Abstract
Lead smelting slag (LSS) has been identified as general industrial solid waste, which is produced from the pyrometallurgical treatment of the Shuikoushan process for primary lead production in China. The LSS-based geopolymer was synthesized after high-energy ball milling. The effect of unconfined compressive strength (UCS) on the synthesis parameters of the geopolymer was optimized. Under the best parameters of the geopolymer (modulus of water glass was 1–1.5, dosage of water glass (W(SiO2+Na2O)) was 5% and water-to-binder ratio was 0.2), the UCS reached 76.09 MPa after curing for 28 days. The toxicity characteristic leaching procedure (TCLP) leaching concentration of Zn from LSS fell from 167.16 to 93.99 mg/L after alkali-activation, which was below the limit allowed. Meanwhile, C-S-H and the geopolymer of the hydration products were identified from the geopolymer. In addition, the behavior of iron was also discussed. Then, the hydration process characteristics of the LSS-based geopolymer were proposed. The obtained results showed that Ca2+ and Fe2+ occupied the site of the network as modifiers in the glass phase and then dissociated from the glass network after the water glass activation. At the same time, C-S-H, the geopolymer and Fe(OH)2 gel were produced, and then the Fe(OH)2 was easily oxidized to Fe(OH)3 under the air curing conditions. Consequently, the conclusion was drawn that LSS was an implementable raw material for geopolymer production.
1. Introduction
China has been the largest producer and consumer of lead in the world for years [1,2]. At present, primary lead production in China is performed via traditional sintering blast furnace smelting, the Shuikoushan process, the Isa smelt system, the Kaldo converter lead smelting process and the Queneau-schuhmann-lurgi lead smelting process [3,4]. Among them, the Shuikoushan process is the main primary lead smelting technology in China, due to its advantages of being energy saving and having a higher metal recovery and longer furnace life [1,5]. In lead smelting, the molten slag is then commonly treated by water quenching to obtain lead smelting slag (LSS), which contains a high content of iron, silicon, calcium and aluminum oxides [6]. For each ton of metallic lead production, 100–350 kg of LSS is generated [7,8]. As a result, a huge amount of LSS is generated from primary lead production. The LSS contains quantities of minor and trace heavy metals [9,10]. It can contaminate the environment through leaching if it is not constrained [11,12,13,14]. In consequence, how to deal with LSS is a serious problem for lead smelters.
Traditionally, LSS was either recycled back into the smelting process or disposed of in piles on site [15]. In recent years, LSS has been used as an aggregate in concrete production [16] and also used in value-added streams, such as in cement clinker production and heavy clay production [17,18,19,20]. Additionally, the immobilization of LSS with a coal fly ash-based geopolymer has been investigated in some recent studies [21,22]. However, the consumption of LSS is limited in these treatments. Therefore, a method that can increase the value-added of IRLSS as well as deal with it in large capacity needs to be developed.
Unlike other applications, the production of a geopolymer primarily based on LSS is possible. The geopolymer has emerged as an alternative to ordinary Portland cement owing to its superior durability and environmental performance [23]. Because of these advantages, the geopolymer has found a variety of applications, such as transportation, industrial, agricultural, residential and mining [24,25,26]. Besides these, one of its major newer applications is in waste management, especially in the immobilization of toxic metals, such as Zn, Cu, Cd, Cr and Pb [27,28,29]. A geopolymer is defined as a synthetic alumino–silicate material and is generated from the reaction of solid alumino-silicate with a highly concentrated aqueous alkali hydroxide or silicate solution [17]. A number of materials have been investigated as candidates for geopolymer production, such as blast furnace slag [30], metakaolin [31], fly ash [32], kaolinitic clays [33], municipal solid waste incineration fly/bottom ashes [34,35] and red mud [36]. However, to our knowledge, there are few correlated studies concerning the geopolymer based on LSS.
Geopolymers are inorganic polymers and are constituted of alternating SiO4 and AlO4 tetrahedra chains connected by a shared oxygen atom and balanced by cations [37]. The presence of iron can substitute for Al and may play important roles in the structure and properties of geopolymers [38,39]. In our previous study, LSS was pretreated as a geopolymer precursor through the high-energy ball milling activation process. It could be used as a geopolymeric solidification/stabilization (S/S) reagent for municipal solid waste incineration fly ash (MSWI FA) [40]. However, to our knowledge, LSS contains a great amount of iron and the behavior of iron in the LSS based geopolymer has not yet been studied. It is necessary to explore the possibility of LSS as a high-performance alkali-activated slag-based cement.
In the present work, the LSS was mixed with water glass for a geopolymer production with a large consumption of LSS. The unconfined compressive strength (UCS) was optimized and the toxicity characteristic leaching procedure (TCLP) was performed. The hydration products were analyzed with x-ray diffractometry (XRD), Fourier transform infrared (FTIR) and Mössbauer. Finally, the hydration process characteristics of the LSS-based geopolymer were proposed and the behavior of iron was discussed.
2. Materials and Methods
2.1. Materials
The LSS used in the experiments was obtained from a lead smelting company in the south of China. The LSS was dried to a constant weight (±0.1 g) at 105 °C. The LSS was milled under the ball-to-material weight ratio of 5 in planetary ball milling at 400 rpm for 3 hours and passed through a 45 µm mesh sieve [41]. The particle size of the LSS powder was analyzed by laser granulometry. As shown in Figure 1, the LSS powder had a particle size of between 0.92 and 41.84 µm. The size of the particles was distributed in three concentrated areas of approximately 1.18, 4.32 and 13.50 μm. The median particle size (D50) of 5.28 μm indirectly reflected that some small particles might be agglomerated into large particles. The chemical compositions of the LSS (Table 1) were detected using x-ray fluorescence (XRF).
