Effects of Individualized Aerobic Exercise Training on Physical Activity and Health-Related Physical Fitness among Middle-Aged and Older Adults with Multimorbidity: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Intervention
2.4. Outcome Measurement
2.5. Sample Size
2.6. Randomization and Blinding
2.7. Ethical Consideration
2.8. Statistical Methods
3. Results
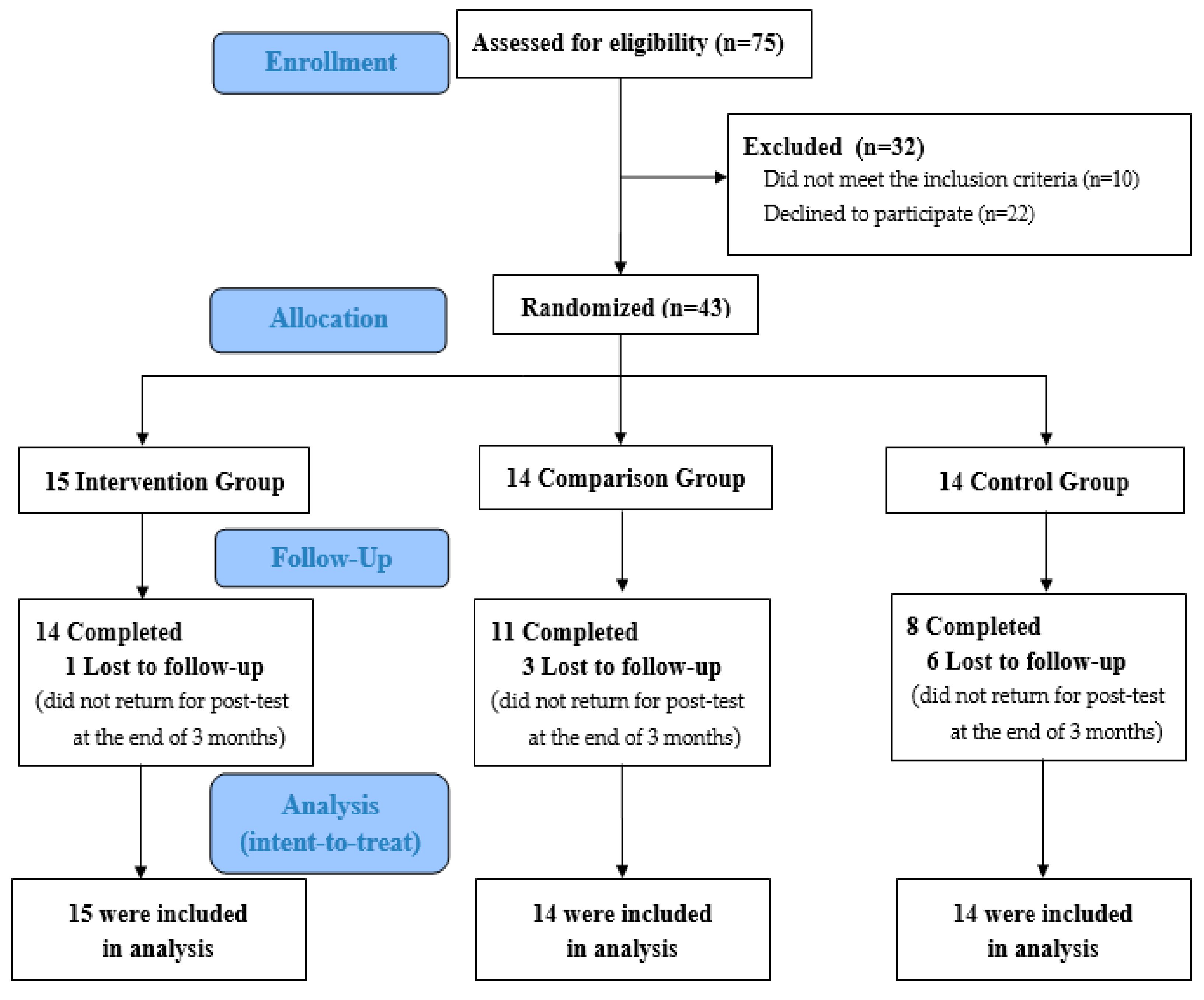
3.1. Recruitment
3.2. Baseline Characteristics of the Participants
3.3. Outcome Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnston, M.C.; Crilly, M.; Black, C.; Prescott, G.J.; Mercer, S.W. Defining and measuring multimorbidity: A systematic review of systematic reviews. Eur. J. Public Health 2018, 29, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Marengoni, A.; Angleman, S.; Melis, R.; Mangialasche, F.; Karp, A.; Garmen, A.; Meinow, B.; Fratiglioni, L. Aging with multimorbidity: A systematic review of the literature. Ageing Res. Rev. 2011, 10, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Willadsen, T.G.; Bebe, A.; Køster-Rasmussen, R.; Jarbøl, D.E.; Guassora, A.D.; Waldorff, F.B.; Reventlow, S.; Olivarius Nde, F. The role of diseases, risk factors and symptoms in the definition of multimorbidity—A systematic review. Scand. J. Prim. Health Care 2016, 34, 112–121. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Noncommunicable Diseases 2014; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Tinetti, M.E.; Fried, T.R.; Boyd, C.M. Designing health care for the most common chronic condition—Multimorbidity. JAMA 2012, 307, 2493–2494. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.F. Chronic Care: Making the Case for Ongoing Care; Robert Wood Johnson Foundation: New Jersey, NJ, USA, 2010. [Google Scholar]
- Bayliss, E.A.; Steiner, J.F.; Fernald, D.H.; Crane, L.A.; Main, D.S. Descriptions of barriers to self-care by persons with comorbid chronic diseases. Ann. Fam. Med. 2003, 1, 15–21. [Google Scholar] [CrossRef]
- Fortin, M.; Bravo, G.; Hudon, C.; Lapointe, L.; Almirall, J.; Dubois, M.F.; Vanasse, A. Relationship between multimorbidity and health-related quality of life of patients in primary care. Qual. Life Res. 2006, 15, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.S.; Li, Y.; Mo, H.Y.; Qiu, D.X.; Zhao, J.; Luo, J.L.; Lin, W.Q.; Wang, J.J.; Wang, P.-X. A community-based cross-sectional study of sleep quality in middle-aged and older adults. Qual Life Res. 2017, 26, 923–933. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Byrd, B.R.; Keith, J.; Keeling, S.M.; Weatherwax, R.M.; Nolan, P.B.; Ramos, J.S.; Dalleck, L.C. Personalized moderate-intensity exercise training combined with high-intensity interval training enhances training responsiveness. Int. J. Environ. Res. Public Health 2019, 16, 2088. [Google Scholar] [CrossRef]
