Public Health Impact of Using Biosimilars, Is Automated Follow up Relevant?
Abstract
1. Introduction
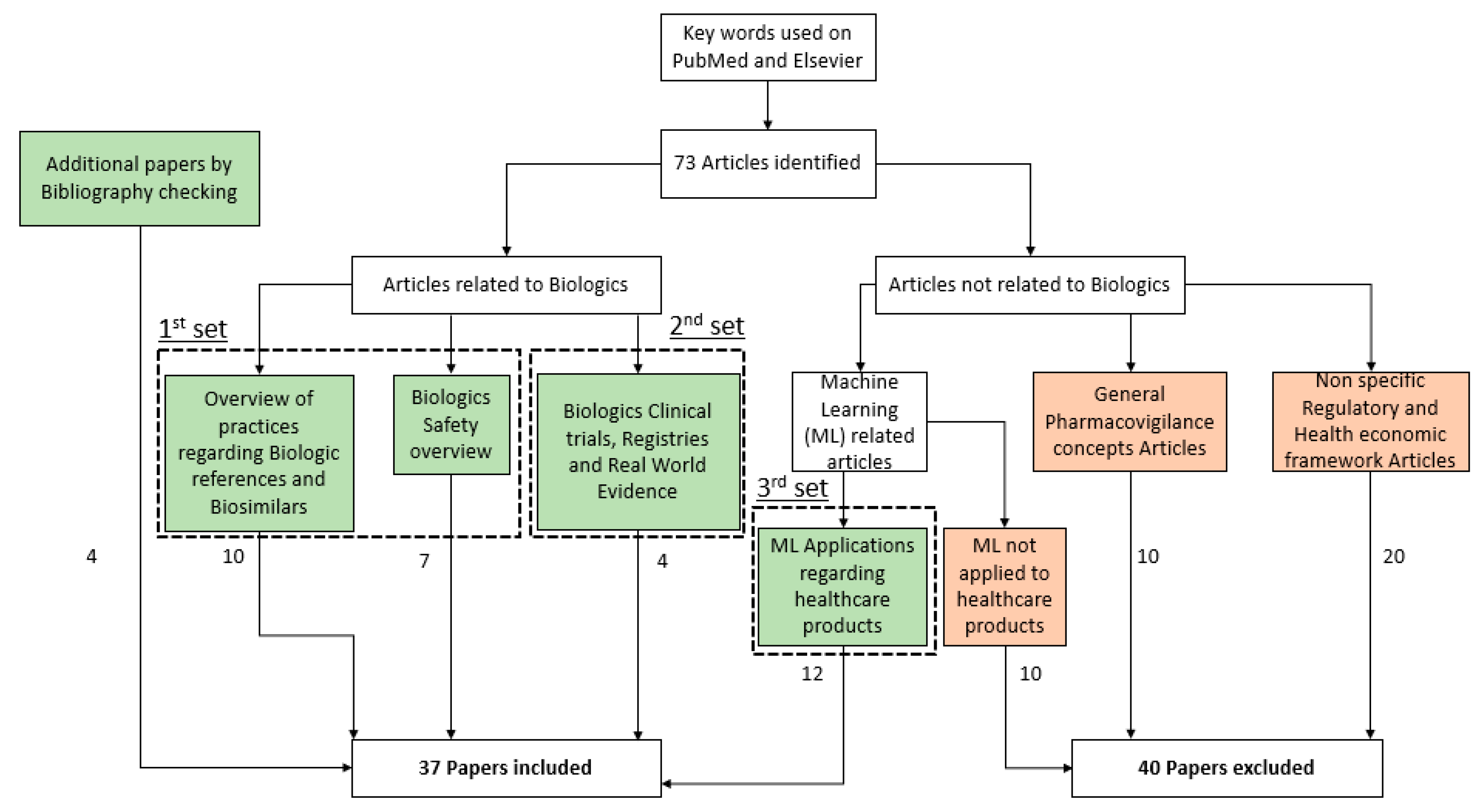
2. Method
3. Results
3.1. Immunogenicity of Biosimilars
3.1.1. Structure of Biologic Drugs
3.1.2. Immunogenicity
3.1.3. Nocebo Effect
3.2. Assessment of Immunogenicity
3.2.1. Assessment
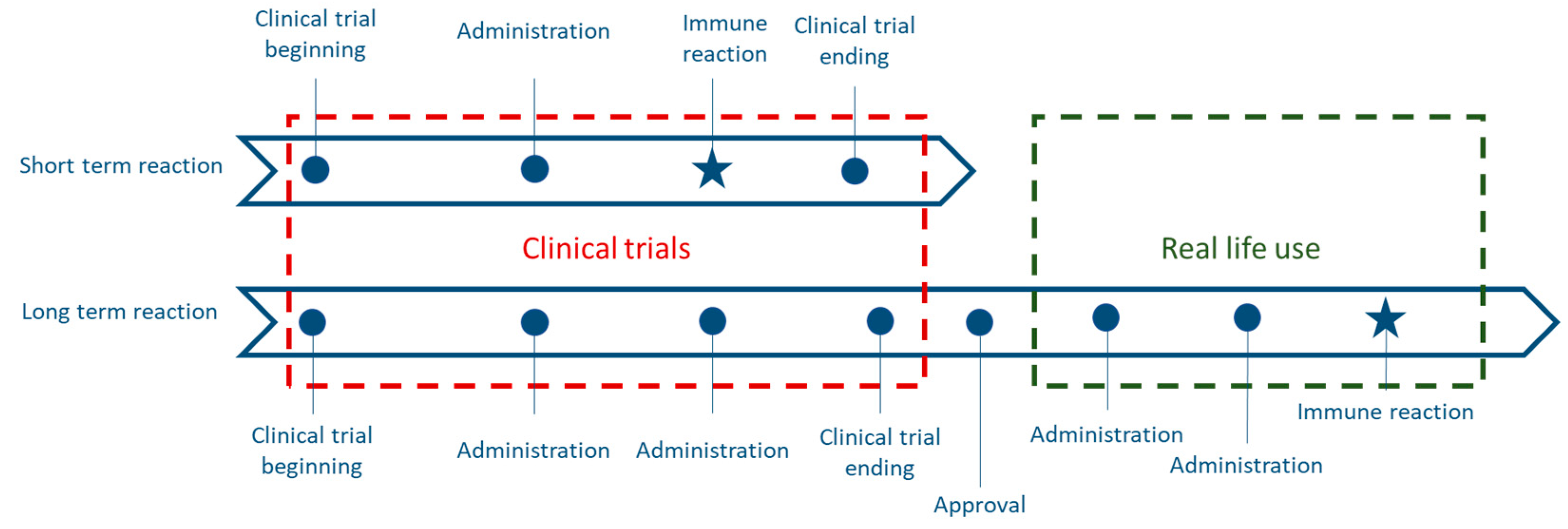
3.2.2. Limitations of Clinical Trials
3.2.3. Real-Life Use of Biosimilars
3.2.4. Immune Risks of a Switch
3.3. Economic Incentives
3.4. Real-Life Data and Big Data
3.4.1. Real-Life Data
3.4.2. Big Data in Healthcare
3.5. Applications of Machine Learning in Healthcare
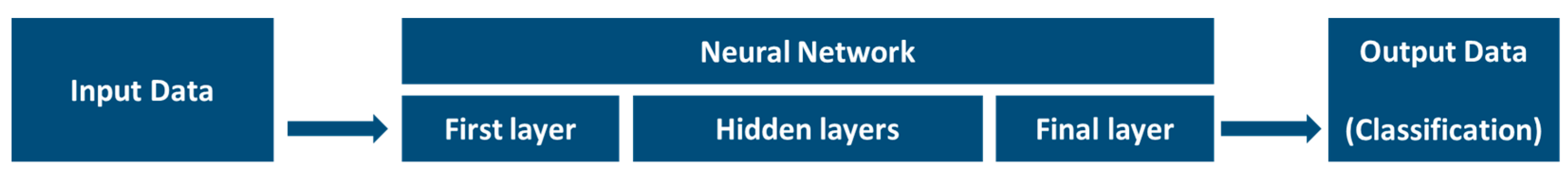
3.5.1. Machine Learning Introduction
3.5.2. Learning Process
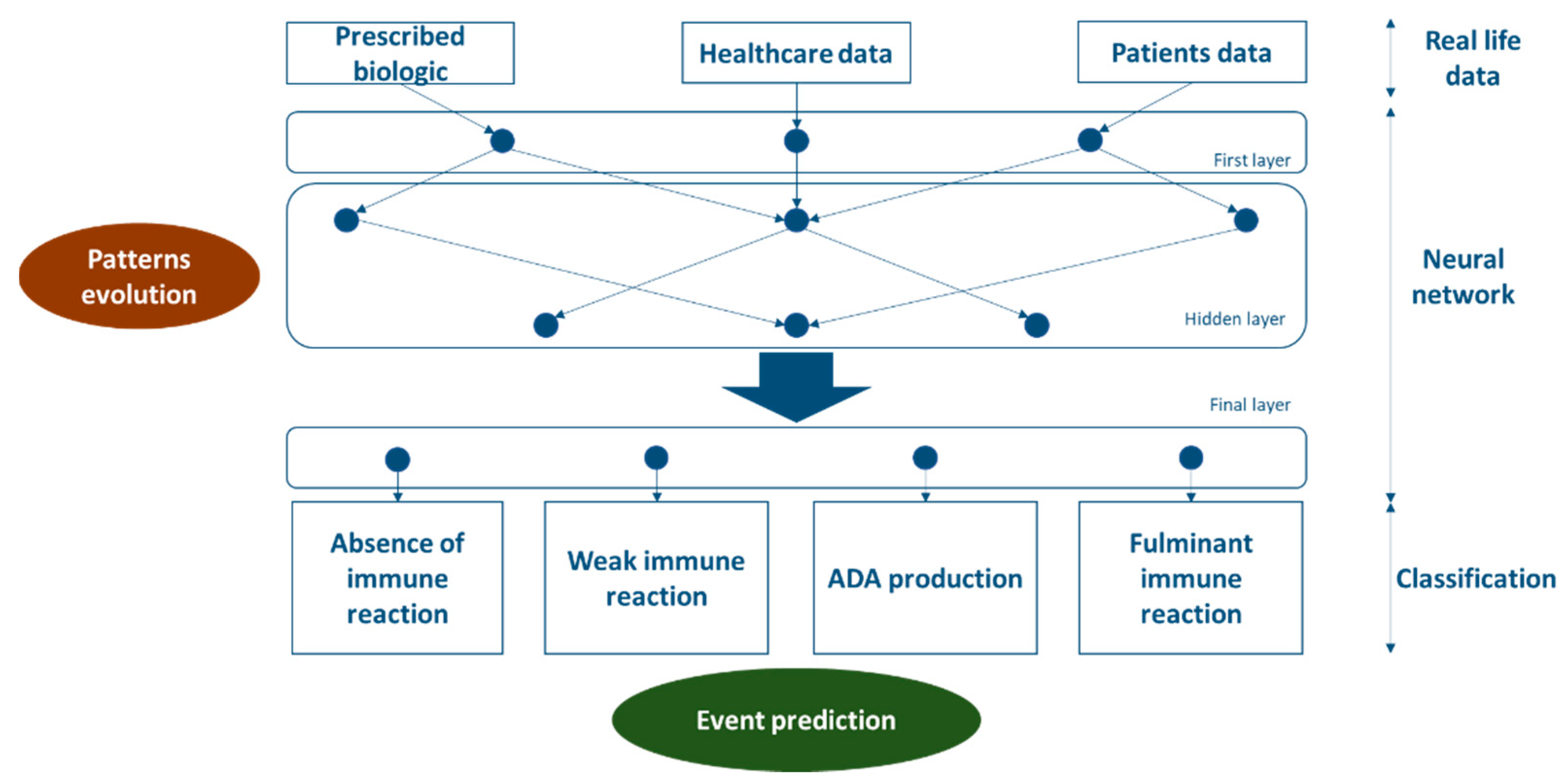
3.5.3. Neural Network Involvement
4. Discussion
4.1. Research on Risk Factors
4.2. First application of Machine Learning in Biosimilars
4.3. Transposability
4.4. Approval of a New Biosimilar
4.5. New Parameters
