Regression Tree Analysis for Stream Biological Indicators Considering Spatial Autocorrelation
Abstract
1. Introduction
2. Materials and Methods
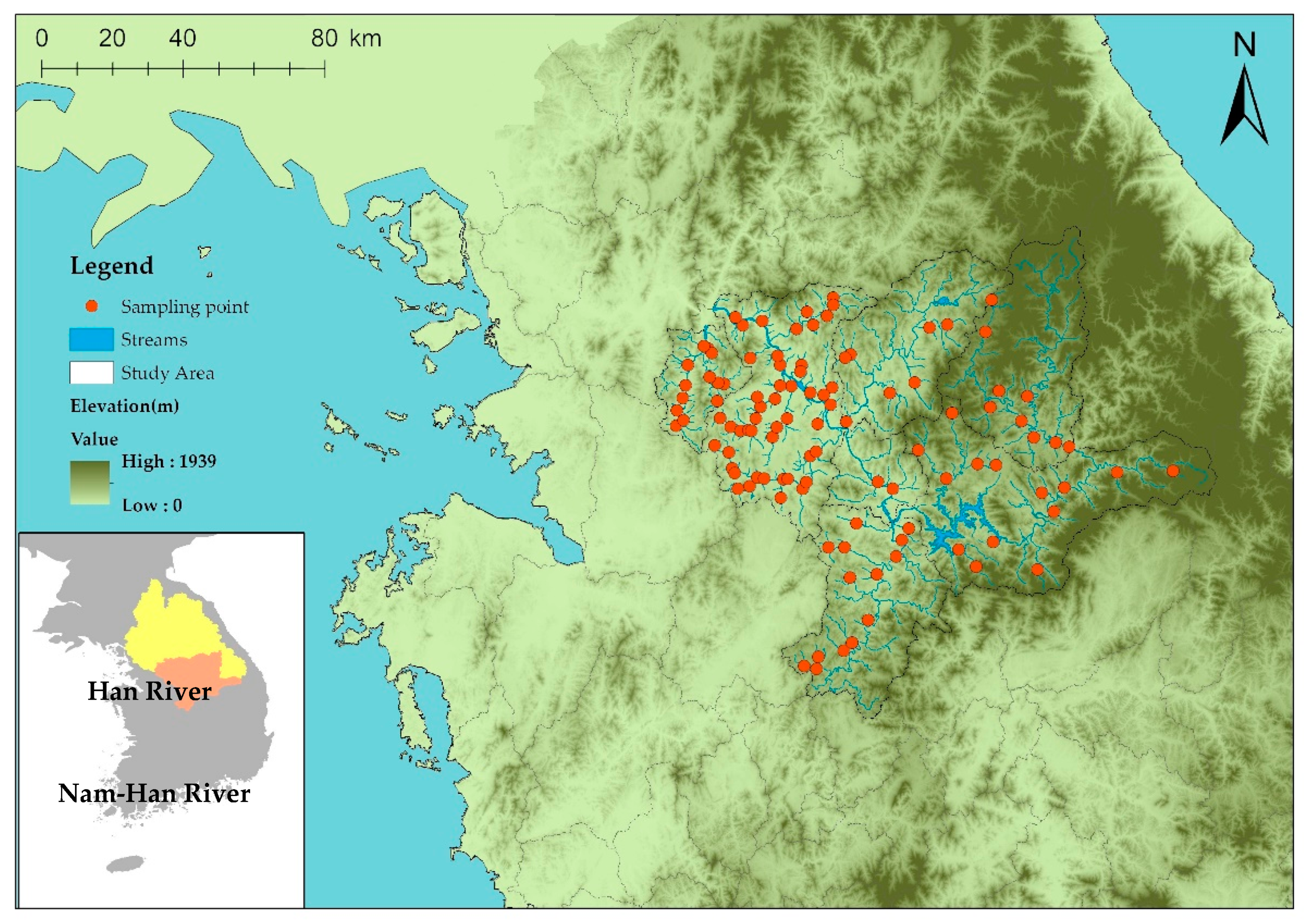
2.1. Study Area and Sampling Site
2.2. Biological Indicators of Streams
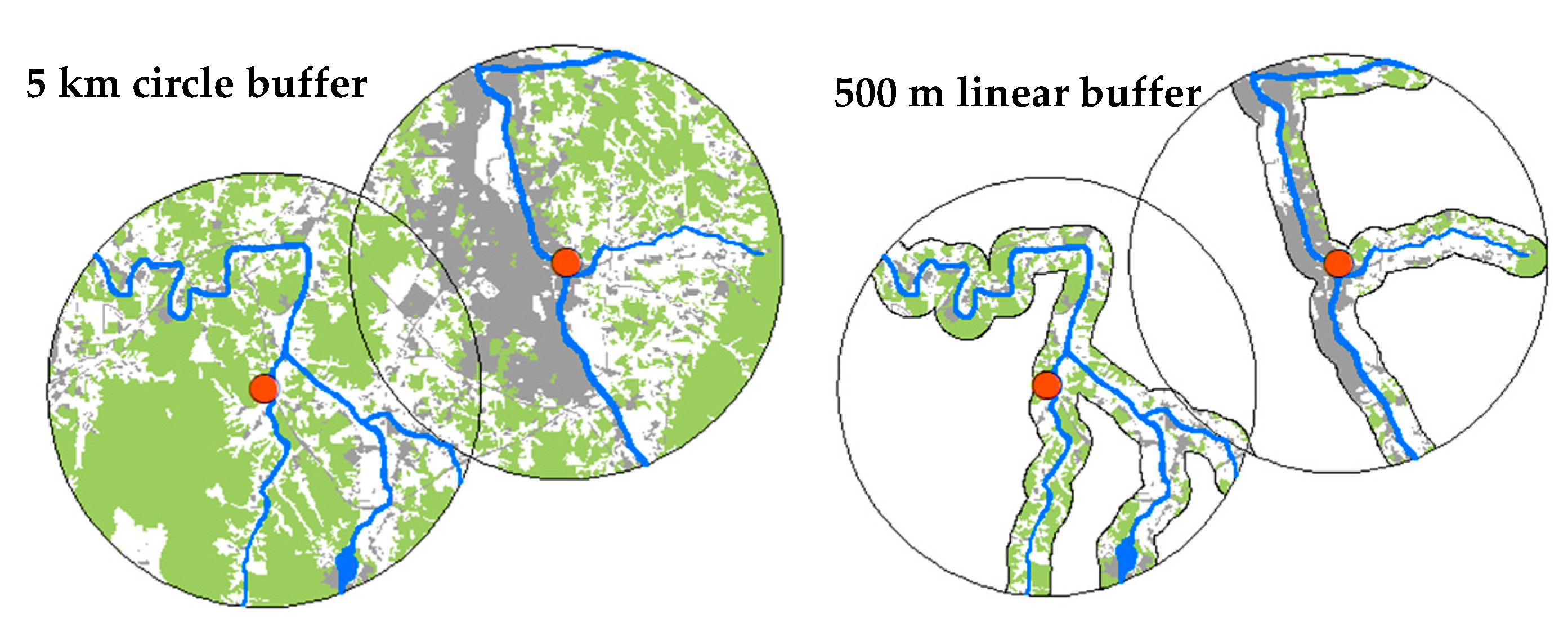
2.3. Measurements and Selection of Scale
2.4. Spatial Autocorrelation and Data Analysis
3. Results
3.1. Descriptive Statistics
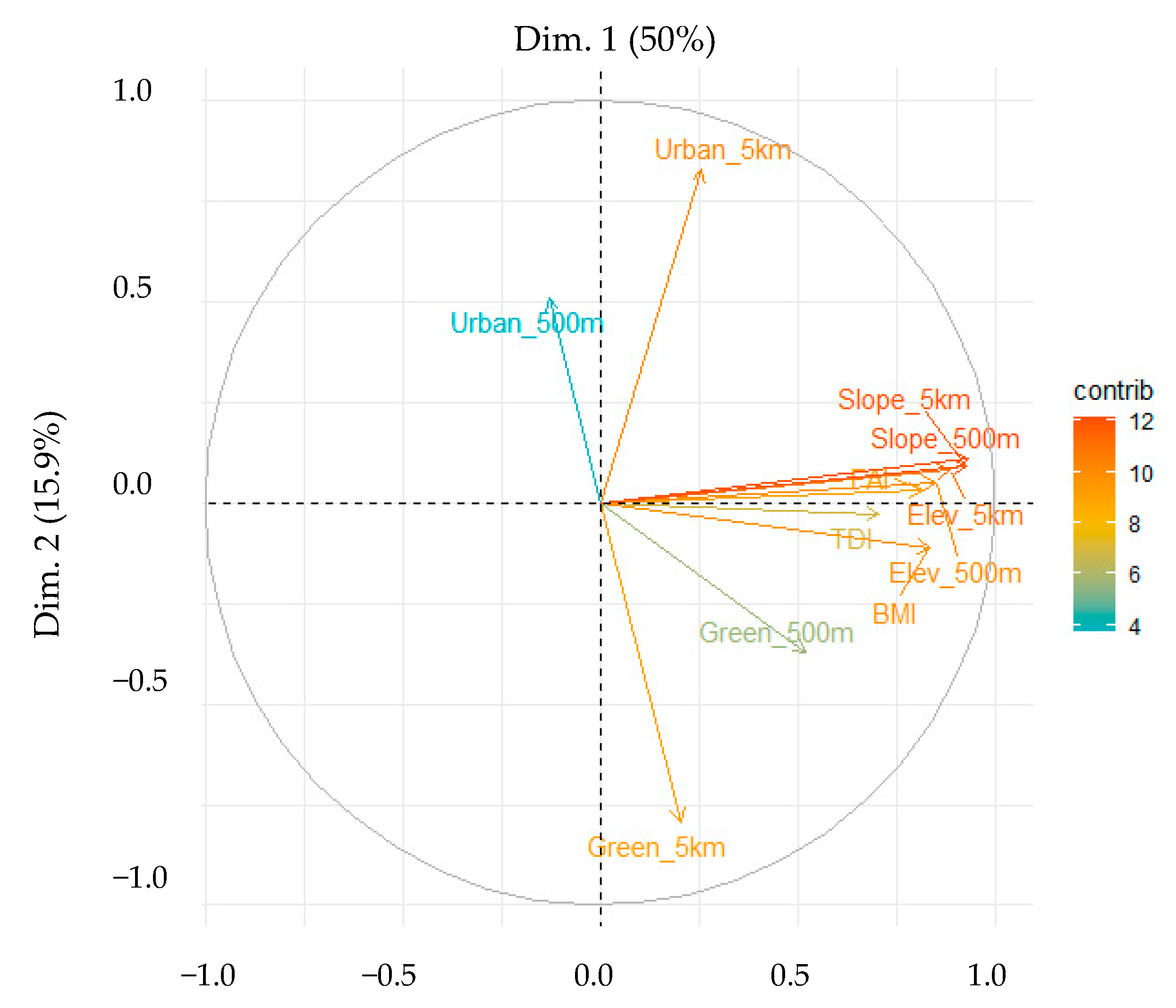
3.2. Principal Component Analysis
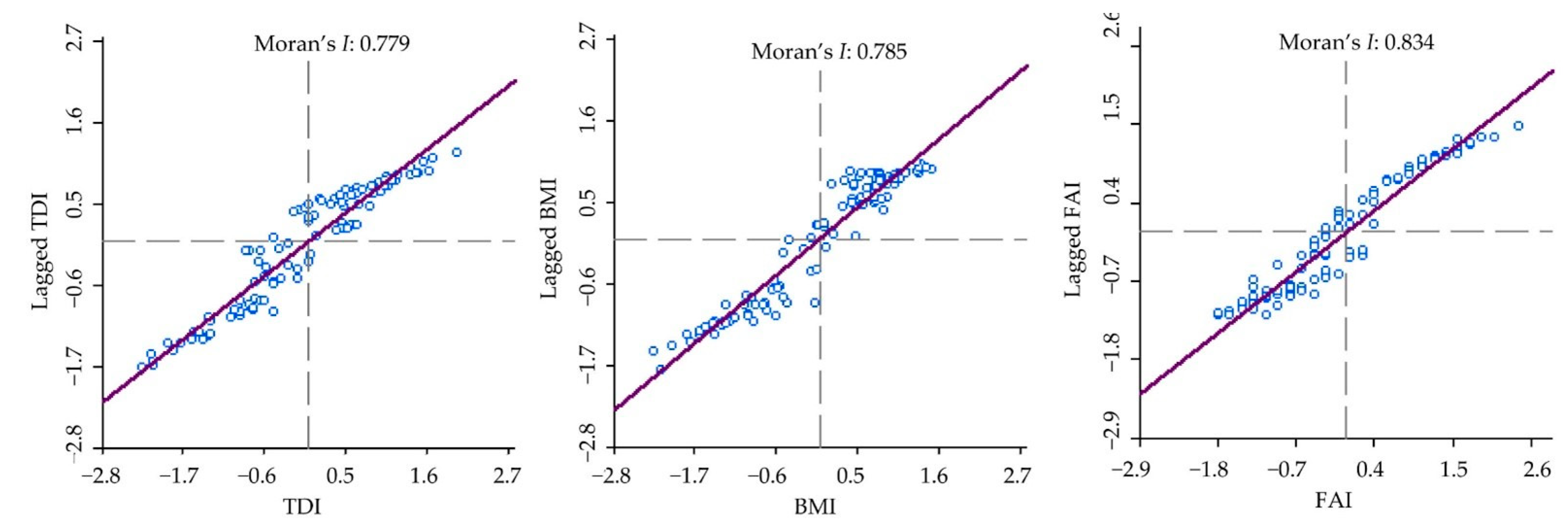
3.3. Spatial Autocorrelation Verification of Biological Indicators
3.4. Regression Tree Analysis
4. Discussion
4.1. Spatial Autocorrelation of Stream Biological Indicators
4.2. Land Use and Topographical Effects on Biological Indicators of Stream
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The River Continuum Concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Yang, B.; Li, S. Design with Nature: Ian McHarg’s Ecological Wisdom as Actionable and Practical Knowledge. Landsc. Urban Plan. 2016, 155, 21–32. [Google Scholar] [CrossRef]
- Stone, M.L.; Whiles, M.R.; Webber, J.A.; Williard, K.W.; Reeve, J.D. Macroinvertebrate Communities in Agriculturally Impacted Southern Illinois Streams: Patterns with Riparian Vegetation, Water Quality, and In-stream Habitat Quality. J. Environ. Qual. 2005, 34, 907–917. [Google Scholar] [CrossRef]
- Rankin, E.T. Habitat Indices in Water Resource Quality Assessments. In Biological Assessment and Criteria: Tools for Water Resource Planning and Decision Making; CRC Press: Boca Raton, FL, USA, 1995; Volume 1995, pp. 181–208. [Google Scholar]
- Wang, L.; Lyons, J.; Kanehl, P.; Gatti, R. Influences of Watershed Land use on Habitat Quality and Biotic Integrity in Wisconsin Streams. Fisheries 1997, 22, 6–12. [Google Scholar] [CrossRef]
- Lamberti, G.A. Grazing Experiments in Artificial Streams. J. N. Am. Benthol. Soc. 1993, 12, 337–342. [Google Scholar]
- Leland, H.V.; Porter, S.D. Distribution of Benthic Algae in the Upper Illinois River Basin in Relation to Geology and Land Use. Freshwat. Biol. 2000, 44, 279–301. [Google Scholar] [CrossRef]
- Lee, S.; Hwang, S. Investigation on the Relationship between Land use and Water Quality with Spatial Dimension, Reservoir Type and Shape Complexity. J. Korean Inst. Landsc. Archit. 2007, 34, 1–9. [Google Scholar]
- Lee, S. Testing Non-Stationary Relationship between the Proportion of Green Areas in Watersheds and Water Quality using Geographically Weighted Regression Model. J. Korean Inst. Landsc. Archit. 2013, 41, 43. [Google Scholar] [CrossRef]
- Meador, M.R.; Coles, J.F.; Zappia, H. American Fisheries Society Symposium; Fish Assemblage Responses to Urban Intensity Gradients in Contrasting Metropolitan Areas: Birmingham, AL, USA, 2005; pp. 409–423. [Google Scholar]
- Mehaffey, M.H.; Nash, M.S.; Wade, T.G.; Ebert, D.W.; Jones, K.B.; Rager, A. Linking Land Cover and Water Quality in New York City’s Water Supply Watersheds. Environ. Monit. Assess. 2005, 107, 29–44. [Google Scholar] [CrossRef]
- Tong, S.T.; Chen, W. Modeling the Relationship between Land use and Surface Water Quality. J. Environ. Manag. 2002, 66, 377–393. [Google Scholar] [CrossRef]
- Tu, J.; Xia, Z. Examining Spatially Varying Relationships between Land use and Water Quality using Geographically Weighted Regression I: Model Design and Evaluation. Sci. Total Environ. 2008, 407, 358–378. [Google Scholar] [CrossRef]
- Weaver, L.A.; Garman, G.C. Urbanization of a Watershed and Historical Changes in a Stream Fish Assemblage. Trans. Am. Fish. Soc. 1994, 123, 162–172. [Google Scholar] [CrossRef]
- Park, S.; Lee, H.; Lee, S.; Hwang, S.; Byeon, M.; Joo, G.; Jeong, K.; Kong, D.; Kim, M. Relationships between Land use and Multi-Dimensional Characteristics of Streams and Rivers at Two Different Scales. Ann. Limnol. Int. J. Limnol. 2011, 47, 107–116. [Google Scholar] [CrossRef]