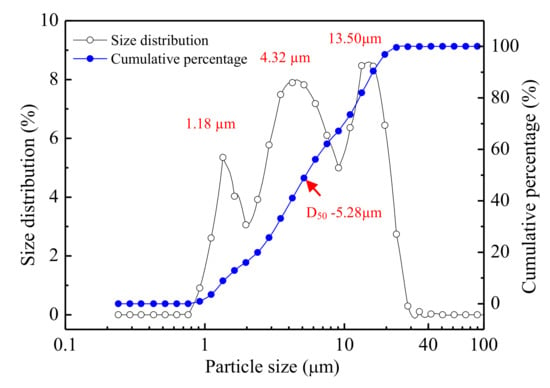
Figure 1.
Particle size distribution of the lead smelting slag (LSS) after ball milling.

Table 1.
Chemical compositions of the LSS (wt.%).
The water glass (SiO2 = 26.5%, Na2O = 8.3%, molar ratio of SiO2/Na2O is 3.3 and density is 1.371 g/cm3) was provided by Shandong Usolf Chemical Technology Co., Ltd. Other chemicals were analytical grade and were purchased from Sinopharm Chemical Reagent Co., Ltd.
2.2. Experimental Procedure
The milled LSS, water glass and deionized water were mixed and stirred evenly in proportion. Then, the slurry was poured into a steel mold (20 × 20 × 20 mm), uniformly shaken, covered with a plastic film and then cured for 24 hours in a cement concrete standard curing box at a 95% relative humidity at 20 ± 2 °C. The matrices were demolded and cured again for different time under the same conditions. Afterwards, the unconfined compressive strength (UCS) of the matrices was measured after curing for 3 and 28 days.
In the experiment, the UCS was taken as an index to optimize the optimal parameters of the LSS-based geopolymer. The influences of the modulus (Ms) of water glass, dosage of water glass (WNa2O + SiO2) and water-to-binder ratio on the UCS were investigated. The formulation design of the LSS-based geopolymer experiments is presented in Table 2.

Table 2.
The formulation design of the LSS-based geopolymer experiments.
2.3. Tests
2.3.1. UCS Test
The UCS tests were performed according to the GB/T17671-1999. The UCS tests of each sample were performed on three cubes as parallel experiments. All the samples were tested after curing for 3 and 28 days. The UCS of the matrices was tested using an unconfined compression machine (TYA-300B, Wuxi Xinluda Instrument Co., Ltd., Wuxi, China) with a loading rate of 2.4 kN/S.
2.3.2. Leaching Test
The TCLP was used to evaluate the leaching ability of heavy metals for the samples. An acetic acid solution with a pH of 2.88 ± 0.02 was selected as the leaching solution [42]. Amounts of 3.0 g of the crushed matrices (<9.5 mm) and 60 mL of the leaching solution were poured into the sealed polyethylene vessels and then shaken on a shaker with a speed of 30 rpm for 18 h. The leachates were filtered with a 0.45 µm membrane filter. Finally, the heavy metals concentrations in the filtrates were analyzed using an inductively coupled plasma-atomic emission spectroscopy (ICP-AES, IRIS Intrepid II XSP). All of the above experiments were carried out in triplicate and the results were calculated to obtain the average values.
2.3.3. Other Tests
The XRF can provide qualitative and semi-quantitative analyses of elements in solid samples. A sample of the LSS was ground to ensure that the particle size was less than 45 µm and then analyzed by XRF (S4-Pioneer, Bruker Ltd., Karlsruhe, Germany). The crystallographic composition of the samples was characterized by x-ray diffraction (XRD, D/max2550 VB + 18 KW) at a speed of 10° min−1 in a 2θ range from 10° to 70°. 57Fe Mössbauer spectra were collected in a standard transmission geometry using a standard constant acceleration EG&G spectrometer with a 57Co(Rh) source. Measurements were performed with a constant acceleration at 20 °C and the calibration was referenced to metallic iron foil. Absorbers were prepared with 1.5 g of the sample powder in a lead sample holder.
3. Results and Discussion
3.1. Strength Optimization of LSS-Based Geopolymer
3.1.1. Effect of Modulus of Water Glass
The effects of the modulus of water glass on the UCS of the LSS based geopolymer are shown in Figure 2. As observed in Figure 2, the UCS was a non-monotonous function of the modulus of water glass. The UCS of the geopolymer increased as the modulus of water glass increased from 0.5 to 1, and reached a maximum value of 73.6 MPa after hydrating for 28 days. Further increasing the modulus of water glass to 2 resulted in the UCS decreasing remarkably. This is because OH− is used for leaching soluble Si and Al from slag [43]. Moreover, the dissolution rate of Si and Al increases with the increase in OH− concentration. Soluble Si is essential for C-S-H and geopolymer gels production. Therefore, increasing the modulus of water glass is equivalent to increasing the content of soluble Si per volume. However, when the modulus of water glass was excessively low, the NaOH in the aqueous solution was superfluous and the frost phenomenon appeared easily. The frost phenomenon could result in many large voids in the geopolymer [44] and a lower UCS. In conclusion, the optimum modulus of water glass was 1–1.5.