4.6. New Practices
4.7. Prediction Hypothesis
4.8. Legal Limitations of Machine Learning
4.9. Technical Limitations of Machine Learning
4.10. Ethical Limitations of Machine Learning
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ginghină, O.; Burcea-Dragomiroiu, G.T.A.; Gălățeanu, B.; Hudiță, A.; Dragomir, S.; Drăgănescu, D.; Bălănescu, A.; Roșca, C.A.; Giuglea, C.; Popa, D.E.; et al. Long-term safety of biosimilar medicinal products—Key for administration? Farmacia 2019, 67, 18–26. [Google Scholar] [CrossRef]
- Bocquet, F.; Paubel, P. Médicaments biosimilaires: Quel cadre juridique pour quel modèle économique? JDSAM 2015, 10, 8–22. [Google Scholar]
- Bocquet, F.; Paubel, P. A long war begins: Biosimilars versus patented biologics. J. Med. Econ. 2015, 18, 1071–1073. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kowalski, S.; Benavides, J.; Roa, P.; Galarza-Maldonado, C.; Caballero-Uribe, C.; Soriano, E.; Pineda, C.; Azevedo, V.; Avila-Pedretti, G.; Babini, A.; et al. Panlar consensus statement on biosimilars. Clin. Rheumatol. 2019, 38, 1485–1496. [Google Scholar] [CrossRef]
