Physician-Level Cost Control Measures and Regional Variation of Biosimilar Utilization in Germany
Abstract
1. Background
2. Biosimilars and Cost Control Measures in Germany
3. Methods
3.1. Data
3.2. Outcome Variables
3.3. Variables Accounting for Heterogeneity of Prescribing Behavior
3.4. Statistical Analysis
3.5. Ethics Approval
4. Results
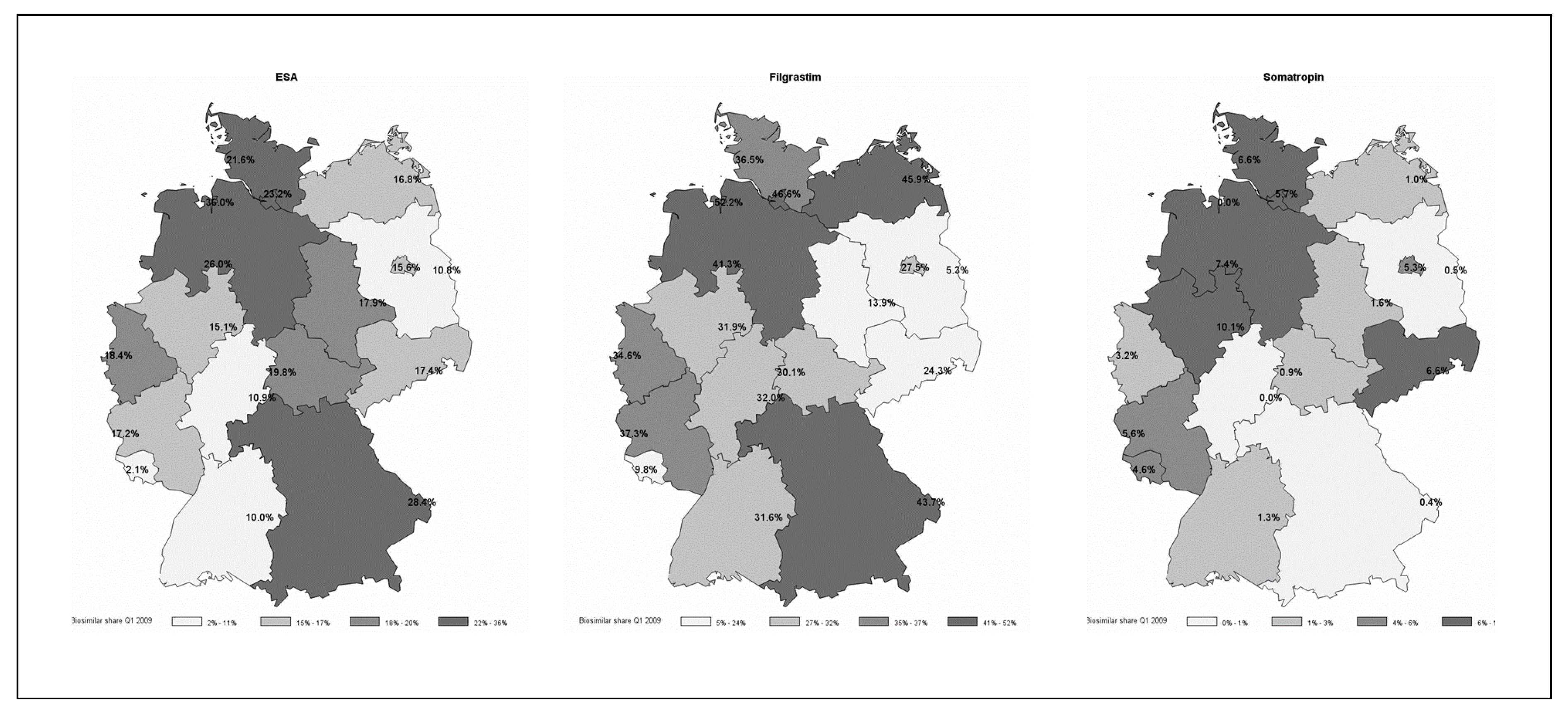
4.1. Descriptives
4.2. Associations between Cost Control Measures and Biosimilar Use
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Food and Drug Administration. Drug Approvals and Databases—Drugs@FDA Glossary of Terms. 2018. Available online: https://www.fda.gov/drugs/informationondrugs/ucm079436.htm (accessed on 3 June 2020).
