.0) | (0.10) | (0.08) | (0.13) | (0.15) | (0.17) | (0.18) | |||||
| SBF (a.u.) | men | 0.01 ± 0 | 0.65 ± 0.39 | 3.74 ± 0.74 | 5.61 ± 1.02 | 6.46 ± 1.07 | 6.98 ± 1.17 | 6.99 ± 1.27 | p < 0.001 | p = 0.74 | p = 0.99 |
| (0) | (0.6) | (0.20) | (0.18) | (0.17) | (0.17) | (0.18) | |||||
| women | 0.01 ± 0 | 0.08 ± 0.17 | 3.83 ± 1.07 | 5.32 ± 1.24 | 6.25 ± 1.28 | 7.01 ± 1.29 | 6.74 ± 1.13 | ||||
| (0) | (2.13) | (0.28) | (0.23) | (0.20) | (0.18) | (0.17) |
| Peak Time (Men) | Peak Time (Women) | p-Value | |
| Left PFC O2Hb | 19.7 ± 2.5 (0.13) | 17.8 ± 2.1 (0.12) | p = 0.56 |
| Right PFC O2Hb | 17.6 ± 2.0 (0.11) | 20.3 ± 2.5 (0.12) | p = 0.40 |
| Left PFC HHb | 28.7 ± 0.5 (0.02) | 22.8 ± 2.7 (0.12) | p = 0.084 |
| Right PFC HHb | 26.7 ± 1.1 (0.04) | 24.3 ± 2.9 (0.12) | p = 0.45 |
| MAP | 6.5 ± 2.2 (0.34) | 6.5 ± 2.4 (0.37) | p = 1 |
| SBF | 22.5 ± 1.6 (0.07) | 22.1 ± 1.9 (0.09) | p = 0.88 |
| Peak Value (Men) | Peak Value (Women) | p-Value | |
| Left PFC O2Hb | 0.17 ± 0.02 (0.12) | 0.09 ± 0.02 (0.22) | p = 0.017 |
| Right PFC O2Hb | 0.15 ± 0.02 (0.13) | 0.08 ± 0.01 (0.13) | p = 0.011 |
| Left PFC HHb | 0.08 ± 0.02 (0.25) | 0.03 ± 0.01 (0.33) | p = 0.002 |
| Right PFC HHb | 0.09 ± 0.01 (0.11) | 0.04 ± 0.01 (0.25) | p = 0.002 |
| MAP | 34.71 ± 3.52 (0.10) | 29.98 ± 2.39 (0.08) | p = 0.27 |
| SBF | 8.14 ± 1.28 (0.16) | 8.47 ± 1.42 (0.17) | p = 0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inagaki, Y.; Sato, R.; Uchiyama, T.; Kojima, S.; Morishita, S.; Qin, W.; Tsubaki, A. Sex Differences in the Oxygenation of the Left and Right Prefrontal Cortex during Moderate-Intensity Exercise. Int. J. Environ. Res. Public Health 2021, 18, 5212. https://doi.org/10.3390/ijerph18105212
Inagaki Y, Sato R, Uchiyama T, Kojima S, Morishita S, Qin W, Tsubaki A. Sex Differences in the Oxygenation of the Left and Right Prefrontal Cortex during Moderate-Intensity Exercise. International Journal of Environmental Research and Public Health. 2021; 18(10):5212. https://doi.org/10.3390/ijerph18105212
Chicago/Turabian StyleInagaki, Yuta, Reo Sato, Takashi Uchiyama, Sho Kojima, Shinichiro Morishita, Weixiang Qin, and Atsuhiro Tsubaki. 2021. "Sex Differences in the Oxygenation of the Left and Right Prefrontal Cortex during Moderate-Intensity Exercise" International Journal of Environmental Research and Public Health 18, no. 10: 5212. https://doi.org/10.3390/ijerph18105212
APA StyleInagaki, Y., Sato, R., Uchiyama, T., Kojima, S., Morishita, S., Qin, W., & Tsubaki, A. (2021). Sex Differences in the Oxygenation of the Left and Right Prefrontal Cortex during Moderate-Intensity Exercise. International Journal of Environmental Research and Public Health, 18(10), 5212. https://doi.org/10.3390/ijerph18105212