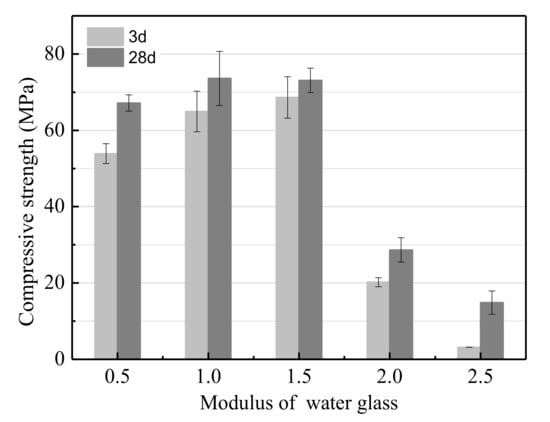
Figure 2.
The effect of the modulus of water glass on the unconfined compressive strength (UCS).
3.1.2. Effect of Dosage of Water Glass
Figure 3 presents the effect of the water glass (W(SiO2+Na2O)) dosage on the UCS of the LSS-based geopolymer. The results show that the UCS increased drastically from 2.32 to 74.45 MPa as the dosage of water glass increased from 2% to 5%. Then, the UCS decreased slowly as the dosage of water glass increased from 5% to 14%, because the concentration of OH- increased as the dosage of water glass increased and accelerated the hydration degree of the LSS. Therefore, increasing the water glass dosage could increase the amount of gels per volume for geopolymer production. This resulted in the UCS of the geopolymer increasing. However, when the dosage of water glass was excessive, the frost phenomenon also occurred, the same as it did when the modulus of water glass was excessively low. It became more serious when the dosage of water glass and curing time increased. Therefore, the UCS of the LSS-based geopolymer cured for 28 days was lower than that of the geopolymer cured for 3 days, and an optimum modulus of water glass of 5% was selected.
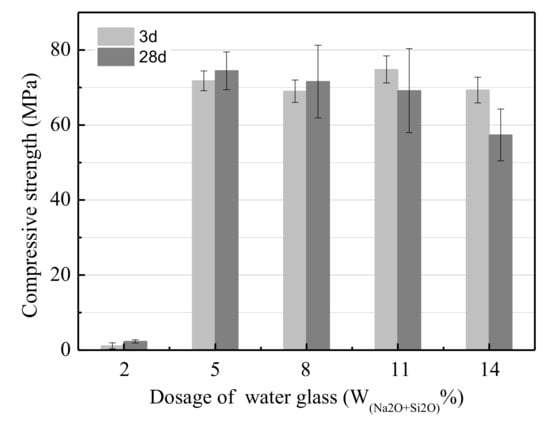
Figure 3.
The effect of the water glass dosage on the UCS.
3.1.3. Effect of Water-To-Binder Ratio
As shown in Figure 4, the effect of the water-to-binder ratio on the UCS of the LSS-based geopolymer was studied in the range of 0.175 to 0.275. The UCS decreased almost linearly as the water-to-binder ratio increased. According to the results, the optimum water-to-binder ratio was 0.2. Using this value, the UCS of the LSS based geopolymer reached 76.09 MPa after curing for 28 days. Essentially, the geopolymer matrix consisted of two solid phases after alkali-activation (i.e., non-dissolved granules of the LSS and gels). When the water-to-binder ratio increased, the concentration of OH− was reduced, resulting in both the hydration degree of the LSS and the gels per volume of the produced geopolymer being reduced. In addition, the porosity of the matrix also increased. Due to these two aspects, the UCS of the geopolymer decreased when the water-to-binder ratio increased.
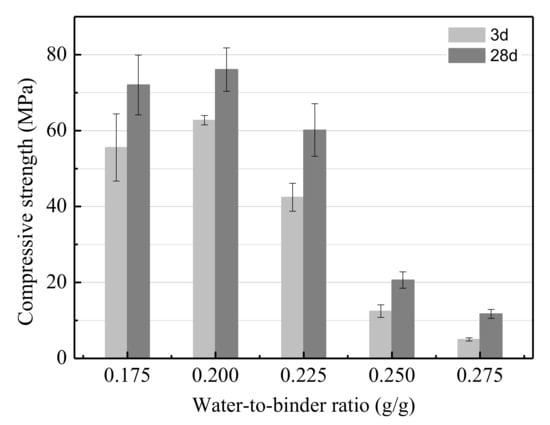
Figure 4.
The effect of the water-to-binder ratio on the UCS.
3.2. Hydration Characteristics Analysis
3.2.1. Hydration Products Analysis
The XRD patterns of LSS and the LSS based geopolymer (R2) cured for 28 days are shown in Figure 5. The initial LSS pattern mainly consisted of an amorphous phase with some crystalline phases of predominantly magnetite (Fe3O4) and a little wuestite (Fe0.872O). The broad and diffuse peaks from the initial LSS around 28–35o (2θ) reflected the short-range order of the CaO–Al2O3–MgO–SiO2 glass structure [45]. This is a common feature of an amorphous phase and is an indication of a rather reactive phase.
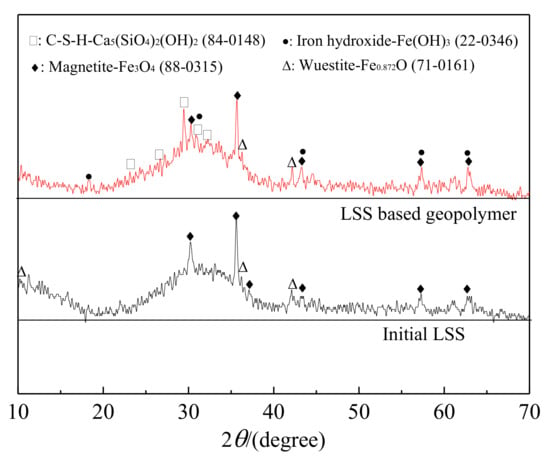
Figure 5.