- Doevendans, E.; Schellekens, H. Immunogenicity of Innovative and Biosimilar Monoclonal Antibodies. Antibodies 2019, 8, 21. [Google Scholar] [CrossRef]
- Rocco, P.; Selletti, S.; Minghetti, P. Biosimilar switching and related medical liability. J. Forensic Leg. Med. 2018, 55, 93–94. [Google Scholar] [CrossRef]
- Tourdot, S.; Abdolzade-Bavil, A.; Bessa, J.; Broët, P.; Fogdell-Hahn, A.; Giorgi, M.; Jawa, V.; Kuranda, K.; Legrand, N.; Pattijn, S.; et al. 10th European immunogenicity platform open symposium on immunogenicity of biopharmaceuticals. mAbs 2020, 12, 1725369. [Google Scholar] [CrossRef]
- Singh, A.; Kalaivani, M.; Srivastava, S.; Goyal, R.K.; Gupta, S.K. Postmarketing Safety of Biosimilars: Current Status, Challenges, and Opportunities in the Spontaneous Reporting System. Ther. Innov. Regul. Sci. 2019, 1–14. [Google Scholar] [CrossRef]
- Megerlin, F.; Lopert, R. Substitution et interchangeabilité des biomédicaments, Prospective d’impact compétitif en droit comparé franco-américain. Tech. Hosp. 2014, 748, 37–43. [Google Scholar]
- Pouillon, L.; Danese, S.; Hart, A.; Fiorino, G.; Argollo, M.; Selmi, C.; Carlo-Stella, C.; Loeuille, D.; Costanzo, A.; Lopez, A.; et al. Consensus report: Clinical recommendations for the prevention and management of the nocebo effect in biosimilar-treated IBD patients. Aliment. Pharmacol. Ther. 2019, 49, 1181–1187. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Misery, L.; Naeyaert, S.; Taylor, P.C. Biological Therapies in Immune-Mediated Inflammatory Diseases: Can Biosimilars Reduce Access Inequities? Front. Pharmacol. 2019, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Agence Européenne du Médicament. Les Médicaments Biosimilaires Dans l’UE [Internet]. 2017. Available online: https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_fr.pdf (accessed on 20 December 2019).