- Scott Morton, F.M.; Stern, A.D.; Stern, S. The Impact of the Entry of Biosimilars: Evidence from Europe. Rev. Ind. Organ. 2018, 53, 173–210. [Google Scholar] [CrossRef]
- Mestre-Ferrandiz, J.; Towse, A.; Berdud, M. Biosimilars: How Can Payers Get Long-Term Savings? Pharmacoeconomics 2016, 34, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Farfan-Portet, M.-I.; Gerkens, S.; Lepage-Nefkens, I.; Vinck, I.; Hulstaert, F. Are biosimilars the next tool to guarantee cost-containment for pharmaceutical expenditures? Eur. J. Health Econ. 2014, 15, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E. S.1695—110th Congress (2007–2008): Biologics Price Competition and Innovation Act of 2007 2008 Nov. Available online: https://www.congress.gov/bill/110th-congress/senate-bill/1695 (accessed on 3 June 2020).
- Dylst, P.; Vulto, A.; Simoens, S. Barriers to the Uptake of Biosimilars and Possible Solutions: A Belgian Case Study. PharmacoEconomics 2014, 32, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.N. Controlling the cost of innovative cancer therapeutics. Nat. Rev. Clin. Oncol. 2009, 6, 550–552. [Google Scholar] [CrossRef] [PubMed]
- Moorkens, E.; Vulto, A.G.; Huys, I.; Dylst, P.; Godman, B.; Keuerleber, S.; Claus, B.; Dimitrova, M.; Petrova, G.; Sovic-Brkicic, L.; et al. Policies for biosimilar uptake in Europe: An overview. PLoS ONE 2017, 12, e0190147. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, H.; Guha, R.; Salgado, M. Biosimilar competition: Lessons from Europe. Nat. Rev. Drug Discov. 2014, 13, 99–100. [Google Scholar] [CrossRef] [PubMed]
- Curto, S.; Ghislandi, S.; van de Vooren, K.; Duranti, S.; Garattini, L. Regional tenders on biosimilars in Italy: An empirical analysis of awarded prices. Health Policy 2014, 116, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Hellerstein, J.K. The Importance of the Physician in the Generic versus Trade-Name Prescription Decision. Rand J. Econ. 1998, 29, 108–136. [Google Scholar] [CrossRef] [PubMed]
- Berndt, E.R.; Gibbons, R.S.; Kolotilin, A.; Taub, A.L. The heterogeneity of concentrated prescribing behavior: Theory and evidence from antipsychotics. J. Health Econ. 2015, 40, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Currie, J.; MacLeod, W.B.; Van Parys, J. Provider practice style and patient health outcomes: The case of heart attacks. J. Health Econ. 2016, 47, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Grandt, D.; Schubert, I. Barmer GEK Arzneimittelreport 2016. Report No.: Band 39. Available online: https://www.barmer.de/blob/36730/5d1b2964c4fe2dc9de815c357fda7dc8/data/pdf-arzneimittelreport-2016.pdf (accessed on 3 June 2020).
- Skinner, J. Causes and Consequences of Regional Variations in Health Care. In Handbook of Health Economics; Pauly, M., McGuire, T.G., Barros, P.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 45–93. [Google Scholar]
- Lücke, J.; Bädeker, M.; Hildinger, M. Biotech-Report: Medizinische Biotechnologie in Deutschland 2017: Biopharmazeutika: Neue Therapiekonzepte in der Onkologie. The Boston Consulting Group und VFA bio. Available online: https://docplayer.org/74812621-Medizinische-biotechnologie-in-deutschland-2017-biopharmazeutika-neue-therapiekonzepte-in-der-onkologie.html (accessed on 3 June 2020).
