Biological Monitoring of Metal Ions Released from Hip Prostheses
Abstract
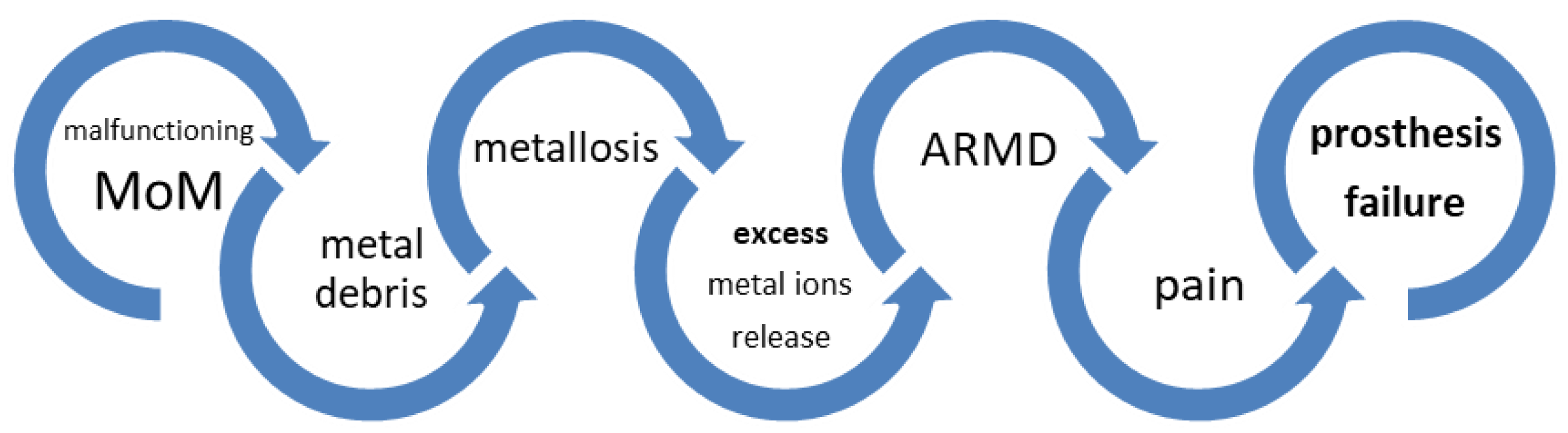
1. Introduction
2. Material and Methods
2.1. Patients
2.2. Ethics Statement
2.3. Urine Sampling and Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liow, M.H.L.; Kwon, Y.M. Metal-on-metal total hip arthroplasty: Risk factors for pseudotumours and clinical systematic evaluation. Int. Orthop. 2017, 41, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Paukkeri, E.L.; Korhonen, R.; Hämäläinen, M.; Pesu, M.; Eskelinen, A.; Moilanen, T.; Moilanen, E. The Inflammatory Phenotype in Failed Metal-On-Metal Hip Arthroplasty Correlates with Blood Metal Concentrations. PLoS ONE 2016, 11, e0155121. [Google Scholar] [CrossRef] [PubMed]
- Charette, R.S.; Neuwirth, A.L.; Nelson, C.L. Arthroprosthetic cobaltism associated with cardiomyopathy. Arthroplast. Today 2017, 3, 225–228. [Google Scholar] [CrossRef]
- Shapiro, J.A.; Eskildsen, S.M.; Gaizo, D.J.D. Systemic cobaltism manifesting as oral mucosal discoloration and metallic gustation after metal-on-metal hip resurfacing. Arthroplast. Today 2018, 4, 436–440. [Google Scholar] [CrossRef]
- Swiatkowska, I.; Martin, N.G.; Henckel, J.; Apthorp, H.; Hamshere, J.; Hart, A.J. Blood and plasma titanium levels associated with well-functioning hip implants. J. Trace Elem. Med. Biol. 2020, 57, 9–17. [Google Scholar] [CrossRef]
- MHRA (Medicines and healthcare Products Regulatory Agency). Medical Device Alert All metal-on-metal (MoM) hip replacement. MHRA MDA/2012/008 (Issued 28 February 2012).
