Xerostomia and Salivary Flow in Patients Taking Antihypertensive Drugs
Abstract
1. Introduction
2. Materials and Methods
2.1. Focused Question
2.2. Search Strategy
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Data Collection and Extraction
2.6. Risk of Bias in Individual Studies
2.7. Categorization of Studies
2.8. Synthesis of the Results
3. Results
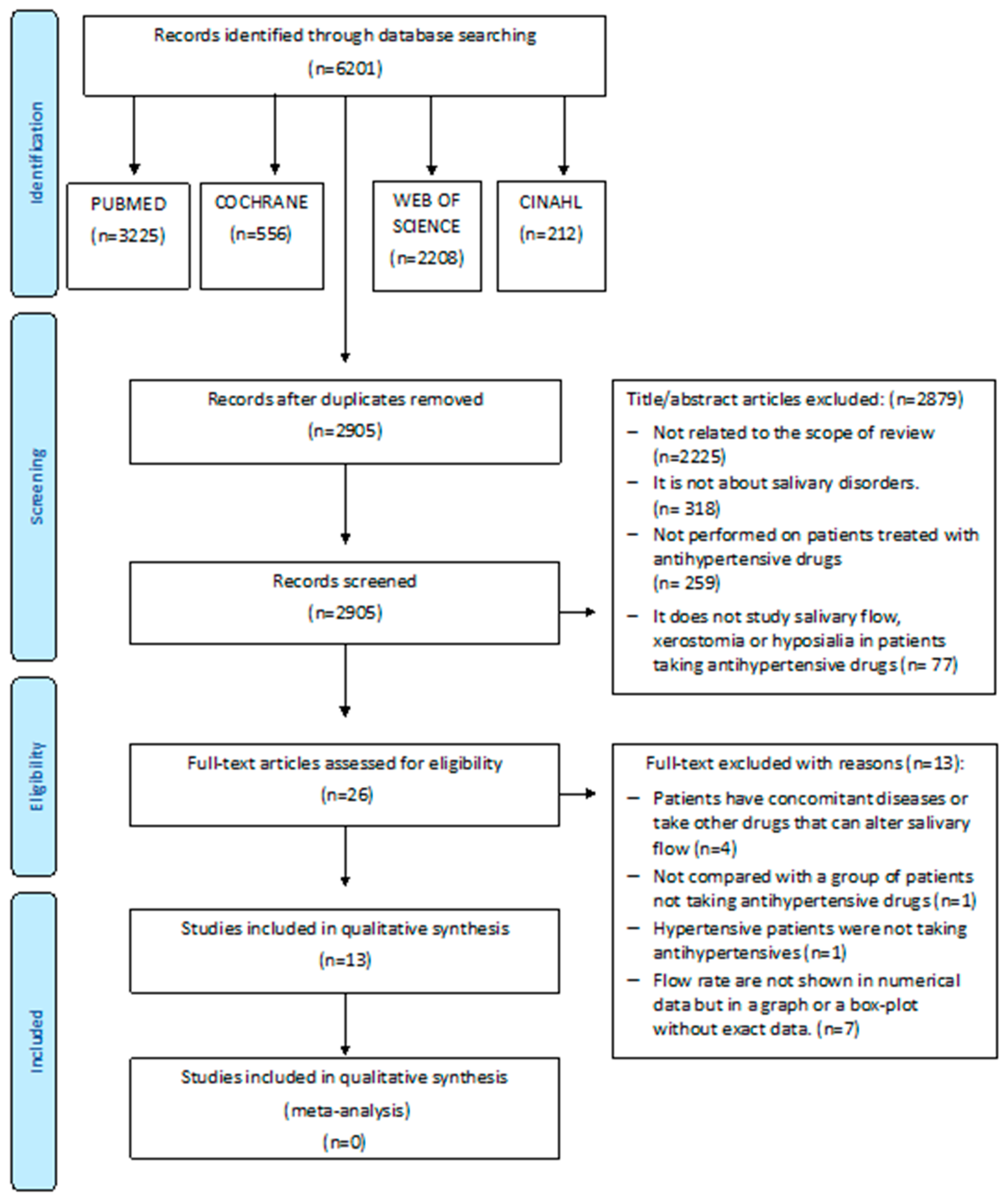
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias within Studies
3.4. Main Findings
3.4.1. Clinical Trials (Table 4)
3.4.2. Case-Control Studies (Table 5)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redón, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension, the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2013, 31, 1281–1357. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; De Palma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation and management of high blood pressure in adults, executive summary, a report of the American college of cardiology/American Heart Association task force on clinical practice guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration. Worldwide trends in blood pressure from 1975 to 2015, a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet 2017, 389, 37–55. [Google Scholar] [CrossRef]
- De Palma, S.M.; Himmelfarb, C.D.; MacLaughlin, E.J.; Taler, S.J. Hypertension guideline update: A new guideline for a new era. J. Am. Acad. Phys. 2018, 31, 16–22. [Google Scholar]
- Niklander, S.; Veas, L.; Barrera, C.; Fuentes, F.; Chiappini, G.; Marshall, M. Risk factors, hyposalivation and impact of xerostomia on oral health-related quality of life. Braz. Oral Res. 2017, 31, e14. [Google Scholar] [CrossRef]
- Carramolino-Cuéllar, E.; Lauritano, D.; Silvestre, F.J.; Carinci, F.; Lucchese, A.; Silvestre-Rangil, J. Salivary flow and xerostomia in patients with type 2 diabetes. J. Oral Pathol. Med. 2018, 47, 526–530. [Google Scholar] [CrossRef]
- Proctor, G.B. The physiology of salivary secretion. Periodontology 2016, 70, 11–25. [Google Scholar] [CrossRef]
- Villa, A.; Connell, C.L.; Abati, S. Diagnosis and management of xerostomia and hyposalivation. Clin. Risk Manag. 2014, 11, 45–51. [Google Scholar] [CrossRef]
- Aliko, A.; Wolff, A.; Dawes, C.; Aframian, D.; Proctor, G.; Ekström, J.; Narayana, N.; Villa, A.; Sia, Y.W.; Joshi, R.K.; et al. World Workshop on Oral Medicine VI, clinical implications of medication-induced salivary gland dysfunction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 120, 185–206. [Google Scholar] [CrossRef]
