A Systematic Review of Network Studies Based on Administrative Health Data
Abstract
1. Introduction
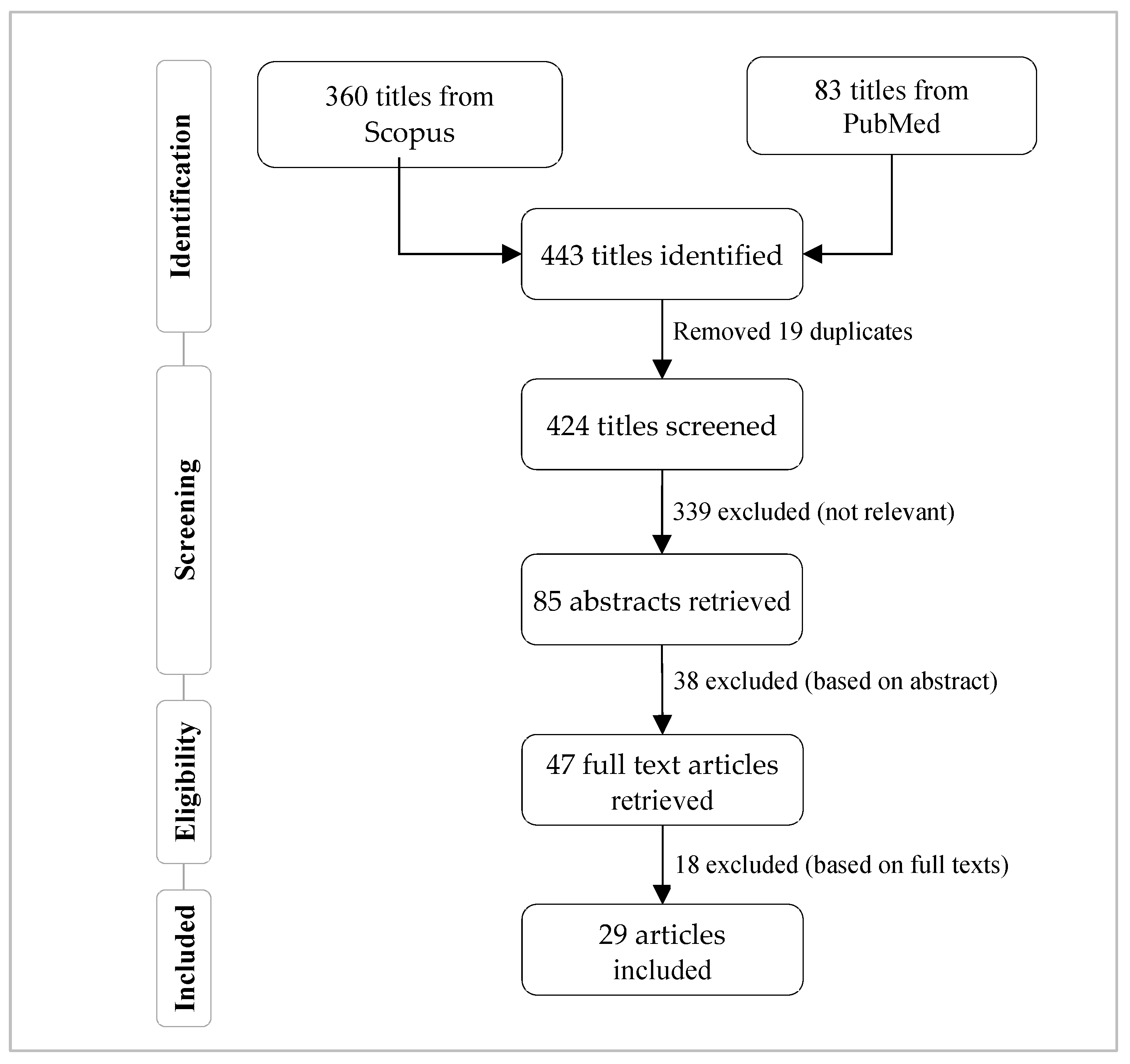
2. Methodology
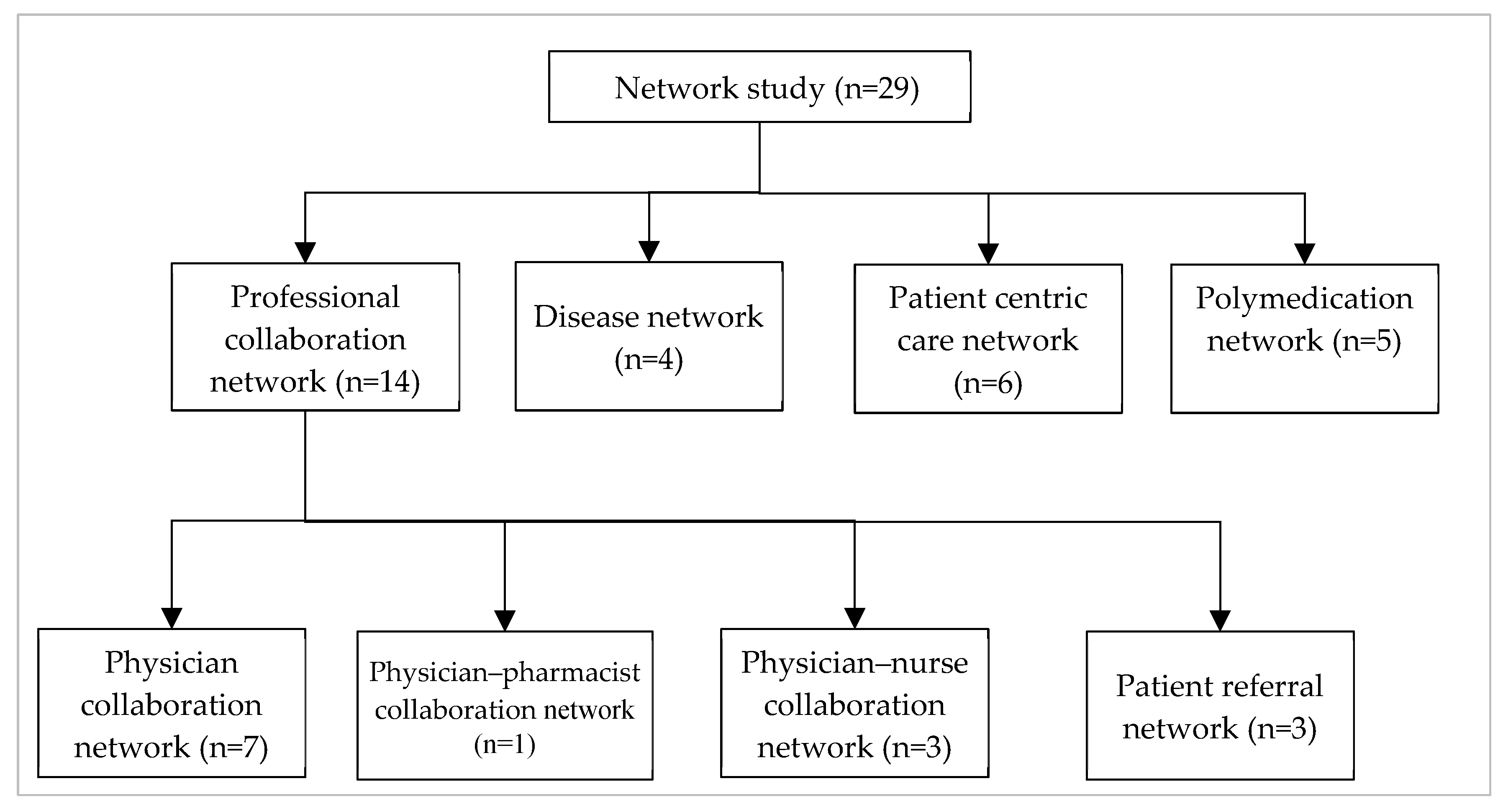
3. Emergence of Network in Healthcare
3.1. Professional Collaboration Network
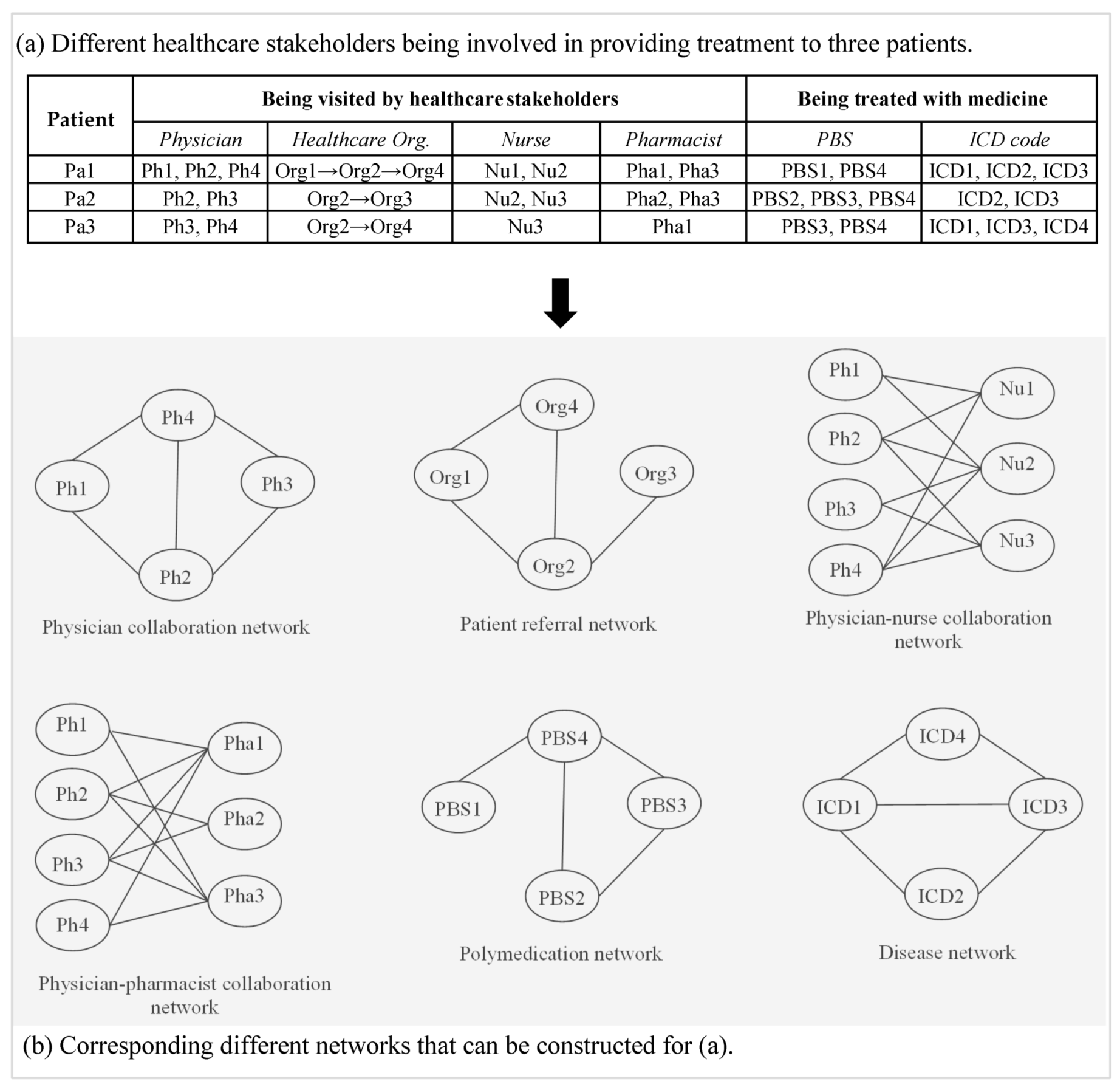
3.1.1. Physician Collaboration Network
3.1.2. Physician–Pharmacist Collaboration Network
3.1.3. Physician–Nurse Collaboration Network
3.1.4. Patient Referral Network
3.2. Disease Network
3.3. Patient-Centric Care Collaboration Network
3.4. Polymedication Network
4. Results and Discussions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Uddin, S.; Kelaher, M.; Srinivasan, U. A framework for administrative claim data to explore healthcare coordination and collaboration. Aust. Health Rev. 2016, 40, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, S.; Awang, R. Intelligent heart disease prediction system using data mining techniques. In Proceedings of the 2008 IEEE/ACS International Conference on Computer Systems and Applications, Doha, Qatar, 31 March–4 April 2008; IEEE: Piscataway, NJ, USA, 2008. [Google Scholar]
- Wu, R.; Peters, W.; Morgan, M. The next generation of clinical decision support: Linking evidence to best practice. J. Healthc. Inf. Manag. JHIM 2002, 16, 50–55. [Google Scholar] [PubMed]
- Ohno-Machado, L.; Kim, J.; Gabriel, R.A.; Kuo, G.M.; Hogarth, M.A. Genomics and electronic health record systems. Hum. Mol. Genet. 2018, 27, R48–R55. [Google Scholar] [CrossRef] [PubMed]
- Culler, S.D.; Parchman, M.L.; Przybylski, M. Factors related to potentially preventable hospitalizations among the elderly. Med. Care 1998, 36, 804–817. [Google Scholar] [CrossRef]
- Uddin, M.S.; Hossain, L. Social networks enabled coordination model for cost management of patient hospital admissions. J. Healthc. Qual. 2011, 33, 37–48. [Google Scholar] [CrossRef]
- Lee, P.P.; Levin, L.A.; Walt, J.G.; Chiang, T.; Katz, L.M.; Dolgitser, M.; Doyle, J.J.; Stern, L.S. Cost of patients with primary open-angle glaucoma: A retrospective study of commercial insurance claims data. Ophthalmology 2007, 114, 1241–1247. [Google Scholar] [CrossRef]
- Davis, D.A.; Chawla, N.V.; Christakis, N.A.; Barabási, A.-L. Time to CARE: A collaborative engine for practical disease prediction. Data Min. Knowl. Discov. 2010, 20, 388–415. [Google Scholar] [CrossRef]