- Chelsea Nagy, R.; Graeme Lockaby, B.; Kalin, L.; Anderson, C. Effects of Urbanization on Stream Hydrology and Water Quality: The Florida Gulf Coast. Hydrol. Process. 2012, 26, 2019–2030. [Google Scholar] [CrossRef]
- An, K.; Lee, S.; Hwang, S.; Park, S.; Hwang, S. Exploring the Non-Stationary Effects of Forests and Developed Land within Watersheds on Biological Indicators of Streams using Geographically-Weighted Regression. Water 2016, 8, 120. [Google Scholar] [CrossRef]
- Hwang, S.; Hwang, S.; Park, S.; Lee, S. Examining the Relationships between Watershed Urban Land use and Stream Water Quality using Linear and Generalized Additive Models. Water 2016, 8, 155. [Google Scholar] [CrossRef]
- Casotti, C.G.; Kiffer Jr, W.P.; Costa, L.C.; Rangel, J.V.; Casagrande, L.C.; Moretti, M.S. Assessing the Importance of Riparian Zones Conservation for Leaf Decomposition in Streams. Nat. Conserv. 2015, 13, 178–182. [Google Scholar] [CrossRef]
- Chellaiah, D.; Yule, C.M. Effect of Riparian Management on Stream Morphometry and Water Quality in Oil Palm Plantations in Borneo. Limnologica 2018, 69, 72–80. [Google Scholar] [CrossRef]
- Pusey, B.J.; Arthington, A.H. Importance of the Riparian Zone to the Conservation and Management of Freshwater Fish: A Review. Mar. Freshw. Res. 2003, 54, 1–16. [Google Scholar] [CrossRef]
- Popov, V.H.; Cornish, P.S.; Sun, H. Vegetated Biofilters: The Relative Importance of Infiltration and Adsorption in Reducing Loads of Water-Soluble Herbicides in Agricultural Runoff. Agric. Ecosyst. Environ. 2006, 114, 351–359. [Google Scholar] [CrossRef]
- Meek, C.S.; Richardson, D.M.; Mucina, L. A River Runs through it: Land-use and the Composition of Vegetation Along a Riparian Corridor in the Cape Floristic Region, South Africa. Biol. Conserv. 2010, 143, 156–164. [Google Scholar] [CrossRef]
- Scott, M.L.; Nagler, P.L.; Glenn, E.P.; Valdes-Casillas, C.; Erker, J.A.; Reynolds, E.W.; Shafroth, P.B.; Gomez-Limon, E.; Jones, C.L. Assessing the Extent and Diversity of Riparian Ecosystems in Sonora, Mexico. Biodivers. Conserv. 2009, 18, 247–269. [Google Scholar] [CrossRef]
- Broadmeadow, S.; Nisbet, T.R. The Effects of Riparian Forest Management on the Freshwater Environment: A Literature Review of Best Management Practice. Hydrol. Earth Syst. Sci. 2004, 8, 286–305. [Google Scholar] [CrossRef]
- Li, M.; Huang, C.; Zhu, Z.; Shi, H.; Lu, H.; Peng, S. Assessing Rates of Forest Change and Fragmentation in Alabama, USA, using the Vegetation Change Tracker Model. For. Ecol. Manag. 2009, 257, 1480–1488. [Google Scholar] [CrossRef]
- Taniwaki, R.H.; Cassiano, C.C.; Filoso, S.; de Barros, F.; Silvio, F.; de Camargo, P.B.; Martinelli, L.A. Impacts of Converting Low-Intensity Pastureland to High-Intensity Bioenergy Cropland on the Water Quality of Tropical Streams in Brazil. Sci. Total Environ. 2017, 584, 339–347. [Google Scholar] [CrossRef]
- Popescu, C.; Oprina-Pavelescu, M.; Dinu, V.; Cazacu, C.; Burdon, F.J.; Forio, M.A.E.; Kupilas, B.; Friberg, N.; Goethals, P.; McKie, B.G. Riparian Vegetation Structure Influences Terrestrial Invertebrate Communities in an Agricultural Landscape. Water 2021, 13, 188. [Google Scholar] [CrossRef]
- Forio, M.A.E.; De Troyer, N.; Lock, K.; Witing, F.; Baert, L.; Saeyer, N.D.; Rîșnoveanu, G.; Popescu, C.; Burdon, F.J.; Kupilas, B. Small Patches of Riparian Woody Vegetation Enhance Biodiversity of Invertebrates. Water 2020, 12, 3070. [Google Scholar] [CrossRef]
- Mutinova, P.T.; Kahlert, M.; Kupilas, B.; McKie, B.G.; Friberg, N.; Burdon, F.J. Benthic Diatom Communities in Urban Streams and the Role of Riparian Buffers. Water 2020, 12, 2799. [Google Scholar] [CrossRef]
- Burdon, F.J.; Ramberg, E.; Sargac, J.; Forio, M.A.E.; De Saeyer, N.; Mutinova, P.T.; Moe, T.F.; Pavelescu, M.O.; Dinu, V.; Cazacu, C. Assessing the Benefits of Forested Riparian Zones: A Qualitative Index of Riparian Integrity is Positively Associated with Ecological Status in European Streams. Water 2020, 12, 1178. [Google Scholar] [CrossRef]