- Smeeding, J.; Malone, D.C.; Ramchandani, M.; Stolshek, B.; Green, L.; Schneider, P. Biosimilars: Considerations for Payers. P T Peer Rev. J. Formul. Manag. 2019, 44, 54–63. [Google Scholar]
- Makady, A.; De Boer, A.; Hillege, H.; Klungel, O.; Goettsch, W. What is real world data? A review of definition based on litterature and stakeholders interviews. Value Health 2017, 20, 858–865. [Google Scholar] [CrossRef] [PubMed]
- De La Forest Divonne, M.; Gottenberg, J.E.; Salliot, C. Revue systématique des registres de polyarthrites rhumatoïdes sous biothérapie dans le monde et méta-analyse sur les données de tolérance. Rev. Rhum. 2017, 84, 199–207. [Google Scholar] [CrossRef]
- Chang, L.-C. The biosimilar pathway in the USA: An analysis of the innovator company and biosimilar company perspectives and beyond. J. Food Drug Anal. 2019, 27, 671–678. [Google Scholar] [CrossRef]
- Frantzen, L.; Cohen, J.-D.; Tropé, S.; Beck, M.; Munos, A.; Sittler, M.-A.; Diebolt, R.; Metzler, I.; Sordet, C.; Sordet, I.C. Patients’ information and perspectives on biosimilars in rheumatology: A French nation-wide survey. Jt. Bone Spine 2019, 86, 491–496. [Google Scholar] [CrossRef]
- Chapman, S.; Fitzpatrick, R.W.; Aladul, M.I. Knowledge, attitude and practice of healthcare professionals towards infliximab and insulin glargine biosimilars: Result of a UK web-based survey. BMJ Open 2017, 7, e016730. [Google Scholar] [CrossRef]
- APM News. Prescription Hospitalière de Biosimilaires Délivrés en Ville: Extension de L’expérimentation à L’adalimumab (Projet D’arrêté) [Internet]. 2019. Available online: https://www.apmnews.com/depeche/132035/331670/prescription-hospitaliere-de-biosimilaires-delivres-en-ville-extension-de-l-experimentation-a-l-adalimumab--projet-d-arrete (accessed on 20 December 2019).
- Bocquet, F. Entre difficultés de statuer sur la question de substitution et les incitations financières à leur prescription: Quelle régulation optimale pour les médicaments biosimilaires? RGDM 2020, 28, 177–194. [Google Scholar]
- Steinwandter, V.; Borchert, D.; Herwig, C. Data science tools and applications on the way to Pharma 4.0. Drug Discov. Today 2019, 24, 1795–1805. [Google Scholar] [CrossRef]
- Berger, M.L.; Sox, H.; Willke, R.J.; Brixner, D.L.; Eichler, H.G.; Goettsch, W.; Madigan, D.; Makady, A.; Schneeweiss, S.; Tarricone, R.; et al. Good Practices for Real-World Data Studies of Treatment and/or Comparative Effectiveness: Recommendations from the Joint ISPOR ISPE Special Task Force on Real World Evidence in Health Care Decision Making. Value Health 2017, 20, 1003–1008. [Google Scholar] [CrossRef]
- Wang, S.V.; Schneeweiss, S.; Berger, M.L.; Brown, J.; de Vries, F.; Douglas, I.; Gagne, J.J.; Gini, R.; Klungel, O.; Mullins, C.D.; et al. Reporting to Improve Reproducibility and Facilitate Validity Assessment for Healthcare Database Studies V1.0. Value Health 2017, 20, 1009–1022. [Google Scholar] [CrossRef] [PubMed]
- Rajkomar, A.; Oren, E.; Chen, K.; Dai, A.M.; Hajaj, N.; Hardt, M.; Liu, P.J.; Liu, X.; Marcus, J.; Sun, M.; et al. Scalable and accurate deep learning for electronic health records. NPJ Digit. Med. 2018, 1, 1–26. [Google Scholar] [CrossRef]
- Crown, W. Real-World Evidence, Causal Inference, and Machine Learning. Value Health 2019, 22, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Berdaï, D.; Thomas-Delecourt, F.; Szwarcensztein, K.; d’Andon, A.; Collignon, C.; Comet, D.; Déal, C.; Dervaux, B.; Gaudin, A.-F.; Lamarque-Garnier, V.; et al. Demandes d’études post-inscription (EPI), suivi des patients en vie réelle: Évolution de la place des bases de données. Therapies 2018, 73, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.E.; Peña, J.-M.; Jayamohan, J.; Jérusalem, A. Mechanistic models versus machine learning, a fight worth fighting for the biological community? Biol. Lett. 2018, 14, 20170660. [Google Scholar] [CrossRef] [PubMed]
- Doupe, P.; Faghmous, J.; Basu, S. Machine Learning for Health Services Researchers. Value Health 2019, 22, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Chaire AgroTIC. Deep Learning et Agriculture. 1-49 (2018) Toulon N. Deep Learning et Agriculture [Internet]. 2019. Available online: https://www.agrotic.org/les-actualites/deep-learning-et-agriculture-une-etude-de-la-chaire-agrotic/ (accessed on 20 December 2019).