- McCormick, T.; Rudin, C.; Madigan, D. A Hierarchical Model for Association Rule Mining of Sequential Events: An Approach to Automated Medical Symptom Prediction. Ann. Appl. Stat. 2011. [Google Scholar] [CrossRef]
- Yiannakoulias, N.; Schopflocher, D.; Svenson, L. Using administrative data to understand the geography of case ascertainment. Chronic Dis. Can. 2009, 30, 20–28. [Google Scholar]
- Fisher, E.S.; Malenka, D.J.; Wennberg, J.E.; Roos, N.P. Technology assessment using insurance claims: Example of prostatectomy. Int. J. Technol. Assess. Health Care 1990, 6, 194–202. [Google Scholar] [CrossRef]
- DuGoff, E.H.; Fernandes-Taylor, S.; Weissman, G.E.; Huntley, J.H.; Pollack, C.E. A scoping review of patient-sharing network studies using administrative data. Transl. Behav. Med. 2018, 8, 598–625. [Google Scholar] [CrossRef] [PubMed]
- Landon, B.E.; Keating, N.L.; Barnett, M.L.; Onnela, J.-P.; Paul, S.; O’Malley, A.J.; Keegan, T.; Christakis, N.A. Variation in patient-sharing networks of physicians across the United States. JAMA 2012, 308, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.J.; Dillon, S.M. Authorship patterns in information systems. Scientometrics 1997, 39, 19. [Google Scholar] [CrossRef]
- Beaver, D.; Rosen, R. Studies in scientific collaboration: Part I. The professional origins of scientific co-authorship. Scientometrics 1978, 1, 65–84. [Google Scholar] [CrossRef]
- Luukkonen, T.; Tijssen, R.; Persson, O.; Sivertsen, G. The measurement of international scientific collaboration. Scientometrics 1993, 28, 15–36. [Google Scholar] [CrossRef]
- Norman, C.; Axelsson, R. Co-operation as a strategy for provision of welfare services—A study of a rehabilitation project in Sweden. Eur. J. Public Health 2007, 17, 532–536. [Google Scholar] [CrossRef]
- Uddin, S.; Hossain, L. Disaster coordination preparedness of soft-target organisations. Disasters 2011, 35, 623–638. [Google Scholar] [CrossRef]
- Uddin, S.; Hossain, L.; Kelaher, M. Effect of physician collaboration network on hospitalization cost and readmission rate. Eur. J. Public Health 2011, 22, 629–633. [Google Scholar] [CrossRef]
- Uddin, S.; Hossain, M.E.; Khan, A. Triad Census and Subgroup Analysis of Patient-Sharing Physician Collaborations. IEEE Access 2018, 6, 72233–72240. [Google Scholar] [CrossRef]