- Haustein, R.; de Millas, C.; Hoer, A.; Haussler, B. Saving money in the European healthcare systems with biosimilars. Generics Biosimilars Initiat. J. 2012, 1, 120. [Google Scholar] [CrossRef]
- Busse, R.; Blümel, M. Germany—Health System review. Health Syst. Transition. 2014, 16, 1–196. [Google Scholar]
- § 84 SGB V Arznei-und Heilmittelvereinbarung. Sozialgesetzbuch Fünftes Buch Gesetzliche Krankenversicherung Zuletzt geändert durch Art. G v. 22.3. I 350. 2019. Available online: https://www.sozialgesetzbuch-sgb.de/sgbv/84.html (accessed on 3 June 2020).
- Wang, Y.R.; Pauly, M.V. Spillover Effects of Restrictive Drug Formularies on Physician Prescribing Behavior: Evidence from Medicaid. J. Econ. Manag. Strategy 2005, 14, 755–773. [Google Scholar] [CrossRef]
- Fischer, K.E.; Koch, T.; Kostev, K.; Stargardt, T. The impact of physician-level drug budgets on prescribing behavior. Eur. J. Health Econ. 2018, 19, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Institut des Bewertungsausschusses. Klassifikationsmodell Morbiditätsbedingte Veränderungsraten gemäß § 87a Abs. 4 SGB V. Available online: https://institut-ba.de/service/klassifikation/km87a2018.html (accessed on 3 June 2020).
- Gonul, F.F.; Carter, F.; Petrova, E.; Srinivasan, K. Promotion of Prescription Drugs and Its Impact on Physicians’ Choice Behavior. J. Marketing 2001, 65, 79–90. [Google Scholar] [CrossRef]
- Atella, V.; Belotti, F.; Depalo, D. Drug therapy adherence and health outcomes in the presence of physician and patient unobserved heterogeneity. Health Econ. 2017, 26, 106–126. [Google Scholar] [CrossRef]
- Koulayev, S.; Simeonova, E.; Skipper, N. Can Physicians Affect Patient Adherence With Medication? Health Econ. 2017, 26, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Bocquet, F.; Loubière, A.; Fusier, I.; Cordonnier, A.-L.; Paubel, P. Competition Between Biosimilars and Patented Biologics: Learning from European and Japanese Experience. Pharmacoeconomics 2016, 34, 1173–1186. [Google Scholar] [CrossRef]
- Rohrbasser, A.; Harris, J.; Mickan, S.; Tal, K.; Wong, G. Quality circles for quality improvement in primary health care: Their origins, spread, effectiveness and lacunae—A scoping review. PLoS ONE 2018, 13, e0202616. [Google Scholar] [CrossRef]
- GKV Spitzenverband. Bekanntmachung des Spitzenverbandes Bund der Krankenkassen (GKV-Spitzenverband) nach § 35 SGB V vom 8. Oktober 2012—Festbetragsgruppe Antianämika, andere. 2012. Available online: https://www.gkv-spitzenverband.de/krankenversicherung/arzneimittel/arzneimittel_festbetraege/festbetragsfestsetzungsbeschluess_2012_2009/arzneimittel_festbetraege_festbetragsanpassung_01_12_2012.jsp (accessed on 3 June 2020).