- Prasanthi, B.; Kannan, N.; Patil, R. Effect of diuretics on salivary flow.; composition and oral health status, A clinico-biochemical study. Ann. Med. Health Sci. Res. 2014, 4, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Saleh, J.; Figueiredo, M.A.Z.; Cherubini, K.; Salum, F.G. Salivary hypofunction: An update on aetiology, diagnosis and therapeutics. Arch. Oral Biol. 2015, 60, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Djukić, L.; Roganović, J.; Brajović, M.; Bokonjić, D.; Stojić, D. The effects of anti-hypertensives and type 2 diabetes on salivary flow and total antioxidant capacity. Oral Dis. 2015, 21, 619–625. [Google Scholar] [CrossRef]
- Higgins, J.; Altman, D.; Gøtzsche, P.; Jüni, P.; Moher, D.; Oxman, A.; Savović, J.; Schulz, K.F.; Weeks, L. The Cochrane´s collaboration tool for assessing risk of bias in randomized trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.; Huskisson, E.C. Graphic representation of pain. J. Pain 1976, 2, 175–184. [Google Scholar] [CrossRef]
- Fox, P.C.; Busch, K.A.; Baum, B.J. Subjective reports of xerostomia and objective measures of salivary gland performance. J. Am. Dent. Assoc. 1987, 115, 581–584. [Google Scholar] [CrossRef]
- De la luz, M.; Barrios, B. Salivary flow and the prevalence of xerostomia in geriatric patients. Rev. Asoc. Dent. Mex. 2013, 70, 25–29. [Google Scholar]
- Modesti, P.A.; Reboldi, G.; Cappuccio, F.P.; Agyemang, C.; Remuzzi, G.; Rapi, S.; Perruolo, E.; Parati, G. Panethnic differences in blood pressure in Europe, a systematic review and meta-analysis. PLoS ONE 2016, 11, e0147601. [Google Scholar] [CrossRef]
- McPheeters, M.L.; Kripalani, S.; Peterson, N.B.; Idowu, R.T.; Jerome, R.N.; Potter, S.A.; Andrews, J.S. Closing the quality gap, revisiting the state of the science (vol. 3, quality improvement interventions to address health disparities). Evid. Rep. Technol. Assess. 2012, 208, 1–475. [Google Scholar]
- Nederfors, T.; Nauntofte, B.; Twetman, S. Effects of furosemide and bendroflumethiazide on saliva flow rate and composition. Arch. Oral Biol. 2004, 49, 507–513. [Google Scholar] [CrossRef]
- Muñoz, L.S.; Narváez, C.G. Salivary pH, buffer capacity, total proteins and salivary flow in controlled hypertensive patients diuretic users. Int. J. Odontostomat. 2012, 6, 11–17. [Google Scholar] [CrossRef][Green Version]
- Ivanovski, K.; Pesevka, S.; Ristoska, S.; Dirjanska, K.; Mindova, S.; Pandilova, M.; Georgieva, S.; Stefanovska, E.; Filipce, V.; Apostolska, S.; et al. The impact of antihypertensive medications on quantitative and qualitative characteristics of saliva. J. Pharm. Biol. Chem. Sci. 2015, 6, 1356–1364. [Google Scholar]