- Uddin, S.; Khan, A.; Piraveenan, M. Administrative claim data to learn about effective healthcare collaboration and coordination through social network. In Proceedings of the 2015 48th Hawaii International Conference on System Sciences, Kauai, HI, USA, 5–8 January 2015; IEEE: Piscataway, NJ, USA, 2015. [Google Scholar]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo Jr, J.L.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA 2003, 289, 2560–2571. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. An evaluation of outcome from intensive care in major medical centers. Ann. Intern. Med. 1986, 104, 410–418. [Google Scholar] [CrossRef] [PubMed]
- An, C.; O’Malley, A.J.; Rockmore, D.N.; Stock, C.D. Analysis of the US patient referral network. Stat. Med. 2018, 37, 847–866. [Google Scholar] [CrossRef] [PubMed]
- Vukmir, R.B.; Kremen, R.; Dehart, D.A.; Menegazzi, J. Compliance with emergency department patient referral. Am. J. Emerg. Med. 1992, 10, 413–417. [Google Scholar] [CrossRef]
- Roberts, R.F.; Innes, K.C.; Walker, S.M. Introducing ICD-10-AM in Australian hospitals. Med J. Aust. 1998, 169, S32–S35. [Google Scholar] [CrossRef]
- Khan, A.; Uddin, S.; Srinivasan, U. Comorbidity network for chronic disease: A novel approach to understand type 2 diabetes progression. Int. J. Med. Inform. 2018, 115, 1–9. [Google Scholar] [CrossRef]
- Elixhauser, A.; Steiner, C.; Harris, D.R.; Coffey, R.M. Comorbidity measures for use with administrative data. Med. Care 1998, 36, 8–27. [Google Scholar] [CrossRef]
- Khan, A.; Srinivasan, U.; Uddin, S. Development and exploration of polymedication network from pharmaceutical and medicare benefits scheme data. In Proceedings of the Australasian Computer Science Week Multiconference, Sydney, NSW, Australia, 29–31 January 2019; ACM: New York, NY, USA, 2019. [Google Scholar]
- Wasserman, S.; Faust, K. Social Network Analysis: Methods and Applications; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Granovetter, M. The strength of weak ties. Am. J. Sociol. 1973, 78, 1360–1380. [Google Scholar] [CrossRef]
- Robins, G.; Pattison, P.; Kalish, Y.; Lusher, D. An introduction to exponential random graph (p*) models for social networks. Soc. Netw. 2007, 29, 173–191. [Google Scholar] [CrossRef]
- Uddin, S.; Hamra, J.; Hossain, L. Mapping and modeling of physician collaboration network. Stat. Med. 2013, 32, 3539–3551. [Google Scholar] [CrossRef]
- Barnett, M.L.; Landon, B.E.; O’Malley, A.J.; Keating, N.L.; Christakis, N.A. Mapping physician networks with self-reported and administrative data. Health Serv. Res. 2011, 46, 1592–1609. [Google Scholar] [CrossRef]