| Drug (Class) | Disease Areas | Prescriptions (2009–2015) | Biosimilar Quota | Priority Prescribing | Combi-Nation | Total |
|---|---|---|---|---|---|---|
| Erythropoesis stimulating agents | Anemia, cancer, postoperative inflammation, reactions after kidney transplantation | 3,784,943 | 14 | 4 | 3 | 15 |
| Filgrastim | Neutropenia, severe neutropenia in cancer, cancer | 330,081 | 3 | 8 | 2 | 9 |
| Somatropin | Growth disorders | 202,519 | 6 | 9 | 5 | 10 |
| Total | 4,317,543 | 23 | 21 | 10 | 44 |
| Drug (Class) | Specialization | Statistic | Biosimilar Share (Prescriptions) | Biosimilar Share (DDD 2) | Share of Patients Using Biosimilars in Practice | Number of Patients Using Biosimilars in Practice | Prescriptions Biologics | Prescription Biosimilars | Prescriptions per 1000 Patients, Biologics | Prescriptions per 1000 Patients, Biosimilars |
|---|---|---|---|---|---|---|---|---|---|---|
| ESAs 1 | GP | mean | 20.64% | 20.49% | 0.31% | 2.53 | 10.9 | 2.99 | 429 | 111 |
| (s.d.) | (6.51%) | (6.38%) | (0.33%) | (2.4) | (10.1) | (2.95) | (419) | (97.3) | ||
| Specialist | mean | 34.73% | 31.92% | 3.37% | 23.8 | 66.3 | 28.4 | 2801 | 947 | |
| (s.d.) | (9.24%) | (8.7%) | (1.14%) | (6.43) | (11.1) | (7.22) | (547) | (273) | ||
| Filgrastim | GP | mean | 61.85% | 61.85% | 0.33% | 4.36 | 7.37 | 5.35 | 28.7 | 20.1 |
| (s.d.) | (15.86%) | (15.97%) | (0.27%) | (3.03) | (3.34) | (3.35) | (15.6) | (14.4) | ||
| Specialist | mean | 75.94% | 75.93% | 1.29% | 7.5 | 11.4 | 9.06 | 110 | 79.6 | |
| (s.d.) | (6.82%) | (6.78%) | (0.63%) | (2.11) | (2.67) | (2.59) | (55.7) | (43.8) | ||
| Somatropin | GP | mean | 3.20% | 3.14% | 0.04% | 0.33 | 6.27 | 0.34 | 409 | 26.5 |
| (s.d.) | (3.99%) | (3.99%) | (0.08%) | (0.42) | (2.5) | (0.42) | (659) | (56.7) | ||
| Specialist | mean | 9.51% | 10.33% | 0.64% | 2.46 | 23.5 | 2.51 | 4456 | 626 | |
| (s.d.) | (4.45%) | (5.12%) | (0.37%) | (1.23) | (7.05) | (1.25) | (2695) | (424) |
| Drug (n = Number of Physician–Quarter Combinations) | Statistic | Erythropoesis-Stimulating Agents (n = 10,948) | Filgrastim (n = 1516) | Somatropin (n = 3193) | Total (n = 19,707) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Number of patients in practice receiving biologic drug | mean | 54.17 | 19.25 | 23.47 | 37.35 | ||||
| (s.d.) | (57.32) | (23.16) | (42.08) | (49.51) | |||||
| Share of patients receiving biologic drug in practice | mean | 0.03 | 0.01 | 0.01 | 0.02 | ||||
| (s.d.) | (0.07) | (0.03) | (0.02) | (0.05) | |||||
| Patient age (biologic patients) | mean | 70.45 | 59.53 | 25.42 | 59.77 | ||||
| (s.d.) | (8.35) | (10.94) | (18.79) | (17.63) | |||||
| Comorbidity index of biologic patients | mean | 17.26 | 13.54 | 9.06 | 14.54 | ||||
| (s.d.) | (3.60) | (3.14) | (3.85) | (4.75) | |||||
| Share of male individuals of biologic patients | mean | 0.50 | 0.39 | 0.57 | 0.49 | ||||
| (s.d.) | (0.23) | (0.28) | (0.26) | (0.25) | |||||