- Nimma, V.; Talla, H.; Poosa, M.; Gopaladas, M.; Meesala, D.; Jayanth, L. Influence of hypertension on pH of saliva and flow rate in elder adults correlating with oral health status. J. Clin. Diagn. Res. 2016, 10, ZC34–ZC36. [Google Scholar] [CrossRef] [PubMed]
- Streckfus, C.F.; Wu, A.J.; Ship, J.A.; Brown, L.J. Stimulated parotid salivary flow rates in normotensive, hypertensive, and hydrochlorothiazide-medicated African-Americans. J. Oral Pathol. Med. 1994, 23, 280–283. [Google Scholar] [CrossRef]
- Nonzee, V.; Manopatanakul, S.; Khovidhunkit, S.; Xerostomia, O. Hyposalivation and oral microbiota in patients using antihypertensive medications. J. Med. Assoc. Thail. 2012, 95, 96–104. [Google Scholar]
- Kagawa, R.; Ikebe, K.; Enoki, K.; Murai, S.; Okada, T.; Matsuda, K.; Maeda, Y. Influence of hypertension on pH of saliva in older adults. Oral Dis. 2013, 19, 525–529. [Google Scholar] [CrossRef]
- Van Hooff, M.; Van Baak, M.A.; Schols, M.; Rahn, K.H. Studies of salivary flow in borderline hypertension, effects of drugs acting on structures innervated by the autonomic nervous system. Clin. Sci. 1984, 66, 599–604. [Google Scholar] [CrossRef]
- Nederfors, T.; Dahlöf, C.; Ericsson, T.; Twetman, S. Effects of the antihypertensive drug captopril on human salivary secretion rate and composition. Eur. J. Oral Sci. 1995, 103, 351–354. [Google Scholar] [CrossRef]
- Ben-Aryeh, H.; Schiller, M.; Shasha, S.; Szargel, R.; Gutman, D. Salivary composition in patients with essential hypertension and the effect of pindolol. J. Oral Med. 1981, 36, 76–78. [Google Scholar]
- Tahrir, N.N.; Aldelaimi, B.D.S. The effect of atenolol (B- blocker) on salivary composition in patients with essential hypertension. J. Baghdad. Coll. Dent. 2006, 18, 57–59. [Google Scholar]
- Thomson, W.M.; Chalmer, J.M.; Spencer, A.J.; Willians, S.M. The Xerostomia Inventory: A multi-item approach to measuring dry mouth. Community Dent. Health 1999, 16, 12–17. [Google Scholar] [PubMed]
- Navazesh, M. Methods for collecting saliva. Ann. N. Y. Acad. Sci. 1993, 20, 694–727. [Google Scholar] [CrossRef] [PubMed]
| Author, Year and Country | Study Design | Duration | Sample | Age and Gender | Type of Salivary Flow Rate | Saliva Sampling | Hyposalivation | Xerostomia Assessment | Type of Antihypertensive |
|---|---|---|---|---|---|---|---|---|---|
| Ben-Aryeh et al., 1981, Israel [29] | Clinical trial | 6 weeks | 10 CG 10 HT | 12 female/8 male Mean age 54 years | UWS | 8–9 am Spitting method 10 min | - | - | β-Adrenergic blocker (Pindolol) |
| Van Hoof et al., 1983, The Netherlands [27] | Clinical trial | 1 day | 23 CG 19 HT | 42 Male 22–34 years | UWS | 15 min cotton-wool method by. Dollery et al. | - | - | β-Adrenergic blocker (Propanolol) α-Adrenergic blocker (Phentolamine) |