- Pollack, C.E.; Weissman, G.E.; Lemke, K.W.; Hussey, P.S.; Weiner, J.P. Patient sharing among physicians and costs of care: A network analytic approach to care coordination using claims data. J. Gen. Intern. Med. 2013, 28, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Khan, A.; Hossain, L.; Piraveenan, M.; Carlsson, S. A topological framework to explore longitudinal social networks. Comput. Math. Organ. Theory 2015, 21, 48–68. [Google Scholar] [CrossRef]
- Uddin, S. Exploring the impact of different multi-level measures of physician communities in patient-centric care networks on healthcare outcomes: A multi-level regression approach. Sci. Rep. 2016, 6, 20222. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Hossain, L. Social networks in exploring healthcare coordination. Asia Pac. J. Health Manag. 2014, 9, 53–62. [Google Scholar]
- Abbasi, A.; Uddin, S.; Hossain, L. Socioeconomic analysis of patient-centric networks: Effects of patients and hospitals’ characteristics and network structure on hospitalization costs. Eur. J. Health Econ. 2012, 13, 267–276. [Google Scholar] [CrossRef]
- Caricati, L.; Mancini, T.; Sollami, A.; Guidi, C.; Prandi, C.; Bianconcini, M.; Silvano, R.; Taffurelli, C.; Artioli, G. Nurse-physician collaboration scale: A contribution to the italian validation. TPM-Test. Psychom. Methodol. Appl. Psychol. 2013, 20, 263–276. [Google Scholar]
- Tschannen, D.; Kalisch, B.J. The impact of nurse/physician collaboration on patient length of stay. J. Nurs. Manag. 2009, 17, 796–803. [Google Scholar] [CrossRef][Green Version]
- Yao, N.; Zhu, X.; Dow, A.; Mishra, V.K.; Phillips, A.; Tu, S.-P. An exploratory study of networks constructed using access data from an electronic health record. J. Interprof. Care 2018, 32, 666–673. [Google Scholar] [CrossRef]
- DeMik, D.E.; Vander Weg, M.W.; Lundt, E.S.; Coffey, C.S.; Ardery, G.; Carter, B.L. Using theory to predict implementation of a physician–pharmacist collaborative intervention within a practice-based research network. Res. Soc. Adm. Pharm. 2013, 9, 719–730. [Google Scholar] [CrossRef]
- Donker, T.; Wallinga, J.; Grundmann, H. Patient referral patterns and the spread of hospital-acquired infections through national health care networks. PLoS Comput. Biol. 2010, 6, e1000715. [Google Scholar] [CrossRef]