- Wilund, K.R.; Viana, J.L.; Perez, L.M. A critical review of exercise training in hemodialysis patients: Personalized activity prescriptions are needed. Exerc. Sport Sci. Rev. 2020, 48, 28–39. [Google Scholar] [CrossRef]
- Wood, W.A.; Phillips, B.; Smith-Ryan, A.E.; Wilson, D.; Deal, A.M.; Bailey, C.; Meeneghan, M.; Reeve, B.B.; Basch, E.M.; Bennett, A.V.; et al. Personalized home-based interval exercise training may improve cardiorespiratory fitness in cancer patients preparing to undergo hematopoietic cell transplantation. Bone Marrow Transpl. 2016, 51, 967–972. [Google Scholar] [CrossRef]
- Armstrong, M.; Vogiatzis, I. Personalized exercise training in chronic lung diseases. Respirology 2019, 24, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Bricca, A.; Harris, L.K.; Jäger, M.; Smith, S.M.; Juhl, C.B.; Skou, S.T. Benefits and harms of exercise therapy in people with multimorbidity: A systematic review and meta-analysis of randomised controlled trials. Ageing Res. Rev. 2020, 63, 101166. [Google Scholar] [CrossRef] [PubMed]
- Budts, W.; Börjesson, M.; Chessa, M.; Van Buuren, F.; Trigo Trindade, P.; Corrado, D.; Heidbuchel, H.; Webb, G.; Holm, J.; Papadakis, M. Physical activity in adolescents and adults with congenital heart defects: Individualized exercise prescription. Eur. Heart J. 2013, 34, 3669–3674. [Google Scholar] [CrossRef] [PubMed]
- Pescatello, L.S.; Riebe, D.; Thompson, P.D. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014. [Google Scholar]
- American Thoracic Society. ATS/ACCP statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Health-Related Physical Fitness Assessment Manual; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Dekker, J.; Buurman, B.M.; van der Leeden, M. Exercise in people with comorbidity or multimorbidity. Health Psychol. 2019, 38, 822–830. [Google Scholar] [CrossRef]
- Bullard, T.; Ji, M.; An, R.; Trinh, L.; Mackenzie, M.; Mullen, S.P. A systematic review and meta-analysis of adherence to physical activity interventions among three chronic conditions: Cancer, cardiovascular disease, and diabetes. BMC Public Health 2019, 19, 636. [Google Scholar] [CrossRef]
- Miller, W.R.; Rollnick, S. Motivational Interviewing: Helping People Change; Guilford Press: New York, NY, USA, 2012. [Google Scholar]
- Louwagie, G.M.; Okuyemi, K.S.; Ayo-Yusuf, O.A. Efficacy of brief motivational interviewing on smoking cessation at tuberculosis clinics in Tshwane, South Africa: A randomized controlled trial. Addiction 2014, 109, 1942–1952. [Google Scholar] [CrossRef]
- Chen, S.M.; Creedy, D.; Lin, H.S.; Wollin, J. Effects of motivational interviewing intervention on self-management, psychological and glycemic outcomes in type 2 diabetes: A randomized controlled trial. Int. J. Nurs. Stud. 2012, 49, 637–644. [Google Scholar] [CrossRef]
- Bennett, J.A.; Lyons, K.S.; Winters-Stone, K.; Nail, L.M.; Scherer, J. Motivational interviewing to increase physical activity in long-term cancer survivors: A randomized controlled trial. Nurs. Res. 2007, 56, 18–27. [Google Scholar] [CrossRef]