| Share of individuals older than 65 years of age of biologic patients | mean | 0.74 | 0.47 | 0.07 | 0.51 | ||||
| (s.d.) | (0.23) | (0.25) | (0.19) | (0.36) | |||||
| Total number of patients in practice | mean | 1362.51 | 1704.44 | 1939.04 | 1584.65 | ||||
| (s.d.) | (1161.10) | (1493.27) | (2202.65) | (1449.43) | |||||
| Price per defined daily dose of packages prescribed by physician (biologics incl. biosimilars) | mean | 10.98 | 175.21 | 53.24 | 45.84 | ||||
| (s.d.) | (3.13) | (94.88) | (18.22) | (70.07) | |||||
| Average household income in region of practice in 1000 EUR | mean | 1699.60 | 1695.37 | 1702.37 | 1702.10 | ||||
| (s.d.) | (173.48) | (177.99) | (167.78) | (173.82) | |||||
| N | % | N | % | N | % | N | % | ||
| Type of practice | |||||||||
| Solo practice | 4206 | 38.42 | 1059 | 69.85 | 1748 | 54.74 | 9360 | 47.5 | |
| Group practice | 6742 | 61.58 | 457 | 30.15 | 1445 | 45.26 | 10,347 | 52.5 | |
| Area | |||||||||
| Metropolitan | 5409 | 49.41 | 814 | 53.69 | 1637 | 51.27 | 9864 | 50.05 | |
| Urbanized | 4129 | 37.71 | 575 | 37.93 | 1165 | 36.49 | 7343 | 37.26 | |
| Rural | 1410 | 12.88 | 127 | 8.38 | 391 | 12.25 | 2500 | 12.69 | |
| Specialist status | |||||||||
| General practitioner | 2470 | 22.56 | 293 | 19.33 | 482 | 15.1 | 3637 | 18.46 | |
| Specialist | 8478 | 77.44 | 1223 | 80.67 | 2711 | 84.9 | 16,070 | 81.54 | |
| ESA | Filgrastim | Somatropin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All Physicians | Balanced Panel | All Physicians | Balanced Panel | All Physicians | Balanced Panel | |||||
| GP | specialist | GP | specialist | GP | specialist | specialist | GP | specialist | specialist | |
| Priority prescribing | −0.0263 | −0.0069 | −0.0776 | −0.0129 | −0.0104 | −0.0331 *** | −0.0180 | 0.0095 | −0.0077 | −0.0199 |
| (0.0202) | (0.0083) | (0.0665) | (0.0106) | (0.0270) | (0.0097) | (0.0230) | (0.0157) | (0.0069) | (0.0105) | |
| Quota | −0.0260 * | −0.0098 * | 0.0924 * | 0.0178 ** | −0.0356 | 0.0612 ** | 0.0411 | −0.0087 | 0.0152 | 0.0118 |
| (0.0105) | (0.0046) | (0.0359) | (0.0056) | (0.0735) | (0.0220) | (0.0589) | (0.0141) | (0.0083) | (0.0138) | |
| Group practice (ref: solo practice) | 0.0153 * | 0.0502 *** | −0.0434 * | 0.0241 *** | 0.1081 *** | 0.0967 *** | 0.0695 *** | −0.0250 ** | −0.0053 | −0.0405 *** |
| (0.0069) | (0.0031) | (0.0181) | (0.0041) | (0.0159) | (0.0062) | (0.0149) | (0.0093) | (0.0056) | (0.0091) | |
| Share of patients receiving biologic drug in practice | 2.6378 *** | 1.9431 *** | 3.9994 *** | 2.2698 *** | 8.4402 *** | 1.8534 *** | 7.1655 *** | 14.0649 | 2.9614 *** | 3.8537 *** |
| (0.2882) | (0.0390) | (0.3167) | (0.0480) | (1.7605) | (0.1609) | (1.5168) | (8.3375) | (0.4713) | (0.4650) | |
| Patient age | 0.0021 *** | 0.0020 *** | −0.0032 | −0.0009 | 0.0064 *** | 0.0041 *** | −0.0044 | −0.0004 | −0.0010 *** | 0.0002 |