- Khan, A.; Uddin, S.; Srinivasan, U. Understanding chronic disease comorbidities from baseline networks: Knowledge discovery utilising administrative healthcare data. In Proceedings of the Australasian Computer Science Week Multiconference, Geelong, Australia, 31 January–3 February 2017; ACM: New York, NY, USA, 2017. [Google Scholar]
- Khan, A.; Uddin, S.; Srinivasan, U. Chronic disease prediction using administrative data and graph theory: The case of type 2 diabetes. Expert Syst. Appl. 2019, 136, 230–241. [Google Scholar] [CrossRef]
- Hossain, M.E.; Uddin, S. Understanding the comorbidity of multiple chronic diseases using a network approach. In Proceedings of the Australasian Computer Science Week Multiconference, Sydney, NSW, Australia, 29–31 January 2019; ACM: New York, NY, USA, 2019. [Google Scholar]
- Zamora, M.; Baradad, M.; Amado, E.; Cordomí, S.; Limón, E.; Ribera, J.; Arias, M.; Gavaldà, R. Characterizing chronic disease and polymedication prescription patterns from electronic health records. In Proceedings of the 2015 IEEE International Conference on Data Science and Advanced Analytics (DSAA), Paris, France, 19–21 October 2015; IEEE: Piscataway, NJ, USA, 2015. [Google Scholar]
- Liu, Q.; Liu, J.; Wang, P.; Zhang, Y.; Li, B.; Yu, Y.; Dang, H.; Li, H.; Zhang, X.; Wang, Z. Poly-dimensional network comparative analysis reveals the pure pharmacological mechanism of baicalin in the targeted network of mouse cerebral ischemia. Brain Res. 2017, 1666, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Medhekar, R.; Aparasu, R.; Bhatara, V.; Johnson, M.; Alonzo, J.; Schwarzwald, H.; Chen, H. Risk factors of psychotropic polypharmacy in the treatment of children and adolescents with psychiatric disorders. Res. Soc. Adm. Pharm. 2019, 15, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Pieroni, S.; Fortunato, L.; Molinaro, S.; Liebman, M. Poly-pharmacy among the elderly: Analyzing the co-morbidity of hypertension and diabetes. Curr. Pharm. Des. 2015, 21, 791–805. [Google Scholar] [CrossRef]
- Carter, B.L.; Bergus, G.R.; Dawson, J.D.; Farris, K.B.; Doucette, W.R.; Chrischilles, E.A.; Hartz, A.J. A cluster randomized trial to evaluate physician/pharmacist collaboration to improve blood pressure control. J. Clin. Hypertens. 2008, 10, 260–271. [Google Scholar] [CrossRef]
- Carter, B.L.; Ardery, G.; Dawson, J.D.; James, P.A.; Bergus, G.R.; Doucette, W.R.; Chrischilles, E.A.; Franciscus, C.L.; Xu, Y. Physician and pharmacist collaboration to improve blood pressure control. Arch. Intern. Med. 2009, 169, 1996–2002. [Google Scholar] [CrossRef]
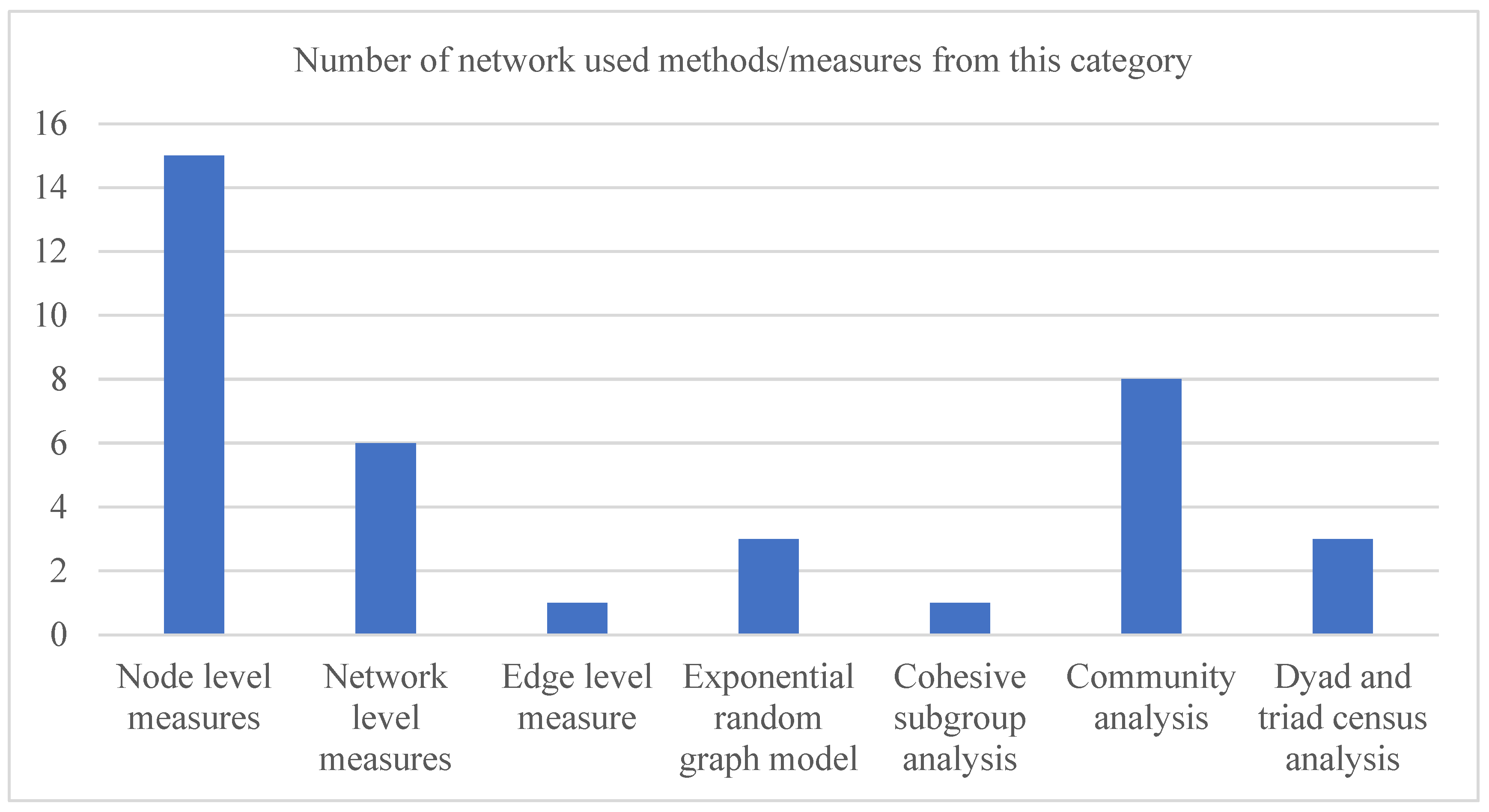