XRD patterns of the initial LSS and LSS based geopolymer (R2) after being hydrated for 28 days.
The XRD pattern of the LSS based geopolymer was slightly different from that of the initial LSS. The broad and diffuse peaks for the geopolymer at around 28–35o (2θ) became wider than those for the initial LSS, indicating that the geopolymer was generated. In addition, some weak peaks of calcium silicate hydroxide (C-S-H) were detected. The results were consistent with those of Li et al. [46,47,48]. Meanwhile, a new phase of iron hydroxide also appeared. However, the peak intensities of magnetite and wuestite were almost unchanged in the LSS based geopolymer compared with the initial LSS, indicating that magnetite and wuestite may not take part in the hydration reaction.
The conclusions of the XRD were strengthened by the analysis of the FTIR (Figure 6). The spectrum of the initial LSS was composed of a broad band at 946 cm−1, ascribed to ν3(Si–O) stretching modes, and another located at 507 cm−1, which was assigned to ν4(O–Si–O) bending modes of the SiO4 tetrahedral.
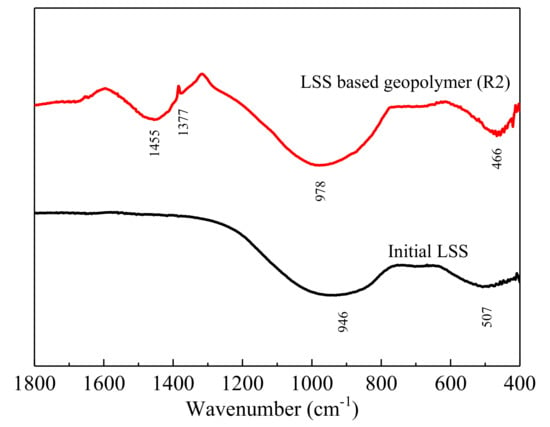
Figure 6.
FTIR patterns of the initial LSS and LSS based geopolymer (R2) after being hydrated for 28 days.
For the LSS-based geopolymer, the spectrum had a sharp and intense absorption band at 1455 cm−1, attributed to the stretching vibrations of O–C–O bonds [43]. This absorption band indicated the presence of carbonates. Calcium carbonate could be the main carbonate compound, mainly because calcium ions in the pore fluid in the hardened body easily react with CO2 in the air to form CaCO3 during the curing process. The ones referred to as asymmetric stretching Si–O–Si vibrations (970–1090 cm−1) comprise the major sign of geopolymerization, according to Panias et al. [43]. The band at 978 cm−1 was attributed to the ν3(Si–O) stretching vibration in the geopolymer and was narrower and higher than anhydrous slag, indicating that the polymerization degree of the Si-O bond increased and that a short-range order was formed in the structure of the geopolymer. This was associated with the ν3(Si–O) stretching vibrations in geopolymer gel [49,50]. Finally, the absorption band was observed in the spectrum of the geopolymer at the wave number 466 cm−1. This was assigned to the ν4(O–Si–O) band and attributed to the formation of the C-S-H phase [51].
3.2.2. Iron Behavior Analysis
Figure 7a shows the Mössbauer spectrum of the initial LSS at room temperature. It consisted of two distinct isomer shifts (IS) and quadrupole splitting (QS). One was equal to Fe3+(IS = 0.78 mm/s and QS = 1.21 mm/s). The value of the IS may be characterized by the Fe3+ with distorted tetrahedral symmetry [52,53]. The QS value of Fe3+ was a little larger than 1.2 mm/s, indicating the presence of a larger distortion of FeO4 tetrahedra. The linewidth value of iron oxide presented in a glass network is generally larger than 0.4 mm/s [52,53]. The linewidth values of Fe3+ and Fe2+ were 0.9 and 0.64 mm/s from the LSS, respectively, indicating that partial iron was present in the glass phase. The IS value of Fe2+ was 1.02 mm/s, indicating a distorted octahedral symmetry [52]. The QS value (1.99 mm/s) of Fe2+ was much smaller than those for typical distorted tetrahedral Fe(II). It may be that the Fe2+ occupied the site of the network as a modifier, as well as Ca2+, Na+, K+, etc. The LSS contained many Ca and Fe but the content of Na and K was low, according to the XRF analysis (Table 1). Therefore, Fe and Ca became the main network modifier in the glass phase.
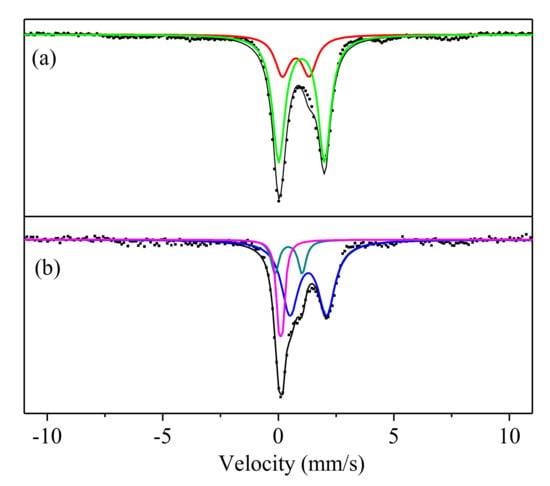
Figure 7.