- Mifsud, J.L.; Galea, J.; Garside, J.; Stephenson, J.; Astin, F. Motivational interviewing to support modifiable risk factor change in individuals at increased risk of cardiovascular disease: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0241193. [Google Scholar] [CrossRef]
- Lilienthal, K.R.; Pignol, A.E.; Holm, J.E.; Vogeltanz-Holm, N. Telephone-based motivational interviewing to promote physical activity and stage of change progression in older adults. J. Aging Phys. Act. 2014, 22, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chiang, S.L.; Heitkemper, M.M.; Hung, Y.J.; Lee, M.S.; Tzeng, W.C.; Chiang, L.C. Effects of telephone-based motivational interviewing in lifestyle modification program on reducing metabolic risks in middle-aged and older women with metabolic syndrome: A randomized controlled trial. Int. J. Nurs. Stud. 2016, 60, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Welfare. 2012 Taiwan Longitudinal Study on Aging Survey Report; Ministry of Health and Welfare, Taiwan (R.O.C): Taipei, Taiwan, 2014.
- Riebe, D.; Franklin, B.A.; Thompson, P.D.; Garber, C.E.; Whitfield, G.P.; Magal, M.; Pescatello, L.S. Updating ACSM’s recommendations for exercise preparticipation health screening. Med. Sci. Sports Exerc. 2015, 47, 2473–2479. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.; Durstine, J.L.; Painter, P.; American College of Sports Medicine. ACSM’s Exercise Management for Persons with Chronic Diseases and Disabilities, 4E; Human Kinetics: Indianapolis, IN, USA, 2016. [Google Scholar]
- Karvonen, M.J.; Kentala, E.; Mustala, O. The effects of training on heart rate; a longitudinal study. Ann. Med. Exp. Biol. Fenn. 1957, 35, 307–315. [Google Scholar]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982. [Google Scholar] [CrossRef]
- Huang, L.H. Research on the cause analysis of related factors of elderly life satisfaction. J. Nurs. 1992, 39, 34–37. [Google Scholar]
- Liou, Y.M.; Jwo, C.J.; Yao, K.G.; Chiang, L.C.; Huang, L.H. Selection of appropriate Chinese terms to represent intensity and types of physical activity terms for use in the Taiwan version of IPAQ. J. Nurs. Res. 2008, 16, 252–263. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Frey, B.B. The SAGE Encyclopedia of Educational Research, Measurement, and Evaluation; Sage Publications: Thousand Oaks, CA, USA, 2018. [Google Scholar]
- Sousa, N.; Mendes, R.; Abrantes, C.; Sampaio, J.; Oliveira, J. Effectiveness of combined exercise training to improve functional fitness in older adults: A randomized controlled trial. Geriatr. Gerontol. Int. 2014, 14, 892–898. [Google Scholar] [CrossRef]
- Chan, Y. Biostatistics 301A. Repeated measurement analysis. Singap. Med. J. 2004, 45, 457. [Google Scholar]