| Aspects | Methods and Measures | Definition |
|---|---|---|
| Node level measure | Degree, closeness, betweenness, eigenvector and other similar measures | Degree centrality: It depicts the number of ties a node (or actor) has with other nodes in a network. It can be of two types (in-degree and out-degree) in a directed network [30]. Closeness centrality: For a node, it represents the extent it is close to the remaining nodes in a network [30]. Betweenness centrality: It represents the extent an actor is in a favoured position in terms of falling on the shortest paths between other actor pairs in the network [30]. Eigenvector: It measures the influence of a node in a network and can distinguish the degree centrality from cases where nodes having a wide range of degree values are connected [30]. |
| Network level measure | Network centralization, density, network diameter and other similar measures | Network centralization: The centralization of a network indicates how central its most central node is compared with how central other nodes within that network are [30]. Network density: It represents the ratio between the number of existing links in a network and the total number of possible links that can be presented among all network actors [30]. Network diameter: It represents the size of the largest path in a network [30]. |
| Edge level measure | Tie strength | Tie strength: It represents the strength of relation between a pair of actors in a network [30] and can be quantified from their duration of relation and the reciprocal services (that specify their tie) they have in common [31]. |
| Exponential random graph model | This model and its different variants | Exponential random graph model: It is a probabilistic model that can identify the building blocks of a given network with respect to different micro-level network substructures (e.g., dyad, triangle and 3-star) [32]. |
| Cohesive subgroup analysis | Clique, clan, n-clique, n-clan and other similar measures | Clique: A clique is a group of actors or nodes in a network that are directly connected with each other [30]. n-clique: An n-clique is also a clique where all member nodes are reachable to each other through at most (n-1) intermediate member nodes [30]. n-clan: An n-clan is also a clique where all member nodes are reachable to each other through at most (n-1) intermediate nodes [30]. The intermediate nodes may or may not be a member of the clique. |