- Hutchinson, L.; Steiert, B.; Soubret, A.; Wagg, J.; Phipps, A.; Peck, R.; Charoin, J.; Ribba, B. Models and Machines: How Deep Learning Will Take Clinical Pharmacology to the Next Level. CPT Pharmacomet. Syst. Pharmacol. 2019, 8, 131–134. [Google Scholar] [CrossRef]
- Ghosh, R.; Kempf, D.; Pufko, A.; Martinez, L.F.B.; Davis, C.M.; Sethi, S. Automation Opportunities in Pharmacovigilance: An Industry Survey. Pharm. Med. 2020, 34, 7–18. [Google Scholar] [CrossRef]
- Sandoval, A.M.; Díaz, J.; Llanos, L.C.; Redondo, T. Biomedical Term Extraction: NLP Techniques in Computational Medicine. Int. J. Interact. Multimed. Artif. Intell. 2019, 5, 51–59. [Google Scholar] [CrossRef]
- Fan, B.; Fan, W.; Smith, C.; Garner, H. “Skip” Adverse drug event detection and extraction from open data: A deep learning approach. Inf. Process. Manag. 2020, 57, 102131. [Google Scholar] [CrossRef]
- Tabuteau, D. 2. La Sécurité Sanitaire; Berger-Levrault: Paris, France, 2002. [Google Scholar]
- République Française. Jurisprudence 2019-097 du 11 Juillet 2019. Délibération 2019-097 du 11 Juillet 2019 Portant avis sur unProjet de loi Relatif à la Bioéthique. Available online: https://www.legifrance.gouv.fr/affichCnil.do?id=CNILTEXT000038848207 (accessed on 14 April 2020).
- Jennath, H.S.; Anoop, V.S.; Asharaf, S. Blockchain for Healthcare: Securing Patient Data and Enabling Trusted Artificial Intelligence. Int. J. Interact. Multimed. Artif. Intell. 2020, 6, 15–23. [Google Scholar] [CrossRef]
- Seymour, K.; Benyahia, N.; Hérent, P.; Malhaire, C. Exploitation des données pour la recherche et l’intelligence artificielle: Enjeux médicaux, éthiques, juridiques, techniques. Imag. Femme 2019, 29, 62–71. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perpoil, A.; Grimandi, G.; Birklé, S.; Simonet, J.-F.; Chiffoleau, A.; Bocquet, F. Public Health Impact of Using Biosimilars, Is Automated Follow up Relevant? Int. J. Environ. Res. Public Health 2021, 18, 186. https://doi.org/10.3390/ijerph18010186
Perpoil A, Grimandi G, Birklé S, Simonet J-F, Chiffoleau A, Bocquet F. Public Health Impact of Using Biosimilars, Is Automated Follow up Relevant? International Journal of Environmental Research and Public Health. 2021; 18(1):186. https://doi.org/10.3390/ijerph18010186
Chicago/Turabian StylePerpoil, Antoine, Gael Grimandi, Stéphane Birklé, Jean-François Simonet, Anne Chiffoleau, and François Bocquet. 2021. "Public Health Impact of Using Biosimilars, Is Automated Follow up Relevant?" International Journal of Environmental Research and Public Health 18, no. 1: 186. https://doi.org/10.3390/ijerph18010186
APA StylePerpoil, A., Grimandi, G., Birklé, S., Simonet, J.-F., Chiffoleau, A., & Bocquet, F. (2021). Public Health Impact of Using Biosimilars, Is Automated Follow up Relevant? International Journal of Environmental Research and Public Health, 18(1), 186. https://doi.org/10.3390/ijerph18010186