- Weinstein, A.A.; Chin, L.M.; Keyser, R.E.; Kennedy, M.; Nathan, S.D.; Woolstenhulme, J.G.; Connors, G.; Chan, L. Effect of aerobic exercise training on fatigue and physical activity in patients with pulmonary arterial hypertension. Respir. Med. 2013, 107, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Kitzman, D.W.; Brubaker, P.; Morgan, T.; Haykowsky, M.; Hundley, G.; Kraus, W.E.; Eggebeen, J.; Nicklas, B.J. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: A randomized clinical trial. JAMA 2016, 315, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, L.F.; Chen, S.C.; Chuang, C.C.; Chai, H.M.; Chen, W.S.; He, Y.C. Supervised aerobic exercise is more effective than home aerobic exercise in female chinese patients with rheumatoid arthritis. J. Rehabil. Med. 2009, 41, 332–337. [Google Scholar] [CrossRef]
- Batterham, A.M.; Bonner, S.; Wright, J.; Howell, S.J.; Hugill, K.; Danjoux, G. Effect of supervised aerobic exercise rehabilitation on physical fitness and quality of life in survivors of critical illness: An exploratory minimized controlled trial (PIX study). Br. J. Anaesth. 2014, 113, 130–137. [Google Scholar] [CrossRef]
- Bouaziz, W.; Schmitt, E.; Kaltenbach, G.; Geny, B.; Vogel, T. Health benefits of cycle ergometer training for older adults over 70: A review. Eur. Rev. Aging Phys. Act. 2015, 12, 8. [Google Scholar] [CrossRef]
- O′Halloran, P.D.; Blackstock, F.; Shields, N.; Holland, A.; Iles, R.; Kingsley, M.; Bernhardt, J.; Lannin, N.; Morris, M.E.; Taylor, N.F. Motivational interviewing to increase physical activity in people with chronic health conditions: A systematic review and meta-analysis. Clin. Rehabil. 2014, 28, 1159–1171. [Google Scholar] [CrossRef]
- Bock, B.C.; Carmona-Barros, R.E.; Esler, J.L.; Tilkemeier, P.L. Program participation and physical activity maintenance after cardiac rehabilitation. Behav. Modif. 2003, 27, 37–53. [Google Scholar] [CrossRef]
- Boesch, C.; Myers, J.; Habersaat, A.; Ilarraza, H.; Kottman, W.; Dubach, P. Maintenance of exercise capacity and physical activity patterns 2 years after cardiac rehabilitation. J. Cardiopulm. Rehabil. 2005, 25, 14–21. [Google Scholar] [CrossRef][Green Version]
- Sawyer, B.J.; Bhammar, D.M.; Angadi, S.S.; Ryan, D.M.; Ryder, J.R.; Sussman, E.J.; Bertmann, F.M.; Gaesser, G.A. Predictors of fat mass changes in response to aerobic exercise training in women. J. Strength Cond. Res. 2015, 29, 297–304. [Google Scholar] [CrossRef]
- Sylvia, L.G.; Bernstein, E.E.; Hubbard, J.L.; Keating, L.; Anderson, E.J. Practical guide to measuring physical activity. J. Acad. Nutr. Diet. 2014, 114, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Strath, S.J.; Kaminsky, L.A.; Ainsworth, B.E.; Ekelund, U.; Freedson, P.S.; Gary, R.A.; Richardson, C.R.; Smith, D.T.; Swartz, A.M. Guide to the assessment of physical activity: Clinical and research applications: A scientific statement from the American Heart Association. Circulation 2013, 128, 2259–2279. [Google Scholar] [CrossRef] [PubMed]