| Streckfus et al., 1994, USA [24] | Case-control study | - | 15 CG 20 HT | NT: 9 female/ 6 male Mean age 69.5 years HCTZ: 10 female/10 male Mean age 68.5 years | SPS | 8–12 am Carlson–Crittenden cups: parotid | - | - | Diuretic (HCTZ) |
| Nederfors et al., 1995, Sweden [28] | Double blind, cross-over randomized trial | 3 months | 24 Healthy patients: Placebo Captopril | 13 female/ 11 male Mean age 24 years | UWS SWS SPS SSS | 7.30–8.30 am UWS: Spitting method 5 min SWS: Spitting method paraffin chewing 5min SPS: Modified Carlson–Crittenden cups SSS: Nederfors modified device 7.30–8.30 am | - | - | ACE inhibitors (Captopril) |
| Nederfors et al., 2004, Sweden [20] | Cross- over clinical trial | 3 months | 12 Healthy patients: Placebo Thiazide Furosemide | 12 female Mean age 28 years | UWS SWS SPS SSS | UWS: Spitting method 5 min SWS: Spitting method paraffin chewing 5min SPS: Modified Carlson–Crittenden cups SSS: Nederfors modified device | - | VAS | Diuretic (Thiazide, furosemide) |
| Tahrir et al., 2006, Irak [30] | Clinical trial | 4 weeks | 48 HT treated with atenolol 48 CG | 20 male /28 female Mean age 49 years | UWS | 8–9 am Spitting method 10 min | - | - | β-Adrenergic blockers: Atenolol |
| Nonzee et al., 2012, Thailand [25] | Case-control study | - | 200 HT 200 CG | CG: 118 female/ 82 male Mean age 58.82 ± 7.84 years HT: 104 female/96 male Mean age 62.41 ± 8.75 years | SWS | 8–12 am UWS: Modified Schirmer test: 3 min SWS: Spitting method paraffin chewing 5min | Fontana et al. Hyposalivation SWS was diagnosed if the color of Schirmer text moved 25 mm at 3 min | Xerostomia questionnaire Fox et al. + VAS | β-Adrenergic blockers (Propanolol, atenolol) Diuretic (HCTZ) ACE inhibitors (Enalapril) Calcium channel blocker (Amlodipine) |
| Muñoz et al., 2012, Chile, [21] | Case-control study | - | 14 HT 10 CG | Gender not available Age not available | UWS | Not available 1 min | - | - | Diuretics |
| De la luz et al., 2013, Mexico [17] | Case-control study | - | 440 Patients: CG HT | 268 female/ 172 male Mean age 68.34 ± 6.19 years | UWS SWS | Morning UWS: Spitting method 3 min SWS: Spitting method Chewing no available 5min | UWS < 0.15 mL/min SWS < 0.5 mL/min | Modified Sreebney and Fox questionnaire | Not available |
| Kagawa et al., 2013, Japan [26] | Case-control study | - | 96 CG 9 Normotensive treated with antihypertensives drugs 18 HT | 92 female/73 male Mean age 66.6 years | UWS SWS | 10 am-3 pm UWS: Spitting method 5 min SWS: Spitting method paraffin chewing 2 min | - | - | Not available |