| Community analysis | Community detection | Community detection: It helps to identify a group of nodes in a network that are densely connected among themselves but sparsely connected with other nodes of that network [30]. |
| Dyad and triad census analysis | Dyad and triad census | A dyad is a subgraph comprising two nodes or actors, while a triad is a subgraph consisting of three actors. Both dyads and triads can be formed with or without any links between their member actors [30]. Various dyadic and triadic structures (known as dyad and triad census) are used to explore the building block of networks. |
| Network Type | Research Question(s) | Reference |
|---|---|---|
| Physician collaboration network (PCN) |
| Uddin et al. [33] |
| Uddin et al. [19] | |
| Barnett et al. [34] | |
| Uddin et al. [1] | |
| Uddin et al. [20] | |
| Landon et al. [13] | |
| Pollack et al. [35] | |
| Patient-centric care coordination network |
| Uddin et al. [36] |
| Uddin [37] | |
| Uddin and Hossain [38] | |
| Uddin and Hossain [6] | |
| Abbasi et al. [39] | |
| Uddin et al. [21] | |
| Physician –nurse collaboration network |
| Caricati et al. [40] |
| Tschannen and Kalisch [41] | |
| Yao et al. [42] | |
| Physician-pharmacist collaboration network |
| DeMik et al. [43] |
| Patient referral network |
| An et al. [24] |
| Vukmir et al. [25] | |
| Donker et al. [44] | |
| Disease network |
| Khan et al. [45] |
| Khan et al. [46] | |
| Khan et al. [27] | |
| Hossain and Uddin [47] | |
| Polymedication network |
| Khan et al. [29] |
| Zamora et al. [48] | |
| Liu et al. [49] | |
| Medhekar et al. [50] | |
| Franchini et al. [51] |
| Network Type | Reference | Network Methods/Measures Used | Key Findings |
|---|---|---|---|
| Physician collaboration network (PCN) | Uddin et al. [33] | Exponential random graph model |
|
| Uddin et al. [19] | Network centralization |
| |
| Barnett et al. [34] | Community detection |
| |
| Uddin et al. [1] | Exponential random graph model |
| |
| Uddin et al. [20] | Triad census, Clique and Clan |
| |
| Landon et al. [13] | Network centrality |
| |
| Patient-centric care coordination network | Uddin et al. [36] | Closeness centrality |
|
| Uddin [37] | Community detection |
| |
| Uddin and Hossain [38] | Dyad and Network centrality |
| |
| Uddin and Hossain [6] | Connectedness, Degree centrality and Tie strength |
| |
| Abbasi et al. [39] | Network centrality |
| |