| Multidisciplinary Individualized Aerobic Exercise Training in the Rehabilitation Center (30–50 Min/Session) | |
|---|---|
| Rehabilitation/sports medicine physician |
1. Collect and evaluate the participants’ medical history and lifestyle 2. Discuss with participants and then design the individualized exercise prescription |
| Physiotherapist |
1. Coach the cycle ergometer aerobic training based on the FITT-VP principles 2. Assess the exercise intensity and fitness level of the participants |
| Trained nurse |
1. Supervise the cycle ergometer aerobic training 2. Introduce the Borg scale 3. Conduct a face-to-face motivational interviewing to set the goals for the next week’s training, motivate the participant to reach WHO physical activity recommendations and ask them how they felt about the training |
| Telephone-based motivational interviewing (15–30 min, once a week) | |
| Four processes | Core content |
|
1. Engaging 2. Focusing 3. Evoking 4. Planning |
1. Determining the physical activity and exercise stage of the participants 2. Providing essential information on exercise benefit 3. Giving personal feedback or encouraging the participants to engage in exercise 4. Setting reasonable and weekly exercise goals for the participants 5. Monitoring the participants’ exercise behavior 6. Reminding the participants to reach the physical activity recommendation 7. Providing rewards for achieving the goals |
| Characteristics | Intervention | Comparison | Control | F/x2 | p |
|---|---|---|---|---|---|
| (n = 15) | (n = 14) | (n = 14) | |||
| Age | 0.15 | 0.927 | |||
| 40–64 (years) | 6 (40.0) | 6 (42.9) | 5 (35.7) | ||
| ≥65 (years) | 9 (60.0) | 8 (57.1) | 9 (64.3) | ||
| Gender | 0.04 | 0.979 | |||
| Male | 7 (46.7) | 7 (50.0) | 7 (50.0) | ||
| Female | 8 (53.3) | 7 (50.0) | 7 (50.0) | ||
| Marital status | 2.47 | 0.650 | |||
| Married | 11 (73.3) | 12 (85.7) | 10 (71.4) | ||
| Single | 1 (6.7) | 0 (0) | 2 (14.3) | ||
| Divorced/widowed | 3 (20.0) | 2 (14.3) | 2 (14.3) | ||
| Have children | 0.61 | 0.736 | |||
| Yes | 14 (93.3) | 13 (92.9) | 12 (85.7) | ||
| No | 1 (6.7) | 1 (7.1) | 2 (14.3) | ||
| Educational level | 8.51 | 0.385 | |||
| Elementary | 1 (6.7) | 2 (14.3) | 3 (21.4) | ||
| Junior high school | 0 (0) | 3 (21.4) | 2 (14.3) | ||
| Senior high school | 9 (60.0) | 4 (28.6) | 3 (21.4) | ||
| College/university | 4 (26.7) | 3 (21.4) | 5 (35.7) | ||
| Graduated | 1 (6.7) | 2 (14.3) | 1 (7.1) | ||
| Currently employed | 3.55 | 0.169 | |||
| Yes | 7 (46.7) | 2 (14.3) | 5 (35.7) | ||
| No | 8 (53.3) | 12 (85.7) | 9 (64.3) | ||
| Number of chronic diseases | 3.7 ± 1.2 | 3.6 ± 0.9 | 3.8 ± 1.1 | 0.15 | 0.861 |
| Self-perceived health status | 15.5 ± 2.2 | 14.6 ± 3.0 | 15.3 ± 2.6 | 0.44 | 0.650 |
| Physical activity amount | |||||
| PA amount (MET-min/week) | |||||
| Total PA | 1132.1 ± 681.1 | 1321.3 ± 873.2 | 989.1 ± 522.5 | 0.78 | 0.465 |