| (0.0004) | (0.0003) | (0.0033) | (0.0006) | (0.0010) | (0.0008) | (0.0025) | (0.0004) | (0.0002) | (0.0006) | |
| Comorbidity index | −0.0001 | 0.0017 ** | 0.0017 | −0.0011 | 0.0041 | 0.0062 *** | −0.0014 | −0.0015 * | −0.0023 ** | −0.0054 * |
| (0.0006) | (0.0006) | (0.0024) | (0.0009) | (0.0022) | (0.0012) | (0.0041) | (0.0007) | (0.0009) | (0.0023) | |
| Share male individuals of biologic patients | −0.0068 | −0.0207 * | 0.0117 | −0.0500 * | −0.0193 | −0.1019 *** | −0.2175 *** | −0.0087 | −0.0148 | −0.0483 |
| (0.0082) | (0.0091) | (0.0947) | (0.0213) | (0.0251) | (0.0126) | (0.0443) | (0.0096) | (0.0094) | (0.0272) | |
| Share individuals older 65 years of age of biologic patients | −0.0144 | 0.0383 *** | 0.4178 *** | 0.1494 *** | −0.0114 | −0.0127 | 0.0417 | −0.0156 | 0.0149 | −0.0479 |
| (0.0114) | (0.0116) | (0.1072) | (0.0233) | (0.0354) | (0.0211) | (0.0760) | (0.0145) | (0.0172) | (0.0437) | |
| Number of patients in practice in 1000 patients | 0.0054 | 0.0182 *** | 0.0445 *** | 0.0478 *** | −0.0091 | 0.0154 *** | 0.0647 *** | 0.0157 *** | 0.0051 ** | 0.0099 *** |
| (0.0033) | (0.0015) | (0.0105) | (0.0027) | (0.0063) | (0.0023) | (0.0116) | (0.0031) | (0.0016) | (0.0025) | |
| Price per DDD of packages prescribed by physician | −0.0014 | −0.0003 *** | −0.0155 *** | −0.0003 | −0.0006 *** | −0.0008 *** | −0.0020 *** | −0.0003 * | −0.0008 *** | −0.0013 *** |
| (0.0011) | (0.0001) | (0.0037) | (0.0003) | (0.0001) | (0.0001) | (0.0002) | (0.0001) | (0.0001) | (0.0001) | |
| Average household income in region of practice in 1000 EUR | −0.0146 | 0.1053 *** | −0.4648 *** | 0.1230 *** | 0.2550 ** | 0.3196 *** | 0.1587 | 0.1009 ** | −0.1499 *** | −0.2061 *** |
| (0.0384) | (0.0156) | (0.1164) | (0.0204) | (0.0867) | (0.0318) | (0.0812) | (0.0343) | (0.0252) | (0.0416) | |
| Urbanized areas (ref: metropolitan) | −0.0056 | 0.0351 *** | 0.0624 ** | 0.0440 *** | −0.1306 *** | −0.0087 | 0.0540 * | 0.0283 * | 0.0036 | −0.0434 *** |
| (0.0078) | (0.0035) | (0.0207) | (0.0045) | (0.0197) | (0.0071) | (0.0225) | (0.0110) | (0.0053) | (0.0092) | |
| Rural (ref: metropolitan) | −0.0233 | 0.0795 *** | 0.0328 | 0.0777 *** | −0.1085 ** | 0.0229 * | 0.1099 *** | 0.0128 | −0.0633 *** | −0.0970 *** |
| (0.0127) | (0.0055) | (0.0480) | (0.0069) | (0.0340) | (0.0113) | (0.0254) | (0.0132) | (0.0078) | (0.0100) | |
| TIME Fixed Effects | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Physician Association Fixed Effects | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Intercept | 0.1310 | −0.1684 *** | 0.7707 ** | −0.0614 | −0.3750 * | −0.2740 ** | 0.8661 *** | −0.1694 ** | 0.3906 *** | 0.7799 *** |
| (0.0692) | (0.0305) | (0.2545) | (0.0448) | (0.1622) | (0.0850) | (0.2267) | (0.0639) | (0.0459) | (0.0753) | |
| N | 15,506 | 50,389 | 1074 | 23,258 | 3021 | 14,758 | 1607 | 1.436 | 7392 | 2569 |
| r2 | 0.070 | 0.319 | 0.537 | 0.456 | 0.308 | 0.247 | 0.504 | 0.186 | 0.216 | 0.376 |