Mössbauer spectrums of the initial LSS (a) and LSS based geopolymer (R2) after being hydrated for 28 days (b).
The Mössbauer spectrum of the LSS based geopolymer is shown in Figure 7b. The variation of the distortion of FeO4 tetrahedra was embodied in the QS. It can be seen that the QS values of Fe2+ decreased from 1.99 to 1.59 mm/s. These smaller QS values of Fe2+ indicated a lessened distortion of the Fe(II)O4 tetrahedra, which showed that Fe2+ dissociated from the glass phase and Fe(OH)2 appeared. The structural relaxation was embodied in the linewidth, as can be seen in Table 3. The linewidth values of Fe3+ in the LSS were reduced from 0.9 to 0.28 mm/s after the alkali-activation. The decrease in linewidth indicated that the homogeneity of the Fe–O bond length and O–Fe–O bond angle increased for the weak crystal phase of Fe(OH)3. This dissociated Fe2+ from the glass network and caused it to be oxidized to Fe3+ under the air conditions.

Table 3.
Mössbauer parameters of the initial LSS (a) and LSS based geopolymer (R2) after being hydrated for 28 days.
By comparing the phase changes between the LSS and LSS-based geopolymer and analyzing the iron behavior, we have deduced that the hydration process characteristics could be described as below. The Ca–O bond and Fe–O bond were broken in the glass network phase under the action of OH- to form Ca(OH)2 and Fe(OH)2, respectively, because the bond energy between the network modifier and oxygen was lower than others were. Meanwhile, the [SiO4] was depolymerized from high degree polymerization to a lower one and became a monomer in the end. A small amount of high degree polymerization of [AlO4] was also depolymerized to a monomer in the same way as [SiO4]. Then, the C-S-H and geopolymer gels were produced. However, Fe(OH)2 was not detected in the LSS-based geopolymer and some weak peaks of Fe(OH)3 were detected. The reason for this was that the Fe(OH)2 was easily oxidized to Fe(OH)3 under the air curing conditions.
3.3. Heavy Metals Solidification
Table 4 lists the concentrations of the main heavy metals of leachates from the initial LSS and geopolymer (R2) cured for 28 days via the TCLP test. Compared with the initial LSS, Hg, Ag and Se were also not detected in the leachate. The concentrations of Be, Cr and Pb did not change substantially and all were far below the limits allowed. The concentrations of Cd and Ba decreased significantly and were below the limits allowed. Moreover, the leachate concentration of Zn for R2 was 93.99 mg/L, which was slightly below the limit allowed. The arsenic concentration in the leachate for R2 was below the limits and increased slightly compared with the initial LSS. Therefore, we can conclude that the LSS-based geopolymer has a certain capacity for the immobilization of Zn.

Table 4.
Toxicity characteristic leaching procedure (TCLP) results of the LSS and geopolymer (R2) cured for 28 days.
C-S-H, the geopolymer and Fe(OH)3 have a good ability to immobilize Zn, Cd, Ni, As, etc. [54,55,56,57,58,59]. Liu et al. [60] reported that the immobilization capacity of C-S-H for Cu2+ and Cd2+ was better than for Zn2+, and that the low Ca/Si of C-S-H could fix Zn2+ better than the high Ca/Si of C-S-H. Therefore, the immobilization effect was better for Cu2+ and Cd2+ than for Zn2+. The Ca/Si of C-S-H in the LSS-based geopolymer was about 0.8, which suggested a good adsorption of Zn2+. Due to the combined actions of these hydration products, Zn and other heavy metals were immobilized effectively.
4. Conclusions
High-energy ball milling was used to activate the potential water-hardness properties of LSS. Afterwards, the LSS-based geopolymer was synthesized via water glass activation. The effect of UCS on the synthesis parameters of the LSS-based geopolymer was optimized. Under the best parameters of the binder (modulus of water glass was 1-1.5, dosage of water glass (W(SiO2 + Na2O)) was 5% and water-to-binder ratio was 0.2), the UCS reached 76.09 MPa after curing for 28 days. Meanwhile, the TCLP leaching concentration of Zn from the LSS fell from 167.16 to 93.99 mg/L after the alkali-activation, which was a significant reduction and slightly below the limit allowed.
Moreover, the C-S-H and geopolymer phases of the hydration products were identified in the LSS-based geopolymer. The behaviors of iron were also discussed. Then, the hydration process characteristics were proposed. The results indicated that calcium and partial iron performed as network modifiers in the glass network phase. The glass phase was dissociated with water glass and then Ca2+ and Fe2+ were dissolved to produce C-S-H, the geopolymer and Fe(OH)2. However, the Fe(OH)2 was easily oxidized to Fe(OH)3 under the air curing conditions. In consequence, we can draw the conclusion that LSS is an implementable raw material for geopolymer production or high-performance alkali-activated cement.