| Vigorous PA | 64.0 ± 179.4 | 34.3 ± 128.3 | 45.7 ± 132.1 | 0.15 | 0.864 |
| Moderate PA | 333.3 ± 421.3 | 545.7 ± 661.9 | 238.6 ± 265.2 | 1.53 | 0.230 |
| Walking PA | 734.8 ± 502.4 | 741.3 ± 455.2 | 704.8 ± 229.9 | 0.03 | 0.970 |
| Sedentary time (min/day) | 350.7 ± 111.3 | 360.0 ± 111.0 | 360.0 ± 93.4 | 0.03 | 0.968 |
| Health-related physical fitness | |||||
| Body composition | |||||
| Weight (kg) | 65.7 ± 11.2 | 71.2 ± 17.8 | 68.5 ± 8.9 | 0.66 | 0.525 |
| BMI (kg/m2) | 24.4 ± 2.9 | 26.2 ± 4.7 | 25.6 ± 3.4 | 0.87 | 0.429 |
| Muscular strength | |||||
| Dominant hand grip (kg) | 28.3 ± 8.7 | 27.4 ± 8.8 | 27.6 ± 8.0 | 0.04 | 0.957 |
| Nondominant hand grip (kg) | 23.8 ± 8.5 | 24.8 ± 7.9 | 25.3 ± 7.5 | 0.13 | 0.878 |
| Muscular endurance | |||||
| 30 s sit-to-stand test (no.) | 13.3 ± 4.5 | 14.5 ± 2.7 | 12.9 ± 1.4 | 0.92 | 0.408 |
| Flexibility | |||||
| Chair sit-and-reach test (cm) | −10.0 ± 9.3 | −6.0 ± 11.8 | −7.6 ± 12.2 | 0.47 | 0.626 |
| Cardiorespiratory fitness | |||||
| FVC (L) | 3.3 ± 0.7 | 3.4 ± 0.9 | 3.5 ± 1.2 | 0.19 | 0.831 |
| FEV1 (L) | 2.6 ± 0.6 | 2.7 ± 0.7 | 2.8 ± 0.9 | 0.31 | 0.732 |
| FEV1/FVC | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.17 | 0.847 |
| VO2 max (ml/kg/min) | 22.0 ± 5.4 | 23.3 ± 5.9 | 24.7 ± 6.0 | 0.79 | 0.459 |
| VO2 max predicted (%) | 88.0 ± 22.0 | 95.9 ± 21.1 | 104.0 ± 25.2 | 1.78 | 0.182 |
| Work (watts) | 73.5 ± 17.9 | 90.6 ± 35.7 | 89.1 ± 24.7 | 1.82 | 0.175 |
| AT (L/min) | 0.8 ± 0.2 | 0.8 ± 0.3 | 0.8 ± 0.2 | 0.27 | 0.767 |
| Physical Activity Amount | Intervention | Comparison | Control | Between-Groups | Within-Times | Group (Intervention) | Group (Intervention) | Group (Comparison) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 15) | (n = 14) | (n = 14) | Fb (p) a | Fw (p) b | ×Time, Fin c | ×Time, Fin c | ×Time, Fin c | ||||||||||
| Mean (SD) | Mean (SD) | Mean (SD) | F | SE | pd | F | SE | pe | F | SE | pd | ||||||
| Total PA | 143.1 (0.530) | 63.7 (0.625) | 481.3 | 189.1 | 0.011 * | −55.4 | 305.7 | 0.858 | 553.5 | 294.3 | 0.077 | ||||||
| Baseline | 1132 (681.1) | 1321 (873.2) | 989 (522.5) | ||||||||||||||
| 12 weeks | 1692 (624.1) | 1919 (803.5) | 1068 (780.5) | ||||||||||||||
| Vigorous-intensity PA | 18.3 (0.755) | 5.4 (0.947) | 298.9 | 90.9 | 0.007 * | 140.5 | 123.6 | 0.268 | 162.1 | 83.8 | 0.079 | ||||||
| Baseline | 64 (179.4) | 34 (128.3) | 46 (132.1) | ||||||||||||||
| 12 weeks | 340 (329.8) | 198 (300.3) | 51 (160.0) | ||||||||||||||
| Moderate-intensity PA | 94.8 (0.573) | 202.5 (0.187) | −66.0 | 171.8 | 0.707 | 51.3 | 187.4 | 0.787 | −117.3 | 230.0 | 0.615 | ||||||
| Baseline | 333 (421.3) | 546 (661.9) | 239 (265.2) | ||||||||||||||
| 12 weeks | 447 (261.7) | 598 (425.2) | 436 (378.2) | ||||||||||||||
| Walking PA | 30.0 (0.877) | −117.6 (0.488) | 287.4 | 194.4 | 0.161 | −218.4 | 253.6 | 0.398 | 505.7 | 189.5 | 0.018 * | ||||||
| Baseline | 735 (502.4) | 741 (455.2) | 705 (229.9) | ||||||||||||||