- Sargac, J.; Johnson, R.K.; Burdon, F.J.; Truchy, A.; Rîşnoveanu, G.; Goethals, P.; McKie, B.G. Forested Riparian Buffers Change the Taxonomic and Functional Composition of Stream Invertebrate Communities in Agricultural Catchments. Water 2021, 13, 1028. [Google Scholar] [CrossRef]
- Kupilas, B.; Burdon, F.J.; Thaulow, J.; Håll, J.; Mutinova, P.T.; Forio, M.A.E.; Witing, F.; Rîșnoveanu, G.; Goethals, P.; McKie, B.G. Forested Riparian Zones Provide Important Habitat for Fish in Urban Streams. Water 2021, 13, 877. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Park, S. A Structural Relationship of Topography, Developed Areas, and Riparian Vegetation on the Concentration of Total Nitrogen in Streams. J. Korean Inst. Landsc. Archit. 2020, 48, 25–34. [Google Scholar] [CrossRef]
- King, R.S.; Baker, M.E.; Whigham, D.F.; Weller, D.E.; Jordan, T.E.; Kazyak, P.F.; Hurd, M.K. Spatial Considerations for Linking Watershed Land Cover to Ecological Indicators in Streams. Ecol. Appl. 2005, 15, 137–153. [Google Scholar] [CrossRef]
- Grau, H.R.; Aide, T.M.; Zimmerman, J.K.; Thomlinson, J.R.; Helmer, E.; Zou, X. The Ecological Consequences of Socioeconomic and Land-use Changes in Postagriculture Puerto Rico. Bioscience 2003, 53, 1159–1168. [Google Scholar] [CrossRef]
- Yirigui, Y.; Lee, S.; Nejadhashemi, A.P.; Herman, M.R.; Lee, J. Relationships between Riparian Forest Fragmentation and Biological Indicators of Streams. Sustainability 2019, 11, 2870. [Google Scholar] [CrossRef]
- Damanik-Ambarita, M.N.; Everaert, G.; Goethals, P.L. Ecological Models to Infer the Quantitative Relationship between Land use and the Aquatic Macroinvertebrate Community. Water 2018, 10, 184. [Google Scholar] [CrossRef]
- Ekka, A.; Pande, S.; Jiang, Y.; der Zaag, P.v. Anthropogenic Modifications and River Ecosystem Services: A Landscape Perspective. Water 2020, 12, 2706. [Google Scholar] [CrossRef]
- Abouali, M.; Nejadhashemi, A.P.; Daneshvar, F.; Woznicki, S.A. Two-Phase Approach to Improve Stream Health Modeling. Ecol. Inform. 2016, 34, 13–21. [Google Scholar] [CrossRef]
- Hrodey, P.J.; Sutton, T.M.; Frimpong, E.A.; Simon, T.P. Land-use Impacts on Watershed Health and Integrity in Indiana Warmwater Streams. Am. Midl. Nat. 2009, 161, 76–95. [Google Scholar] [CrossRef]
- Dahm, V.; Hering, D. A Modeling Approach for Identifying Recolonisation Source Sites in River Restoration Planning. Landscape Ecol. 2016, 31, 2323–2342. [Google Scholar] [CrossRef]
- de Paula, F.R.; Gerhard, P.; de Barros, S.F.; Wenger, S.J. Multi-Scale Assessment of Forest Cover in an Agricultural Landscape of Southeastern Brazil: Implications for Management and Conservation of Stream Habitat and Water Quality. Ecol. Ind. 2018, 85, 1181–1191. [Google Scholar] [CrossRef]
- Bae, M.; Kwon, Y.; Hwang, S.; Chon, T.; Yang, H.; Kwak, I.; Park, J.; Ham, S.; Park, Y. Relationships between Three Major Stream Assemblages and their Environmental Factors in Multiple Spatial Scales. Ann. Limnol. Int. J. Limnol. 2011, 47, 91–105. [Google Scholar] [CrossRef]
- Jontos, R. Vegetative Buffers for Water Quality Protection: An Introduction and Guidance Document. Conn. Assoc. Wetl. Sci. White Pap. Veg. Buffers 2004, 1, 22. [Google Scholar]
- Lee, S.; Hwang, S.; Lee, S.; Hwang, H.; Sung, H. Landscape Ecological Approach to the Relationships of Land use Patterns in Watersheds to Water Quality Characteristics. Landsc. Urban Plann. 2009, 92, 80–89. [Google Scholar] [CrossRef]
- Kim, J.; An, K.; Hwang, S.; Hwang, G.; Kim, D.; Kim, C.; Lee, S. Mediating Effect of Stream Geometry on the Relationship between Urban Land use and Biological Index. Paddy Water Environ. 2014, 12, 157–168. [Google Scholar] [CrossRef]
- Cai, X.; Wang, D. Spatial Autocorrelation of Topographic Index in Catchments. J. Hydrol. 2006, 328, 581–591. [Google Scholar] [CrossRef]
- Dormann, C.F. Effects of Incorporating Spatial Autocorrelation into the Analysis of Species Distribution Data. Glob. Ecol. Biogeogr. 2007, 16, 129–138. [Google Scholar] [CrossRef]