- Lodge, F.; Khatun, R.; Lord, R.; John, A.; Fraser, A.G.; Yousef, Z. Prevalence of subclinical cardiac abnormalities in patients with metal-on-metal hip replacements. Int. J. Cardiol. 2018, 271, 274–280. [Google Scholar] [CrossRef]
- Sangaletti, R.; Spreafico, A.; Barbieri, F.; Ferrari, R.; Castelli, C.C. Metal ion trend in patients with metal-on-metal total hip arthroplasty: A 10-year prospective study. HIP Int. 2018, 28, 43–47. [Google Scholar] [CrossRef]
- Chen, L.; Xing, F.; Wang, Y.; He, R.; He, J.; Xu, Y.; Wang, C.; Li, Z.; Sun, Z.; Li, Y.; et al. Outcome analysis of various bearing surface materials used in total hip replacement. Mater. Express 2020, 10, 301–313. [Google Scholar] [CrossRef]
- Sarmiento-González, A.; Merchante-Gayón, J.M.; Tejerina-Lobo, J.M.; Paz-Jeménez, J.; Sanz-Mendel, A. High-resolution ICP–MS determination of Ti, V, Cr, Co, Ni, and Mo in human blood and urine of patients implanted with a hip or knee prosthesis. Anal. Bioanal. Chem. 2008, 391, 2583–2589. [Google Scholar] [CrossRef]
- Savarino, L.; Greco, M.; Cenni, E.; Cavasinni, L.; Rotini, R.; Baldini, N.; Giunti, A. Differences in ion release after ceramic-on-ceramic and metal-on-metal total hip replacement. J. Bone Joint Surg Br. 2006, 88, 472–476. [Google Scholar] [CrossRef]
- Aprea, M.C.; Apostoli, P.; Bettinelli, M.; Lovreglio, P.; Negri, S.; Perbellini, L.; Perico, A.; Ricossa, M.C.; Salamon, F.; Scapellato, M.L.; et al. Urinary levels of metal elements in the non-smoking general population in Italy: SIVR study 2012-2015. Toxicol. Lett. 2018, 298, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Nicolli, A.; Bisinella, G.; Padovani, G.; Vitella, A.; Chiara, F.; Trevisan, A. Predictivity and fate of metal ion release from metal-on-metal total hip prostheses. J. Arthroplasty 2014, 29, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.J.; Skipor, A.K.; Patterson, L.M.; Hallab, N.J.; Paprosky, W.G.; Galante, J.O. Metal release in patients who have had a primary total hip arthroplasty. A prospective, controlled, longitudinal study. J. Bone Joint Surg. Am. 1998, 80, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.J.; Skipor, A.K.; Doorn, P.F.; Campbell, P.; Schmalzried, T.P.; Black, J.; Amstutz, H.C. Cobalt and chromium concentrations in patients with metal on metal total hip replacements. Clin. Orthopaed. Related Res. 1996, 329, S256–S263. [Google Scholar] [CrossRef]
- Brodner, W.; Bitzan, P.; Meisinger, V.; Kaider, A.; Gottsauner-Wolf, F.; Kptz, R. Elevated serum cobalt with metal-on-metal articulating surfaces. J. Bone Joint Surg. Br. 1997, 79, 316–321. [Google Scholar] [CrossRef]