Author Contributions
Conceptualization, L.Y., D.L. and X.M.; Data curation, L.Y. and D.L.; Formal analysis, L.Y.; Funding acquisition, X.M.; Investigation, L.Y. and D.L.; Methodology, D.L. and Z.W.; Project administration, Y.K. and X.M.; Resources, J.F.; Software, Y.L., Z.W. and H.X.; Validation, Y.K., Y.L. and X.M.; Visualization, Y.K.; Writing—original draft, L.Y.; Writing—review & editing, L.Y. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by National Key R&D Program of China (2017YFC0210402), the key project of National Natural Science Foundation of China (51634010), and the Natural Science Foundation of China (51474247 and 51474102), and Science and Technology Project of Hunan Province (2017RS3010).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gao, W.; Wang, C.Y.; Yin, F.; Chen, Y.Q.; Yang, W.J. Situation and technology progress of lead smelting in China. Adv. Mater. Res. 2012, 581–582. [Google Scholar] [CrossRef]
- Ke, Y.; Peng, N.; Xue, K.; Min, X.; Chai, L.; Pan, Q.; Liang, Y.; Xiao, R.; Wang, Y.; Tang, C.; et al. Sulfidation behavior and mechanism of zinc silicate roasted with pyrite. Appl. Surf. Sci. 2018, 435, 1011–1019. [Google Scholar] [CrossRef]
- Norgate, T.; Jahanshahi, S.; Rankin, W. Assessing the environmental impact of metal production processes. J. Clean. Prod. 2007, 15, 838–848. [Google Scholar] [CrossRef]
- Min, X.; Zhou, B.; Ke, Y.; Chai, L.; Xue, K.; Zhang, C.; Zhao, Z.; Shen, C. Sulfidation behavior of ZnFe2O4 roasted with pyrite: Sulfur inducing and sulfur-oxygen interface exchange mechanism. Appl. Surf. Sci. 2016, 371, 67–73. [Google Scholar] [CrossRef]
- Chai, L.Y.; Shi, M.Q.; Liang, Y.J.; Tang, J.W.; Li, Q.Z. Behavior, distribution and environmental influence of arsenic in a typical lead smelter. J. Cent. South Univ. 2015, 22, 1276–1286. [Google Scholar] [CrossRef]
- Li, Y.C.; Min, X.B.; Chai, L.Y.; Shi, M.Q.; Tang, C.J.; Wang, Q.W.; Liang, Y.J.; Lei, J.; Liyang, W.J. Co-treatment of gypsum sludge and Pb/Zn smelting slag for the solidification of sludge containing arsenic and heavy metals. J. Environ. Manag. 2016, 181, 756–761. [Google Scholar] [CrossRef] [PubMed]
- De Andrade Lima, L.; Bernardez, L. Characterization of the lead smelter slag in Santo Amaro, Bahia, Brazil. J. Hazard. Mater. 2011, 189, 692–699. [Google Scholar] [CrossRef]
- Kreusch, M.; Ponte, M.; Ponte, H.; Kaminari, N.; Marino, C.; Mymrin, V. Technological improvements in automotive battery recycling. Resour. Conserv. Recycl. 2007, 52, 368–380. [Google Scholar] [CrossRef]
- Ettler, V.; Komárková, M.; Jehlička, J.; Coufal, P.; Hradil, D.; Machovič, V.; Delorme, F. Leaching of lead metallurgical slag in citric solutions—implications for disposal and weathering in soil environments. Chemosphere 2004, 57, 567–577. [Google Scholar] [CrossRef]
- Saikia, N.; Cornelis, G.; Mertens, G.; Elsen, J.; Van Balen, K.; Van Gerven, T.; Vandecasteele, C. Assessment of Pb-slag, MSWI bottom ash and boiler and fly ash for using as a fine aggregate in cement mortar. J. Hazard. Mater. 2008, 154, 766–777. [Google Scholar] [CrossRef]
- Xie, X.D.; Min, X.B.; Chai, L.Y.; Tang, C.J.; Liang, Y.J.; Li, M.; Ke, Y.; Chen, J.; Wang, Y. Quantitative evaluation of environmental risks of flotation tailings from hydrothermal sulfidation-flotation process. Environ. Sci. Pollut. Res. 2013, 20, 6050–6058. [Google Scholar] [CrossRef]
- Wang, Z.X.; Chai, L.Y.; Wang, Y.Y.; Yang, Z.H.; Wang, H.Y.; Wu, X. Potential health risk of arsenic and cadmium in groundwater near Xiangjiang River, China: A case study for risk assessment and management of toxic substances. Environ. Monit. Assess. 2011, 175, 167–173. [Google Scholar] [CrossRef]
- Wang, Z.; Chai, L.; Yang, Z.; Wang, Y.; Wang, H. Identifying sources and assessing potential risk of heavy metals in soils from direct exposure to children in a mine-impacted city, Changsha, China. J. Environ. Qual. 2010, 39, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.C.; Min, X.B.; Wang, Z.X.; Pang, Z.H.; Liang, Y.J.; Ke, Y. Health and ecological risk assessment of heavy metals pollution in an antimony mining region: A case study from South China. Environ. Sci. Pollut. Res. 2017, 24, 27573–27586. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Chai, L.Y.; Min, X.B.; Tang, C.J.; Chen, J.; Wang, Y.; Liang, Y.J. Sulfidation of heavy-metal-containing neutralization sludge using zinc leaching residue as the sulfur source for metal recovery and stabilization. Miner. Eng. 2014, 61, 105–112. [Google Scholar] [CrossRef]