- Teittinen, A.; Kallajoki, L.; Meier, S.; Stigzelius, T.; Soininen, J. The Roles of Elevation and Local Environmental Factors as Drivers of Diatom Diversity in Subarctic Streams. Freshwat. Biol. 2016, 61, 1509–1521. [Google Scholar] [CrossRef]
- Soininen, J.; Paavola, R.; Muotka, T. Benthic Diatom Communities in Boreal Streams: Community Structure in Relation to Environmental and Spatial Gradients. Ecography 2004, 27, 330–342. [Google Scholar] [CrossRef]
- Legendre, P. Spatial Autocorrelation: Trouble or New Paradigm? Ecology 1993, 74, 1659–1673. [Google Scholar] [CrossRef]
- Schabenberger, O.; Gotway, C.A. Statistical Methods for Spatial Data Analysis; Chapman Hall/CRC: London, UK, 2005. [Google Scholar]
- Lee, S.; Won, M.; Lee, H. Testing Spatial Autocorrelation of Burn Severity. J. Korean Soc. For. Sci. 2012, 101, 203–212. [Google Scholar]
- Allen, T.F.; Starr, T.B. Hierarchy: Perspectives for Ecological Complexity; University of Chicago Press: Chicago, IL, USA, 2017. [Google Scholar]
- Dorner, B.; Lertzman, K.; Fall, J. Landscape Pattern in Topographically Complex Landscapes: Issues and Techniques for Analysis. Landsc. Ecol. 2002, 17, 729–743. [Google Scholar] [CrossRef]
- Detenbeck, N.E.; Cincotta, D.; Denver, J.M.; Greenlee, S.K.; Olsen, A.R.; Pitchford, A.M. Watershed-Based Survey Designs. Environ. Monit. Assess. 2005, 103, 59–81. [Google Scholar] [CrossRef]
- Peterson, E.E.; Merton, A.A.; Theobald, D.M.; Urquhart, N.S. Patterns of Spatial Autocorrelation in Stream Water Chemistry. Environ. Monit. Assess. 2006, 121, 571–596. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Kim, I.; Ryoo, S. Evaluation of Raingauge Density and Spatial Distribution: A Case Study for Nam Han River Basin. J. Korea Water Resour. Assoc. 2003, 36, 173–181. [Google Scholar] [CrossRef][Green Version]
- Lee, J.; Choi, J.; An, K. Influence of Landuse Pattern and Seasonal Precipitation on the Long-Term Physico-Chemical Water Quality in Namhan River Watershed. J. Environ. Sci. Int. 2012, 21, 1115–1129. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; An, K.; Hwang, S.; Kim, N. An Estimated Structural Equation Model to Assess the Effects of Land use on Water Quality and Benthic Macroinvertebrates in Streams of the Nam-Han River System, South Korea. Int. J. Environ. Res. Public Health 2020, 17, 2116. [Google Scholar] [CrossRef]
- Lee, S.; Hwang, S.; Lee, J.; Jung, D.; Park, Y.; Kim, J. Overview and Application of the National Aquatic Ecological Monitoring Program (NAEMP) in Korea. Ann. Limnol. Int. J. Limnol. 2011, 47, 3–14. [Google Scholar] [CrossRef]
- Won, D.; Byun, M.; Park, J.; Lee, S.; Hwang, S. Evaluation the current status of aquatic ecosystem health in five major rivers in Korea. Korean Soc. Civ. Eng. 2010, 3, 149–160. [Google Scholar]
- National Institute of Environmental Research, (NIER). The Survey and Evaluation of Aquatic Ecosystem Health in Korea; NIER: Seong-Kyu Yoon, Korea, 2008. [Google Scholar]
- Homes, N. Integrated Watershed Management: Ecohydrology and Phytotechnology. Wetl. Ecol. Manag. 2005, 13, 209–210. [Google Scholar]
- Kelly, M.G.; Whitton, B.A. The Trophic Diatom Index: A New Index for Monitoring Eutrophication in Rivers. J. Appl. Phycol. 1995, 7, 433–444. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S. Stream Classification Based on the Ecological Characteristics for Effective Stream Management-in the Case of Nakdong River. J. Korean Soc. Environ. Restor. Technol. 2012, 15, 103–114. [Google Scholar] [CrossRef]
- Allan, J.D.; Castillo, M.M. Stream Ecology: Structure and Function of Running Waters; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Jun, Y.; Won, D.; Lee, S.; Kong, D.; Hwang, S. A Multimetric Benthic Macroinvertebrate Index for the Assessment of Stream Biotic Integrity in Korea. Int. J. Environ. Res. Public Health 2012, 9, 3599–3628. [Google Scholar] [CrossRef] [PubMed]
- Karr, J.R. Assessing Biological Integrity in Running Waters: A Method and its Rationale; Special publication/Illinois Natural History Survey: Champaign, IL, USA, 1986.