- Jacobs, J.J.; Skipor, A.K.; Black, G.; Urban, R.M.; Galante, J. Release and excretion of metal in patients who have a total hip-replacement component made of titanium-base alloy. J. Bone Joint Surg. Am. 1991, 73, 1475–1486. [Google Scholar] [CrossRef]
- Campbell, J.R.; Estey, M.P. Metal release from hip prostheses: Cobalt and chromium toxicity and the role of the clinical laboratory. Clin. Chem. Lab. Med. 2013, 51, 213–220. [Google Scholar] [CrossRef]
- Estey, M.P.; Diamandis, E.P.; Van Der Straeten, C.; Tower, S.S.; Hart, A.J.; Moyer, T.P. Cobalt and Chromium Measurement in Patients with Metal Hip Prostheses. Clin. Chem. 2013, 59, 880–886. [Google Scholar] [CrossRef]
- De Smet, K.; De Haan, R.; Calistri, A.; Campbell, P.A.; Ebramzadeh, E.; Pattyn, C.; Gill, H.S. Metal ion measurement as a diagnostic tool to identify problems with metal-on-metal hip resurfacing. J. Bone Joint Surg. Am. 2008, 90, 202–208. [Google Scholar] [CrossRef]
- Finley, B.L.; Monnot, A.D.; Gaffney, S.H.; Paustenbach, D.J. Dose-response relationships for blood cobalt concentrations and health effects: A review of the literature and application of a biokinetic model. J. Toxicol. Environ. Health B Crit. Rev. 2012, 15, 493–523. [Google Scholar] [CrossRef]
- Trevisan, A. Concentration adjustment of spot samples in analysis of urinary xenobiotic metabolites. Am. J. Ind. Med. 1990, 17, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, A.; Nicoletto, G.; Maso, S.; Grandesso, G.; Odynets, A.; Secondin, L. Biological monitoring of cadmium exposure: Reliability of spot urine samples. Int. Arch. Occup. Environ. Health 1994, 65, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Carrieri, M.; Trevisan, A.; Bartolucci, G.B. Adjustment to concentration-dilution of spot urine samples: Correlation between specific gravity and creatinine. Int. Arch. Occup. Environ. Health 2001, 74, 63–67. [Google Scholar] [CrossRef]
- Dumbleton, J.H.; Manley, M.T. Metal on-metal total hip replacement: What does the literature say? J. Arthroplasty 2005, 20, 174–188. [Google Scholar] [CrossRef]
| Variable. as Mean (Range) | Group A | Group B | ||||
|---|---|---|---|---|---|---|
| Total (n = 25) | Males (n = 19) | Females (n = 6) | Total (n = 28) | Males (n = 11) | Females (n = 17) | |
| Age at surgery (years) | 56.9 (30–74) | 57.3 (38–68) | 55.8 (30–74) | 64.7 (19–82) | 64.0 (19–79) | 65.2 (33–82) |
| Age at metal ion measurement (years) | 66.4 (44–84) | 66.6 (48–76) | 65.8 (44–84) | 71.6 (25–89) | 70.3 (25–86) | 72.3 (40–89) |
| Interval between surgery and metal ion measurement (years) | 8.9 (6–13) | 8.7 (6–11) | 9.3 (6–13) | 6.4 (2–11) | 6.3 (4–10) | 6.5 (2–11) |
| Metal | Group A | Group B | SIVR Values | ||||
|---|---|---|---|---|---|---|---|
| Mean ± sd | Median | 5°–95° | Mean ± sd | Median | 5°–95° | 5°–95° | |
| As | 37.0 ± 42.6 | 22.3 | 4.62–113.1 | 42.1 ± 80.9 | 11.9 | 1.4–242.3 | nd–16.1 |