- Shi, C.; Meyer, C.; Behnood, A. Utilization of copper slag in cement and concrete. Resou. Conserv. Recycl. 2008, 52, 1115–1120. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Van Deventer, J.S.J.; Provis, J.L.; Duxson, P.; Brice, D.G. Chemical research and climate change as drivers in the commercial adoption of alkali activated materials. Waste Biomass Valoriz. 2010, 1, 145–155. [Google Scholar] [CrossRef]
- Criado, M.; Fernández-Jiménez, A.; Palomo, A. Alkali activation of fly ash. Part III: Effect of curing conditions on reaction and its graphical description. Fuel 2010, 89, 3185–3192. [Google Scholar] [CrossRef]
- Albitar, M.; Mohamed Ali, M.S.; Visintin, P.; Drechsler, M. Effect of granulated lead smelter slag on strength of fly ash-based geopolymer concrete. Constr. Build. Mater. 2015, 83, 128–135. [Google Scholar] [CrossRef]
- Ogundiran, M.B.; Nugteren, H.W.; Witkamp, G.J. Immobilisation of lead smelting slag within spent aluminate-fly ash based geopolymers. J. Hazard. Mater. 2013, 248, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Onisei, S.; Pontikes, Y.; Van Gerven, T.; Angelopoulos, G.N.; Velea, T.; Predica, V.; Moldovan, P. Synthesis of inorganic polymers using fly ash and primary lead slag. J. Hazard. Mater. 2012, 205, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Fernando, P.; João, C.; Said, J. Durability and Environmental Performance of Alkali-Activated Tungsten Mine Waste Mud Mortars. J. Mater. Civil Eng. 2010, 22, 897–904. [Google Scholar] [CrossRef]
- Danutė, V.; Janavičius, E.; Kielė, A.; Parthiban, S.; Aras, K. The influence of aluminum hydroxide (Al(OH)3) additive on the physical and mechanical properties of alkali-activated slag. J. Sustain. Archit. Civil Eng. 2016, 17, 77–83. [Google Scholar] [CrossRef]
- Van Deventer, J.S.J.; Provis, J.L.; Duxson, P. Technical and commercial progress in the adoption of geopolymer cement. Miner. Eng. 2012, 29, 89–104. [Google Scholar] [CrossRef]
- Gharzouni, A.; Ouamara, L.; Sobrados, I.; Rossignol, S. Alkali-activated materials from different aluminosilicate sources: Effect of aluminum and calcium availability. J. Non-Cryst. Solids 2018, 484, 14–25. [Google Scholar] [CrossRef]
- Pan, Z.H.; Zhang, J.; Liu, W.Q. Solidification/stabilization of zinc-lead tailings by alkali activated slag cement. J. Wuhan Univ. Technol. 2015, 30, 105–108. [Google Scholar] [CrossRef]
- Koplík, J.; Kalina, L.; Másilko, J.; Šoukal, F. The characterization of fixation of Ba, Pb, and Cu in alkali-activated fly ash/blast furnace slag matrix. Materials 2016, 9, 533. [Google Scholar] [CrossRef]
- Fan, G.; Zhang, D.; Wang, X. Reduction and utilization of coal mine waste rock in China: A case study in Tiefa coalfield. Resour. Conserv. Recycl. 2014, 83, 24–33. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, G.R.; Wang, J.; Chen, Q. Effect of polyacrylic resin on mechanical properties of granulated blast furnace slag based geopolymer. J. Non-Cryst. Solids 2018, 481, 4–9. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; MacKenzie, K.J.D. Preparation and characterisation of fly ash based geopolymer mortars. Constr. Build. Mater. 2010, 24, 1906–1910. [Google Scholar] [CrossRef]
- Cioffi, R.; Maffucci, L.; Santoro, L. Optimization of geopolymer synthesis by calcination and polycondensation of a kaolinitic residue. Resour. Conserv. Recycl. 2003, 40, 27–38. [Google Scholar] [CrossRef]
- Akyıldız, A.; Köse, E.T.; Yıldız, A. Compressive strength and heavy metal leaching of concrete containing medical waste incineration ash. Constr. Build. Mater. 2017, 138, 326–332. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Carcelen-Taboada, V.; Fernández-Jiménez, A.; Palomo, A. Manufacture of hybrid cements with fly ash and bottom ash from a municipal solid waste incinerator. Constr. Build. Mater. 2016, 105, 218–226. [Google Scholar] [CrossRef]
- Li, Y.C.; Min, X.B.; Ke, Y.; Chai, L.Y.; Shi, M.Q.; Tang, C.J.; Wang, Q.W.; Liang, Y.J.; Lei, J.; Liu, D.G. Utilization of red mud and Pb/Zn smelter waste for the synthesis of a red mud-based cementitious material. J. Hazard. Mater. 2018, 344, 343–349. [Google Scholar] [CrossRef]
- Palomo, A.; dela Fuente, J.I.L. Alkali-activated cementitous materials_ Alternative matrices for the immobilisation of hazardous wastes_ Part I. Stabilisation of boron. Cem. Concr. Res. 2003, 33, 281–288. [Google Scholar] [CrossRef]
- Soro, N.; Aldon, L.; Olivier-Fourcade, J.; Jumas, J.C.; Laval, J.P.; Blanchart, P. Role of Iron in Mullite Formation from Kaolins by Mössbauer Spectroscopy and Rietveld Refinement. J. Eur. Ceram. Soc. 2003, 86, 129–134. [Google Scholar] [CrossRef]