| F | 13.339 | 313.778 | 58.227 | 312.700 | 25.908 | 66.053 | 93.470 | 3.877 | 27.386 | 23.007 |
| RMSE | 0.3568 | 0.2857 | 0.2041 | 0.2396 | 0.3768 | 0.3307 | 0.2594 | 0.1305 | 0.1675 | 0.1481 |
| Biologic Drug (Class) | Outcome | Biosimilar Share (Prescriptions) | Prescriptions Biosimilars | Prescriptions Original | Prescriptions Total | ||||
|---|---|---|---|---|---|---|---|---|---|
| Type of Measure | GP | Specialist | GP | Specialist | GP | Specialist | GP | Specialist | |
| ESAs (n = 15,506 (GPs), n = 50,389 (specialists)) | Priority prescribing | −0.0161 | −0.0230 * | −1.7419 * | −6.8939 * | −0.6267 | −4.1181 | −2.3686 | −11.0120 * |
| (0.0180) | (0.0106) | (0.8732) | (3.4506) | (1.3009) | (3.2010) | (1.7723) | (4.9739) | ||
| Quota | −0.0044 | 0.0247 *** | −0.5899 | 8.5505 ** | −1.3458 | 2.2037 | −1.9357 | 10.7542 * | |
| (0.0148) | (0.0075) | (1.4571) | (2.7040) | (1.9901) | (2.7980) | (2.9063) | (4.3682) | ||
| Filgrastim (n = 3021 (GPs), n = 14,758 (specialists)) | Priority prescribing | −0.0196 | −0.0055 | 0.2571 | 3.3308 * | 0.903 | 0.6469 | 1.1601 | 3.9777 * |
| (0.0361) | (0.0126) | (0.8610) | (1.6303) | (0.6409) | (1.2059) | (0.9966) | (1.9138) | ||
| Quota | −0.1228 | 0.0442 | 2.1511 | −1.3236 | 8.313 | −1.1173 | 10.464 | −2.4409 | |
| (0.1073) | (0.0319) | (1.1118) | (3.5723) | (6.9305) | (1.8316) | (7.2926) | (4.4568) | ||
| Somatropin (n = 1436 (GPs), n = 7392 (specialists)) | Priority prescribing | 0.0055 | −0.0018 | 0.0834 | 0.069 | 0.7486 | −10.5695 | 0.832 | −10.5005 |
| (0.0231) | (0.0087) | (0.1211) | (1.8285) | (1.3554) | (11.9035) | (1.4192) | (13.1738) | ||
| Quota | −0.0224 | 0.0136 | 0.0997 | 0.4783 | 1.6937 | −12.3819 | 1.7933 | −11.9036 | |
| (0.0340) | (0.0106) | (0.1958) | (1.6254) | (2.3429) | (11.4075) | (2.490) | (12.3867) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blankart, K.E.; Arndt, F. Physician-Level Cost Control Measures and Regional Variation of Biosimilar Utilization in Germany. Int. J. Environ. Res. Public Health 2020, 17, 4113. https://doi.org/10.3390/ijerph17114113
Blankart KE, Arndt F. Physician-Level Cost Control Measures and Regional Variation of Biosimilar Utilization in Germany. International Journal of Environmental Research and Public Health. 2020; 17(11):4113. https://doi.org/10.3390/ijerph17114113
Chicago/Turabian StyleBlankart, Katharina E., and Friederike Arndt. 2020. "Physician-Level Cost Control Measures and Regional Variation of Biosimilar Utilization in Germany" International Journal of Environmental Research and Public Health 17, no. 11: 4113. https://doi.org/10.3390/ijerph17114113
APA StyleBlankart, K. E., & Arndt, F. (2020). Physician-Level Cost Control Measures and Regional Variation of Biosimilar Utilization in Germany. International Journal of Environmental Research and Public Health, 17(11), 4113. https://doi.org/10.3390/ijerph17114113