- Ahn, C.H.; Joo, J.C.; Kwon, J.H.; Song, H.M. Evaluation on Functional Assessment for Fish Habitat of Underground Type Eco-Artificial Fish Reef using the Index of Biological Integrity (IBI) and Qualitative Habitat Evaluation Index (QHEI). J. Korean Soc. Civil Eng. 2011, 31, 565–575. [Google Scholar]
- McGarigal, K. FRAGSTATS: Spatial Pattern Analysis Program for Categorical Maps. Computer Software Program Produced by the Authors at the University of Massachusetts. 2002. Available online: http://www.umass.edu/landeco/research/fragstats/fragstats.html (accessed on 12 March 2021).
- Frate, L.; Carranza, M.L. Quantifying Landscape-Scale Patterns of Temperate Forests Over Time by Means of Neutral Simulation Models. ISPRS Int. J. Geo Inf. 2013, 2, 94–109. [Google Scholar] [CrossRef]
- Tolessa, T.; Senbeta, F.; Kidane, M. Landscape Composition and Configuration in the Central Highlands of Ethiopia. Ecol. Evol. 2016, 6, 7409–7421. [Google Scholar] [CrossRef] [PubMed]
- Yirigui, Y.; Lee, S.; Nejadhashemi, A.P. Multi-Scale Assessment of Relationships between Fragmentation of Riparian Forests and Biological Conditions in Streams. Sustainability 2019, 11, 5060. [Google Scholar] [CrossRef]
- Poiani, K.A.; Richter, B.D.; Anderson, M.G.; Richter, H.E. Biodiversity Conservation at Multiple Scales: Functional Sites, Landscapes, and Networks. Bioscience 2000, 50, 133–146. [Google Scholar] [CrossRef]
- Hawes, E.; Smith, M. Riparian Buffer Zones: Functions and Recommended Widths. Eightmile River Wild Scen. Study Comm. 2005, 15, 2005. [Google Scholar]
- Fortin, M.; Drapeau, P.; Legendre, P. Spatial autocorrelation and sampling design in plant ecology. In Progress in Theoretical Vegetation Science; Springer: Berlin/Heidelberg, Germany, 1990; pp. 209–222. [Google Scholar]
- Griffith, D.A. Spatial autocorrelation in spatial interaction. In Complexity and Spatial Networks; Springer: Berlin/Heidelberg, Germany, 2009; pp. 221–237. [Google Scholar]
- Anselin, L.; Syabri, I.; Kho, Y. GeoDa: An introduction to spatial data analysis. In Handbook of Applied Spatial Analysis; Springer: Berlin/Heidelberg, Germany, 2010; pp. 73–89. [Google Scholar]
- Anselin, L. Local Indicators of Spatial association—LISA. Geogr. Anal. 1995, 27, 93–115. [Google Scholar] [CrossRef]
- Miralha, L.; Kim, D. Accounting for and Predicting the Influence of Spatial Autocorrelation in Water Quality Modeling. ISPRS Int. J. Geo Inf. 2018, 7, 64. [Google Scholar] [CrossRef]
- De’ath, G.; Fabricius, K.E. Classification and Regression Trees: A Powerful Yet Simple Technique for Ecological Data Analysis. Ecology 2000, 81, 3178–3192. [Google Scholar] [CrossRef]
- Kurita, T. Principal Component Analysis (PCA). In Computer Vision: A Reference Guide; Springer: Tokyo, Japan, 2019; pp. 1–4. [Google Scholar]
- Abdi, H.; Williams, L.J. Wiley interdisciplinary reviews: Computational statistics. Princ. Compon. Anal. 2010, 2, 433–459. [Google Scholar]
- Cablk, M. Assessment of Spatial Autocorrelation in Empirical Models in Ecology. Predicting Species Occurrences: Issues of Accuracy and Scale; Island Press: Washington, DC, USA, 2001; pp. 429–440. [Google Scholar]
- Collins, B.M.; Kelly, M.; Van Wagtendonk, J.W.; Stephens, S.L. Spatial Patterns of Large Natural Fires in Sierra Nevada Wilderness Areas. Landsc. Ecol. 2007, 22, 545–557. [Google Scholar] [CrossRef]
- Ripley, B.D. Pattern Recognition and Neural Networks; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Goethals, P.L.; Dedecker, A.P.; Gabriels, W.; Lek, S.; De Pauw, N. Applications of Artificial Neural Networks Predicting Macroinvertebrates in Freshwaters. Aquat. Ecol. 2007, 41, 491–508. [Google Scholar] [CrossRef]
- Boets, P.; Lock, K.; Messiaen, M.; Goethals, P.L. Combining Data-Driven Methods and Lab Studies to Analyse the Ecology of Dikerogammarus Villosus. Ecol. Inform. 2010, 5, 133–139. [Google Scholar] [CrossRef]
- Dominguez-Granda, L.; Lock, K.; Goethals, P.L. Using Multi-Target Clustering Trees as a Tool to Predict Biological Water Quality Indices Based on Benthic Macroinvertebrates and Environmental Parameters in the Chaguana Watershed (Ecuador). Ecol. Inform. 2011, 6, 303–308. [Google Scholar] [CrossRef]
- Everaert, G.; Boets, P.; Lock, K.; Džeroski, S.; Goethals, P.L. Using Classification Trees to Analyze the Impact of Exotic Species on the Ecological Assessment of Polder Lakes in Flanders, Belgium. Ecol. Model. 2011, 222, 2202–2212. [Google Scholar] [CrossRef]
- Everaert, G.; Pauwels, I.S.; Boets, P.; Verduin, E.; de la Haye Michelle, A.A.; Blom, C.; Goethals, P.L. Model-Based Evaluation of Ecological Bank Design and Management in the Scope of the European Water Framework Directive. Ecol. Eng. 2013, 53, 144–152. [Google Scholar] [CrossRef]
- De’Ath, G. Multivariate Regression Trees: A New Technique for Modeling Species–environment Relationships. Ecology 2002, 83, 1105–1117. [Google Scholar]
- Everaert, G.; Pauwels, I.; Bennetsen, E.; Goethals, P.L. Development and Selection of Decision Trees for Water Management: Impact of Data Preprocessing, Algorithms and Settings. Communications 2016, 29, 711–723. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.; Stone, C.J.; Olshen, R.A. Classification and Regression Trees; CRC Press: Boca Raton, FL, USA, 1984. [Google Scholar]
- Therneau, T.M.; Atkinson, E.J. An Introduction to Recursive Partitioning Using the RPART Routines; Technical report Mayo Foundation. 1997. Available online: https://r.789695.n4.nabble.com/attachment/3209029/0/zed.pdf (accessed on 14 March 2021).