| 12 weeks | 897 (665.1) | 1128 (765.2) | 586 (378.2) | ||||||||||||||
| Sedentary time(min/day) | −9.3 (0.824) | −12.7 (0.693) | −52.5 | 37.2 | 0.179 | 40.4 | 46.1 | 0.391 | −92.9 | 37.7 | 0.025 * | ||||||
| Baseline | 351 (111.3) | 360 (111.0) | 360 (93.4) | ||||||||||||||
| 12 weeks | 290 (76.2) | 243 (101.0) | 348 (108.8) | ||||||||||||||
| Health-Related Physical Fitness | Intervention | Comparison | Control | Between-Groups | Within-Times | Group (Intervention) | Group (Intervention) | Group (Comparison) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 15) | (n = 14) | (n = 14) | Fb (p) a | Fw (p) b | ×Time, Fin c | ×Time, Fin c | ×Time, Fin c | ||||||||||
| Mean (SD) | Mean (SD) | Mean (SD) | F | SE | pd | F | SE | pe | F | SE | pd | ||||||
| Weight (kg) | −2.9 (0.563) | 0.3 (0.660) | 0.576 | 0.896 | 0.529 | 1.91 | 0.808 | 0.028 * | −1.33 | 0.801 | 0.115 | ||||||
| Baseline | 65.7 (11.2) | 71.2 (17.8) | 68.5 (8.9) | ||||||||||||||
| 12 weeks | 66.5 (10.9) | 70.3 (19.2) | 68.8 (6.6) | ||||||||||||||
| Body mass index (kg/m2) | −1.2 (0.403) | 0.1 (0.644) | 0.108 | 0.331 | 0.748 | 0.685 | 0.295 | 0.030 * | −0.578 | 0.294 | 0.069 | ||||||
| Baseline | 24.4 (2.9) | 26.2 (4.7) | 25.6 (3.4) | ||||||||||||||
| 12 weeks | 24.7 (3.4) | 25.8 (5.1) | 25.7 (2.4) | ||||||||||||||
| Dominant hand grip (kg) | 0.6 (0.847) | −1.0 (0.238) | 1.96 | 0.770 | 0.019 * | 1.13 | 1.18 | 0.960 | 0.834 | 1.18 | 0.489 | ||||||
| Baseline | 28.3 (8.7) | 27.4 (8.8) | 27.6 (8.0) | ||||||||||||||
| 12 weeks | 29.2 (8.2) | 27.2 (10.1) | 26.5 (7.9) | ||||||||||||||
| Nondominant hand grip (kg) | −1.5 (0.620) | −1.7 (0.109) | 2.19 | 0.910 | 0.027 * | 1.42 | 1.37 | 0.316 | 0.775 | 1.35 | 0.575 | ||||||
| Baseline | 23.8 (8.5) | 24.8 (7.9) | 25.3 (7.5) | ||||||||||||||
| 12 weeks | 24.3 (7.3) | 23.7 (8.2) | 23.5 (8.2) | ||||||||||||||
| 30 s sit-to-stand test (no.) | 0.4 (0.726) | −0.6 (0.474) | 1.02 | 0.589 | 0.101 | 0.04 | 1.073 | 0.970 | 0.979 | 0.931 | 0.314 | ||||||
| Baseline | 13.3 (4.5) | 14.5 (2.7) | 12.9 (1.4) | ||||||||||||||
| 12 weeks | 14.0 (3.2) | 15.0 (3.8) | 12.5 (1.6) | ||||||||||||||
| Cardiorespiratory Fitness | Intervention | Comparison | Control | Between-Groups | Within-Times | Group (Intervention) | Group (Intervention) | Group (Comparison) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 15) | (n = 14) | (n = 14) | Fb (p) a | Fw (p) b | × Time, Fin c | × Time, Fin c | × Time, Fin c | ||||||||||
| Mean (SD) | Mean (SD) | Mean (SD) | F | SE | pd | F | SE | pe | F | SE | pd | ||||||
| FVC (L) | −0.2 (0.540) | 0.4 (0.011) * | −0.061 | 0.163 | 0.712 | 0.048 | 0.176 | 0.788 | −0.109 | 0.168 | 0.524 | ||||||
| Baseline | 3.3 (0.7) | 3.4 (0.9) | 3.5 (1.2) | ||||||||||||||
| 12 weeks | 3.7 (0.5) | 3.6 (0.9) | 3.8 (1.3) | ||||||||||||||
| FEV1(L) | −0.2 (0.431) | 0.1 (0.280) | 0.079 | 0.132 | 0.562 | −0.048 | 0.171 | 0.782 | 0.126 | 0.151 | 0.415 | ||||||