| Uddin et al. [21] | Network centrality and Exponential random graph model |
| |
| Physician–nurse collaboration network | Caricati et al. [40] | Community detection |
|
| Tschannen and Kalisch [41] | Network centrality |
| |
| Yao et al. [42] | Network centrality |
| |
| Physician–pharmacist collaboration network | DeMik et al. [43] | Community detection |
|
| Patient referral network | An et al. [24] | Network centrality and Triad census |
|
| Vukmir et al. [25] | Community detection |
| |
| Donker et al. [44] | Degree centrality and Community detection |
| |
| Disease network | Khan et al. [45] | Network centrality |
|
| Khan et al. [46] | Network centrality |
| |
| Khan et al. [27] | Network centrality |
| |
| Hossain and Uddin [47] | Network centrality |
| |
| Polymedication network | Khan et al. [29] | Network centrality |
|
| Zamora et al. [48] | Betweenness centrality and Community detection |
| |
| Liu et al. [49] | Network centrality |
| |
| Medhekar et al. [50] | Community detection |
| |
| Franchini et al. [51] | Network density |
|
| Network Type | Strength | Weakness |
|---|---|---|
| Physician collaboration network |
|
|
| Patient-centric care coordination network |
|
|
| Physician–nurse collaboration network |
|
|
| Physician–pharmacist collaboration network |
|
|
| Patient referral network |
|
|
| Disease network |
|
|
| Polymedication network |
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karim, S.; Uddin, S.; Imam, T.; Moni, M.A. A Systematic Review of Network Studies Based on Administrative Health Data. Int. J. Environ. Res. Public Health 2020, 17, 2568. https://doi.org/10.3390/ijerph17072568
Karim S, Uddin S, Imam T, Moni MA. A Systematic Review of Network Studies Based on Administrative Health Data. International Journal of Environmental Research and Public Health. 2020; 17(7):2568. https://doi.org/10.3390/ijerph17072568
Chicago/Turabian StyleKarim, Shakir, Shahadat Uddin, Tasadduq Imam, and Mohammad Ali Moni. 2020. "A Systematic Review of Network Studies Based on Administrative Health Data" International Journal of Environmental Research and Public Health 17, no. 7: 2568. https://doi.org/10.3390/ijerph17072568
APA StyleKarim, S., Uddin, S., Imam, T., & Moni, M. A. (2020). A Systematic Review of Network Studies Based on Administrative Health Data. International Journal of Environmental Research and Public Health, 17(7), 2568. https://doi.org/10.3390/ijerph17072568