- Wood, S.N. Generalized Additive Models: An Introduction with R; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Ayllón, D.; Almodóvar, A.; Nicola, G.G.; Parra, I.; Elvira, B. A New Biological Indicator to Assess the Ecological Status of Mediterranean Trout Type Streams. Ecol. Ind. 2012, 20, 295–303. [Google Scholar] [CrossRef]
- Hill, B.H.; Stevenson, R.J.; Pan, Y.; Herlihy, A.T.; Kaufmann, P.R.; Johnson, C.B. Comparison of Correlations between Environmental Characteristics and Stream Diatom Assemblages Characterized at Genus and Species Levels. J. N. Am. Benthol. Soc. 2001, 20, 299–310. [Google Scholar] [CrossRef]
- Wang, B.; Liu, D.; Liu, S.; Zhang, Y.; Lu, D.; Wang, L. Impacts of Urbanization on Stream Habitats and Macroinvertebrate Communities in the Tributaries of Qiangtang River, China. Hydrobiologia 2012, 680, 39–51. [Google Scholar] [CrossRef]
- Ward, J.V. The Four-Dimensional Nature of Lotic Ecosystems. J. N. Am. Benthol. Soc. 1989, 8, 2–8. [Google Scholar] [CrossRef]
- Gomi, T.; Sidle, R.C.; Richardson, J.S. Understanding Processes and Downstream Linkages of Headwater Systems: Headwaters Differ from Downstream Reaches by their Close Coupling to Hillslope Processes, More Temporal and Spatial Variation, and their Need for Different Means of Protection from Land Use. Bioscience 2002, 52, 905–916. [Google Scholar]
- Dodds, W.K.; Oakes, R.M. Headwater Influences on Downstream Water Quality. Environ. Manag. 2008, 41, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Le Pichon, C.; Tales, É.; Belliard, J.; Torgersen, C.E. Spatially Intensive Sampling by Electrofishing for Assessing Longitudinal Discontinuities in Fish Distribution in a Headwater Stream. Fish. Res. 2017, 185, 90–101. [Google Scholar] [CrossRef]
- Beven, K.J.; Kirkby, M.J. A Physically Based, Variable Contributing Area Model of Basin Hydrology/Un Modèle À Base Physique De Zone D’Appel Variable De L’Hydrologie Du Bassin Versant. Hydrol. Sci. J. 1979, 24, 43–69. [Google Scholar] [CrossRef]
- O’loughlin, E.M. Prediction of Surface Saturation Zones in Natural Catchments by Topographic Analysis. Water Resour. Res. 1986, 22, 794–804. [Google Scholar] [CrossRef]
- Goh, K.; Härdter, R.; Fairhurst, T. Fertilizing for Maximum Return. Oil Palm: Management for Large and Sustainable Yields; International Plant Nutrition Institute: São Paulo, Brazil, 2003; pp. 279–306. [Google Scholar]
- Gharabaghi, B.; Rudra, R.; Whiteley, H.R.; Dickinson, W.T. Development of a Management Tool for Vegetative Filter Strips. J. Water Manag. Model. 2002. [Google Scholar] [CrossRef][Green Version]
- Yu, S.; Xu, Z.; Wu, W.; Zuo, D. Effect of Land use Types on Stream Water Quality Under Seasonal Variation and Topographic Characteristics in the Wei River Basin, China. Ecol. Ind. 2016, 60, 202–212. [Google Scholar] [CrossRef]
- Karp, D.S.; Chaplin-Kramer, R.; Meehan, T.D.; Martin, E.A.; DeClerck, F.; Grab, H.; Gratton, C.; Hunt, L.; Larsen, A.E.; Martínez-Salinas, A. Crop Pests and Predators Exhibit Inconsistent Responses to Surrounding Landscape Composition. Proc. Natl. Acad. Sci. USA 2018, 115, 7863–7870. [Google Scholar] [CrossRef]
- Henebry, G.M. Spatial Model Error Analysis using Autocorrelation Indices. Ecol. Model. 1995, 82, 75–91. [Google Scholar] [CrossRef]
- Villeneuve, B.; Souchon, Y.; Usseglio-Polatera, P.; Ferréol, M.; Valette, L. Can we Predict Biological Condition of Stream Ecosystems? A Multi-Stressors Approach Linking Three Biological Indices to Physico-Chemistry, Hydromorphology and Land Use. Ecol. Ind. 2015, 48, 88–98. [Google Scholar] [CrossRef]