| Baseline | 2.6 (0.6) | 2.7 (0.7) | 2.8 (0.9) | ||||||||||||||
| 12 weeks | 2.9 (0.4) | 3.0 (0.8) | 3.0 (1.0) | ||||||||||||||
| FEV1/FVC | −0.02 (0.505) | −0.05 (0.044) * | 0.045 | 0.021 | 0.043 * | −0.010 | 0.031 | 0.746 | 0.055 | 0.030 | 0.075 | ||||||
| Baseline | 0.8 (0.07) | 0.8 (0.09) | 0.8 (0.06) | ||||||||||||||
| 12 weeks | 0.8 (0.1) | 0.8 (0.04) | 0.8 (0.03) | ||||||||||||||
| VO2max (ml/kg/min) | −2.7 (0.232) | −1.8 (0.140) | 5.30 | 1.26 | 0.001 * | 2.59 | 1.67 | 0.134 | 2.72 | 1.38 | 0.066 | ||||||
| Baseline | 22.0 (5.4) | 23.3 (5.9) | 24.7 (6.0) | ||||||||||||||
| 12 weeks | 25.0 (4.8) | 24.2 (7.9) | 22.7 (6.5) | ||||||||||||||
| VO2 max predicted (%) | −16.0 (0.075) | −6.7 (0.199) | 21.6 | 5.76 | 0.002 * | 11.3 | 7.17 | 0.129 | 10.3 | 5.53 | 0.081 | ||||||
| Baseline | 88.0 (22.0) | 95.9 (21.1) | 104.0 (25.2) | ||||||||||||||
| 12 weeks | 100.8 (22.6) | 96.0 (26.6) | 96.4 (28.6) | ||||||||||||||
| Work (watts) | −15.7 (0.137) | −1.7 (0.766) | 22.5 | 5.61 | 0.001 * | 21.7 | 7.88 | 0.012 * | 0.845 | 7.32 | 0.910 | ||||||
| Baseline | 73.5 (17.9) | 90.6 (35.7) | 89.1 (24.7) | ||||||||||||||
| 12 weeks | 94.7 (21.7) | 89.3 (36.3) | 87.7 (29.0) | ||||||||||||||
| AT (L/min) | −0.06 (0.496) | 0.03 (0.517) | 0.165 | 0.059 | 0.011 * | 0.175 | 0.052 | 0.004 * | −0.010 | 0.045 | 0.828 | ||||||
| Baseline | 0.77 (0.2) | 0.81 (0.3) | 0.83 (0.2) | ||||||||||||||
| 12 weeks | 0.96 (0.2) | 0.88 (0.3) | 0.88 (0.2) | ||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, Y.-P.; Chiang, S.-L.; Lin, C.-H.; Liu, H.-C.; Chiang, L.-C. Effects of Individualized Aerobic Exercise Training on Physical Activity and Health-Related Physical Fitness among Middle-Aged and Older Adults with Multimorbidity: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 101. https://doi.org/10.3390/ijerph18010101
Lo Y-P, Chiang S-L, Lin C-H, Liu H-C, Chiang L-C. Effects of Individualized Aerobic Exercise Training on Physical Activity and Health-Related Physical Fitness among Middle-Aged and Older Adults with Multimorbidity: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2021; 18(1):101. https://doi.org/10.3390/ijerph18010101
Chicago/Turabian StyleLo, Yi-Pang, Shang-Lin Chiang, Chia-Huei Lin, Hung-Chang Liu, and Li-Chi Chiang. 2021. "Effects of Individualized Aerobic Exercise Training on Physical Activity and Health-Related Physical Fitness among Middle-Aged and Older Adults with Multimorbidity: A Randomized Controlled Trial" International Journal of Environmental Research and Public Health 18, no. 1: 101. https://doi.org/10.3390/ijerph18010101
APA StyleLo, Y.-P., Chiang, S.-L., Lin, C.-H., Liu, H.-C., & Chiang, L.-C. (2021). Effects of Individualized Aerobic Exercise Training on Physical Activity and Health-Related Physical Fitness among Middle-Aged and Older Adults with Multimorbidity: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 18(1), 101. https://doi.org/10.3390/ijerph18010101