- Casteleina, O.; Aldon, L.; Olivier-Fourcade, J.; Jumas, J.C.; Bonnet, J.P.; Blanchart, P. 57Fe Mössbauer study of iron distribution in a kaolin raw material: Influence of the temperature and the heating rate. J. Eur. Ceram. Soc. 2002, 22, 1767–1773. [Google Scholar] [CrossRef]
- Liu, D.G.; Ke, Y.; Min, X.B.; Liang, Y.J.; Wang, Z.B.; Li, Y.C.; Fei, J.C.; Yao, L.W.; Xu, H.; Jiang, G.H. Cotreatment of MSWI fly ash and granulated lead smelting slag using a geopolymer system. Int. J. Environ. Res. Public Health 2019, 16, 156. [Google Scholar] [CrossRef]
- Aydın, S.; Baradan, B. The effect of fiber properties on high performance alkali-activated slag/silica fume mortars. Compos. Part B-Eng. 2013, 45, 63–69. [Google Scholar] [CrossRef]
- Lei, J.; Peng, B.; Min, X.; Liang, Y.; You, Y.; Chai, L. Modeling and optimization of lime-based stabilization in high alkaline arsenic-bearing sludges with a central composite design. J. Environ. Sci. Health Part A 2017, 52, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Panias, D.; Giannopoulou, I.P.; Perraki, T. Effect of synthesis parameters on the mechanical properties of fly ash-based geopolymers. Colloid. Surf. A 2007, 301, 246–254. [Google Scholar] [CrossRef]
- Mardani-Aghabaglou, A.; Kalıpcılar, İ.; İnan Sezer, G.; Sezer, A.; Altun, S. Freeze–thaw resistance and chloride-ion penetration of cement-stabilized clay exposed to sulfate attack. Appl. Clay Sci. 2015, 115, 179–188. [Google Scholar] [CrossRef]
- Deja, J. Immobilization of Cr6+, Cd2+, Zn2+ and Pb2+ in alkali-activated slag binders. Cem. Concr. Res. 2002, 32, 1971–1979. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si+Ca) and metakaolin (Si+Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Wang, S.D.; Scrivener, K.L. 29Si and 27Al NMR study of alkali-activated slag. Cem. Concr. Res. 2003, 33, 769–774. [Google Scholar] [CrossRef]
- Mozgawa, W.; Deja, J. Spectroscopic studies of alkaline activated slag geopolymers. J. Mol. Struct. 2009, 924, 434–441. [Google Scholar] [CrossRef]
- Puertas, F.; Palacios, M.; Manzano, H.; Dolado, J.S.; Rico, A.; Rodríguez, J. A model for the C-A-S-H gel formed in alkali-activated slag cements. J. Eur. Ceram. Soc. 2011, 31, 2043–2056. [Google Scholar] [CrossRef]
- Cui, X.; He, Y.; Liu, L.; Chen, J. NaA zeolite synthesis from geopolymer precursor. MRS Commun. 2011, 1, 49–51. [Google Scholar] [CrossRef]
- Puertas, F.; Martinez-Ramirez, S.; Alonso, S.; Vázquez, T. Alkali-activated fly ash_slag cements_ Strength behaviour and hydration products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Homonnay, Z.; Music, S.; Nishida, T.; Kopelev, N.S.; Vertes, A. Mössbauer Spectroscopy of Sophisticated Oxides. Akadémiai Kiadó Bp. 1997, 305, 159–193. [Google Scholar]
- Nishida, T.; Tokunaga, M.; Sugata, Y.; Kubuki, S. Mössbauer study of semiconducting and ferrimagnetic fly ash-recycled glass. J. Radioanal. Nucl. Chem. 2005, 266, 171–177. [Google Scholar] [CrossRef]
- Stumm, A.; Garbev, K.; Beuchle, G.; Black, L.; Stemmermann, P.; Nüesch, R. Incorporation of zinc into calcium silicate hydrates, Part I: Formation of C-S-H(I) with C/S=2/3 and its isochemical counterpart gyrolite. Cem. Concr. Res. 2005, 35, 1665–1675. [Google Scholar] [CrossRef]
- Kara, İ.; Yilmazer, D.; TunaliAkar, S. Metakaolin based geopolymer as an effective adsorbent for adsorption of zinc(II) and nickel(II) ions from aqueous solutions. Appl. Clay Sci. 2017, 139, 54–63. [Google Scholar] [CrossRef]
- Liu, D.G.; Min, X.B.; Chai, L.Y.; Ke, Y.; Liang, Y.J.; Li, Y.C.; Yao, L.W.; Wang, Z.B. Co-treatment of flotation waste, neutralization sludge and arsenic-containing gypsum sludge from copper smelting: Solidification/stabilization of arsenic and heavy metals with minimal cement clinker. Environ. Sci. Pollut. Res. 2018, 25, 7600–7607. [Google Scholar] [CrossRef]
- Min, X.; Li, Y.; Ke, Y.; Shi, M.; Chai, L.; Xue, K. Fe-FeS2 adsorbent prepared with iron powder and pyrite by facile ball milling and its application for arsenic removal. Water Sci. Technol. 2017, 76, 192–200. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Gao, N.Y.; Lin, Y.C.; Xu, B.; Le, L.S. Removal of arsenic(V) from aqueous solutions using iron-oxide-coated modified activated carbon. Water Environ. Res. 2007, 79, 931–936. [Google Scholar] [CrossRef]
- Liang, Y.J.; Min, X.B.; Chai, L.Y.; Wang, M.; Liyang, W.J.; Pan, Q.; Okido, M. Stabilization of arsenic sludge with mechanochemically modified zero valent iron. Chemosphere 2017, 168, 1142–1151. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Wang, L.; Kao, H. Effect of absorption and binding of Cd2+, Zn2+ and Cu2+ with C-S-H gel. Cement 2012, 49, 151–185. (In Chinesse) [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).