- Zuellig, R.E.; Carlisle, D.M.; Meador, M.R.; Potapova, M. Variance Partitioning of Stream Diatom, Fish, and Invertebrate Indicators of Biological Condition. Freshw. Sci. 2012, 31, 182–190. [Google Scholar] [CrossRef][Green Version]
- Lee, S.; Seo, J.; Lee, Y.; Kim, S.; Chun, K. Analysis of Research Trends on Mountain Streams in the Republic of Korea: Comparison to International Research Trends. Korean J. Environ. Ecol. 2019, 33, 216–227. [Google Scholar] [CrossRef]
- Aguiar, F.C.; Martins, M.J.; Silva, P.C.; Fernandes, M.R. Riverscapes Downstream of Hydropower Dams: Effects of Altered Flows and Historical Land-use Change. Landsc. Urban Plan. 2016, 153, 83–98. [Google Scholar] [CrossRef]
- Min, H.; Kim, N.; Kim, M.; Lee, S.; Hwang, K.; Hwang, S. Relation of Stream Shape Complexity to Land use, Water Quality and Benthic Diatoms in the Seom River Watershed. Korean J. Ecol. Environ. 2012, 45, 110–122. [Google Scholar]
- Teittinen, A.; Taka, M.; Ruth, O.; Soininen, J. Variation in Stream Diatom Communities in Relation to Water Quality and Catchment Variables in a Boreal, Urbanized Region. Sci. Total Environ. 2015, 530, 279–289. [Google Scholar] [CrossRef]
- Beyene, A.; Addis, T.; Kifle, D.; Legesse, W.; Kloos, H.; Triest, L. Comparative Study of Diatoms and Macroinvertebrates as Indicators of Severe Water Pollution: Case Study of the Kebena and Akaki Rivers in Addis Ababa, Ethiopia. Ecol. Ind. 2009, 9, 381–392. [Google Scholar] [CrossRef]
- Karr, J.R. Assessment of Biotic Integrity using Fish Communities. Fisheries 1981, 6, 21–27. [Google Scholar] [CrossRef]
- Schmalz, B.; Kuemmerlen, M.; Kiesel, J.; Cai, Q.; Jähnig, S.C.; Fohrer, N. Impacts of Land use Changes on Hydrological Components and Macroinvertebrate Distributions in the Poyang Lake Area. Ecohydrology 2015, 8, 1119–1136. [Google Scholar] [CrossRef]
- Nerbonne, B.A.; Vondracek, B. Effects of Local Land use on Physical Habitat, Benthic Macroinvertebrates, and Fish in the Whitewater River, Minnesota, USA. Environ. Manag. 2001, 28, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Miserendino, M.L.; Casaux, R.; Archangelsky, M.; Di Prinzio, C.Y.; Brand, C.; Kutschker, A.M. Assessing Land-use Effects on Water Quality, in-Stream Habitat, Riparian Ecosystems and Biodiversity in Patagonian Northwest Streams. Sci. Total Environ. 2011, 409, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Clapcott, J.E.; Collier, K.J.; Death, R.G.; Goodwin, E.O.; Harding, J.S.; Kelly, D.; Leathwick, J.R.; Young, R.G. Quantifying Relationships between Land-use Gradients and Structural and Functional Indicators of Stream Ecological Integrity. Freshwat. Biol. 2012, 57, 74–90. [Google Scholar] [CrossRef]
- Prasad, A.M.; Iverson, L.R.; Liaw, A. Newer Classification and Regression Tree Techniques: Bagging and Random Forests for Ecological Prediction. Ecosystems 2006, 9, 181–199. [Google Scholar] [CrossRef]
- Poor, C.J.; Ullman, J.L. Using Regression Tree Analysis to Improve Predictions of Low-Flow Nitrate and Chloride in Willamette River Basin Watersheds. Environ. Manag. 2010, 46, 771–780. [Google Scholar] [CrossRef]
- Breslow, L.A.; Aha, D.W. Simplifying Decision Trees: A Survey. Knowl. Eng. Rev. 1997, 12, 1–40. [Google Scholar] [CrossRef]
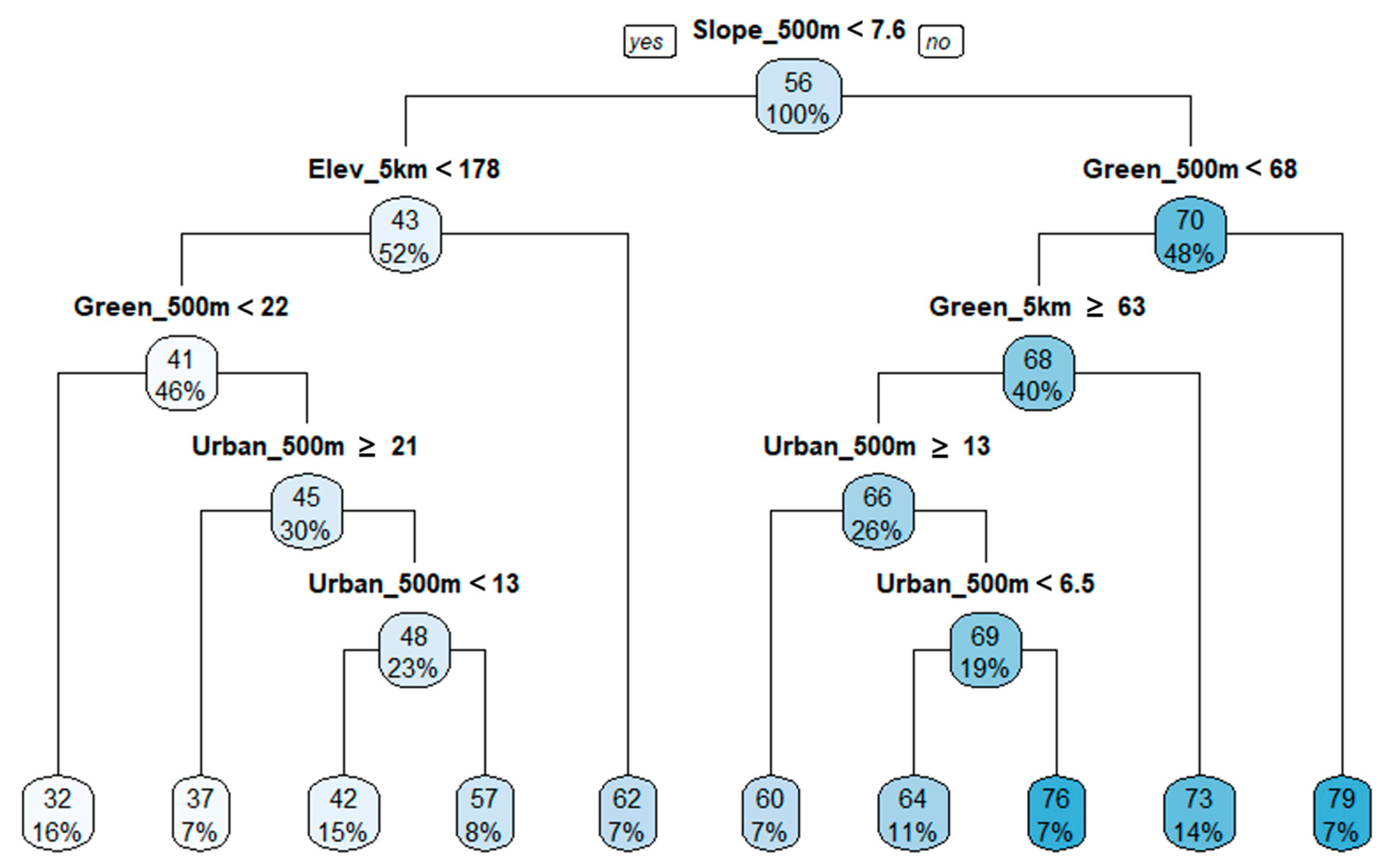
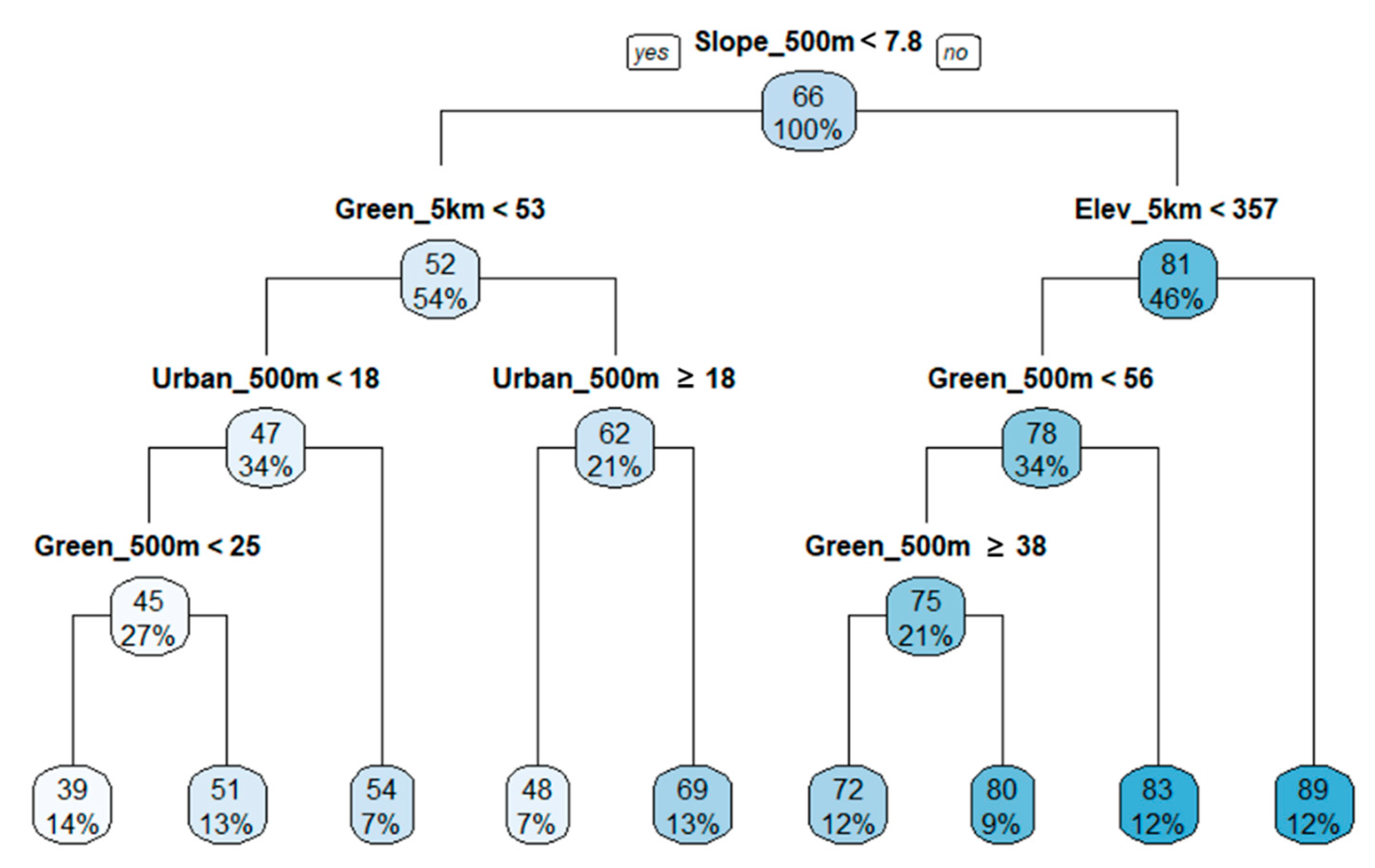
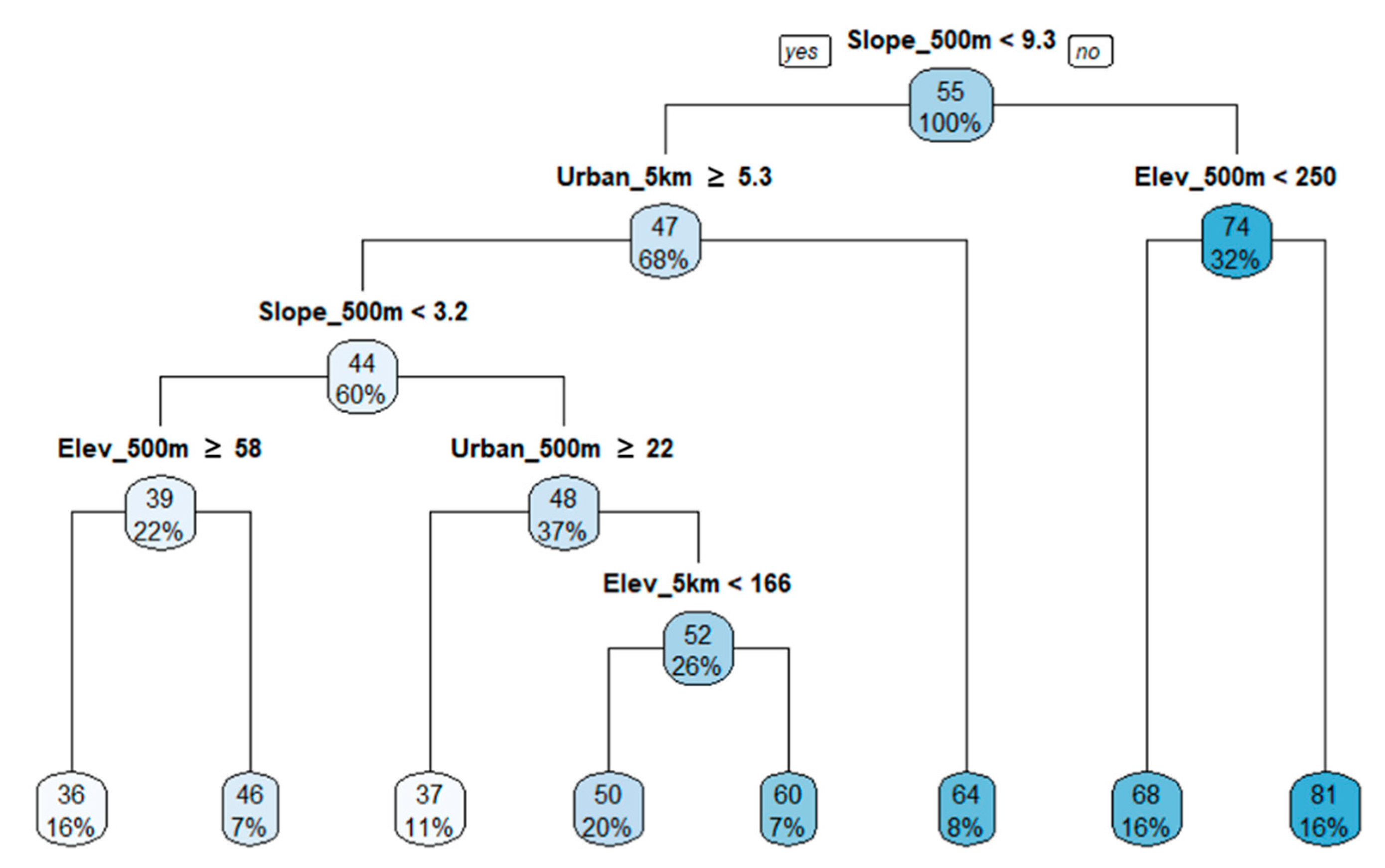
| Biological Indicators | Equations |
|---|---|
| TDI (Trophic Diatom Index) | TDI = 100 − {(WMS × 25) − 25} WMS: weighted mean sensitivity Where, j = species Aj = abundance (proportion) of species j in the sample (%) Sj = pollution sensitivity (1 ≤ S ≤ 5) of species j Vj = indicator value (1 ≤ V ≤ 3) |