| Be | 0.023 ± 0.012 | 0.022 | 0.011–0.041 | 0.018 ± 0.013 | 0.012 | 0.006–0.042 | <0.01–0.034 |
| Bi | 0.02 ± 0.02 | 0.01 | 0.002–0.034 | 0.02 ± 0.02 | 0.01 | 0.002–0.051 | nd–0.03 |
| Cd | 0.44 ± 0.29 | 0.39 | 0.12–1.05 | 0.56 ± 0.55 | 0.40 | 0.13–1.53 | 0.1–0.9 |
| Co | 55.7 ± 41.5 | 60.8 | 5.01–108.5 | 20.6 ± 23.5 | 11.5 | 1.57–67.00 | 0.077–2.2 |
| Cr | 17.7 ± 11.8 | 15.8 | 5.4–42.8 | 9.9 ± 8.9 | 7.4 | 0.8–23.0 | 0.05–0.6 |
| Cu | 13.3 ± 5.2 | 10.4 | 8.7–22.5 | 9.6 ± 6.8 | 7.5 | 2.5–22.0 | 5.01–24 |
| Hg | 1.10 ± 0.93 | 0.92 | 0.13–2.88 | 0.48 ± 0.66 | 0.26 | 0.03–1.49 | 0.1–5 |
| Mn | 0.808 ± 0.316 | 0.792 | 0.39–1.41 | 0.273 ± 0.244 | 0.209 | 0.04–0.60 | 0.04–1.5 |
| Ni | 2.67 ± 0.89 | 2.84 | 1.24–3.80 | 1.41 ± 1.01 | 1.31 | 0.32–3.33 | 0.372–4.44 |
| Pb | 2.07 ± 0.90 | 2.15 | 0.98–3.74 | 1.20 ± 1.47 | 0.54 | 0.05–2.74 | 0.17–2.64 |
| Se | 35.4 ± 14.6 | 35.5 | 14.2–62.7 | 26.7 ± 22.7 | 18.9 | 6.5–78.6 | nd–61.6 |
| Tl | 0.279 ± 0.310 | 0.215 | 0.12–0.42 | 0.186 ± 0.142 | 0.161 | 0.03–0.40 | 0.06–0.759 |
| V | 0.643 ± 0.507 | 0.527 | 0.30–1.13 | 0.443 ± 0.290 | 0.361 | 0.18–1.10 | 0.025–0.855 |
| Zn | 953 ± 438 | 825 | 408–1720 | 632 ± 621 | 343 | 44–1836 | nd–1048 |
| % Co Levels > 30 µg/g Creatinine | % Cr Levels > 21 µg/g Creatinine | % Co > 30 µg/g Creatinine and Cr Levels > 21 µg/g Creatinine (both) | |
|---|---|---|---|
| Group A | 72 | 32 | 28 |
| Group B | 39 | 36 | 32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolli, A.; Trevisan, A.; Bortoletti, I.; Pozzuoli, A.; Ruggieri, P.; Martinelli, A.; Gambalunga, A.; Carrieri, M. Biological Monitoring of Metal Ions Released from Hip Prostheses. Int. J. Environ. Res. Public Health 2020, 17, 3223. https://doi.org/10.3390/ijerph17093223
Nicolli A, Trevisan A, Bortoletti I, Pozzuoli A, Ruggieri P, Martinelli A, Gambalunga A, Carrieri M. Biological Monitoring of Metal Ions Released from Hip Prostheses. International Journal of Environmental Research and Public Health. 2020; 17(9):3223. https://doi.org/10.3390/ijerph17093223
Chicago/Turabian StyleNicolli, Annamaria, Andrea Trevisan, Isabella Bortoletti, Assunta Pozzuoli, Pietro Ruggieri, Andrea Martinelli, Alberto Gambalunga, and Mariella Carrieri. 2020. "Biological Monitoring of Metal Ions Released from Hip Prostheses" International Journal of Environmental Research and Public Health 17, no. 9: 3223. https://doi.org/10.3390/ijerph17093223
APA StyleNicolli, A., Trevisan, A., Bortoletti, I., Pozzuoli, A., Ruggieri, P., Martinelli, A., Gambalunga, A., & Carrieri, M. (2020). Biological Monitoring of Metal Ions Released from Hip Prostheses. International Journal of Environmental Research and Public Health, 17(9), 3223. https://doi.org/10.3390/ijerph17093223