| BMI (Benthic Macroinvertebrates Index) | where, j = number assigned to species n = number of species Kj = unit saprobic value of species j Hj = frequency of species j Gj = indicators weight value of species j |
| FAI (Fish Assessment Index) | FAI = sum of 8 metrics Metric 1 (M1): number of Korean native species Metric 2 (M2): number of rifle benthic species Metric 3 (M3): number of sensitive species Metric 4 (M4): percentage of tolerant species Metric 5 (M5): percentage of omnivores Metric 6 (M6): percentage of insectivores Metric 7 (M7): the amount of collection native species Metric 8 (M8): percentage of fish abnormalities |
| Classification | Variables | Min. | Max. | Mean | S.D. |
|---|---|---|---|---|---|
| Biological indicators | TDI | 11.20 | 95.20 | 56.08 | 19.78 |
| BMI | 22.60 | 94.70 | 65.52 | 19.19 | |
| FAI | 21.90 | 100.0 | 55.44 | 18.56 | |
| Topographical features | 5 km Mean elevation (m) | 48.85 | 764.73 | 212.00 | 139.73 |
| 500 m Mean elevation (m) | 38.90 | 589.63 | 158.91 | 109.00 | |
| 5 km Mean slope (%) | 1.99 | 23.29 | 10.06 | 4.91 | |
| 500 m Mean slope (%) | 1.64 | 21.54 | 7.85 | 4.87 | |
| Green spaces | 5 km Forest and Grass area (%) | 4.58 | 90.91 | 50.10 | 23.87 |
| 500 m Forest and Grass area (%) | 4.82 | 78.21 | 41.06 | 19.73 | |
| Urban areas | 5 km Urban area (%) | 0.98 | 91.74 | 14.56 | 19.48 |
| 500 m Urban area (%) | 2.33 | 66.06 | 13.85 | 12.52 |
| Biological Conditions | Class | TDI | BMI | FAI |
|---|---|---|---|---|
| Good | A | 60 ≤ TDI ≤ 100 | 80 ≤ BMI ≤ 100 | 87.5 ≤ FAI ≤ 100 |
| Fair | B | 45 ≤ TDI < 60 | 60 ≤ BMI < 80 | 56.2 ≤ FAI < 87.5 |
| Poor | C | 30 ≤ TDI < 45 | 45 ≤ BMI < 60 | 25 ≤ FAI < 56.2 |
| Very Poor | D | 0 ≤ TDI < 30 | 0 ≤ BMI < 45 | 0 ≤ FAI < 25 |
| Variable | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | PC8 | PC9 | PC10 | PC11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Proportion of Variance | 0.50 | 0.15 | 0.09 | 0.07 | 0.06 | 0.03 | 0.02 | 0.02 | 0.02 | 0.00 | 0.00 |
| Cumulative Proportion | 0.50 | 0.65 | 0.75 | 0.82 | 0.88 | 0.92 | 0.95 | 0.97 | 0.99 | 0.99 | 1.00 |
| Variable | Principal Component | ||||
|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | |
| TDI | 0.70 | −0.02 | 0.11 | 0.48 | −0.28 |
| BMI | 0.83 | −0.11 | 0.14 | 0.16 | −0.21 |
| FAI | 0.81 | 0.03 | 0.01 | −0.07 | −0.35 |
| Urban_5 km | 0.25 | 0.82 | −0.21 | 0.17 | 0.18 |
| Green_5 km | 0.20 | −0.79 | 0.39 | −0.12 | 0.19 |
| Elev_5 km | 0.88 | 0.07 | −0.15 | −0.32 | 0.16 |
| Slope_5 km | 0.92 | 0.09 | 0.14 | −0.02 | 0.10 |
| Urban_500 m | −0.12 | 0.50 | 0.78 | 0.05 | 0.24 |
| Green_500 m | 0.52 | -0.37 | −0.28 | 0.49 | 0.47 |
| Elev_500 m | 0.85 | 0.05 | −0.15 | −0.39 | 0.13 |
| Slope_500 m | 0.93 | 0.10 | 0.14 | −0.03 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-Y.; Lee, S.-W. Regression Tree Analysis for Stream Biological Indicators Considering Spatial Autocorrelation. Int. J. Environ. Res. Public Health 2021, 18, 5150. https://doi.org/10.3390/ijerph18105150
Kim M-Y, Lee S-W. Regression Tree Analysis for Stream Biological Indicators Considering Spatial Autocorrelation. International Journal of Environmental Research and Public Health. 2021; 18(10):5150. https://doi.org/10.3390/ijerph18105150
Chicago/Turabian StyleKim, Mi-Young, and Sang-Woo Lee. 2021. "Regression Tree Analysis for Stream Biological Indicators Considering Spatial Autocorrelation" International Journal of Environmental Research and Public Health 18, no. 10: 5150. https://doi.org/10.3390/ijerph18105150
APA StyleKim, M.-Y., & Lee, S.-W. (2021). Regression Tree Analysis for Stream Biological Indicators Considering Spatial Autocorrelation. International Journal of Environmental Research and Public Health, 18(10), 5150. https://doi.org/10.3390/ijerph18105150