| Prasanthi et al., 2014, India [11] | Case-control study | - | 50 CG 50 HT | CG 27 female/23 male Mean age 43.9 ±2.4 years HT 23 female/27 male Mean age 46.3 ±2.7 years | UWS SWS | 9–10 am UWS: Spitting method 5 min SWS: Spitting method paraffin chewing 5 min | UWS<0.3 mL/min SWS<0.7 mL/min | - | Diuretics |
| Ivanovski et al., 2015, Republic of Macedonia [22] | Case-control study | - | 30 CG 30 HT | Gender not available 30–70 years | UWS | Navazesh method 10 min. | USW<0.2 mL/min | - | Diuretic β-Adrenergic blockers α-Adrenergic blocker ACE inhibitors Calcium channel blocker Heart glycosides Antihypertensives drugs with central effect |
| Nimma et al. 2016, India [23] | Case-control study | - | 20 CG 20 HT | Gender not available 60–75 years | UWS SWS | Moment no available UWS: Spitting method 5 min SWS: Spitting method paraffin chewing 5 min | - | - | Not available |
| Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Other Bias | Quality | |
|---|---|---|---|---|---|---|---|---|
| Ben-Aryeh 1981 | High | High | High | High | Unclear | Unclear | Unclear | Poor quality |
| Van Hoof et al. 1983 | Unclear | Unclear | High | Unclear | Unclear | Unclear | Unclear | Poor quality |
| Nederfors et al. 1995 | Unclear | Unclear | Low | Unclear | Unclear | Low | Unclear | Poor quality |
| Nederfors et al. 2004 | Unclear | Unclear | Low | Unclear | Unclear | Low | Unclear | Poor quality |
| Tahir et al. 2006 | High | High | High | High | Unclear | Unclear | Unclear | Poor quality |
| Selection (1) | Selection (2) | Selection (3) | Selection (4) | Comparability (1) | Outcome (1) | Outcome (2) | Score | Quality | |
|---|---|---|---|---|---|---|---|---|---|
| Streckfus et al. 1994b [24] | * | - | - | * | * | ** | * | 6/10 | Fair |
| Nonzee et al. 2012 [25] | * | - | - | - | * | ** | * | 5/10 | Poor |
| Muñoz et al. 2012 [21] | * | - | - | - | * | * | * | 4/10 | Poor |
| De la luz et al. 2013 [17] | - | - | - | - | * | ** | * | 4/10 | Poor |
| Kagawa et al. 2013 [26] | * | - | - | * | * | ** | * | 6/10 | Fair |
| Prasanthi et al. 2014 [11] | * | - | - | - | * | * | * | 4/10 | Poor |
| Ivanovski et al 2015 [22] | * | - | - | * | * | ** | * | 6/10 | Fair |
| Nimma et al. 2016 [23] | - | - | - | * | * | * | * | 4/10 | Poor |
| Author/Year/Country | Antihypertensive Medications | Salivary Flow Rate (mL/min) (g/min) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | No Treatment | Placebo | Results | ||||||||
| Before | After | Before | After | ||||||||
| Ben-Aryeh et al., 1981, Israel [29] | β-Adrenergic blocker (Pindolol) | UWS: 0.24 ± 0.14 | 3 h | 24 h | 6 weeks | UWS: 0.39 ± 0.18 | The UWS flow rate increased, no significantly, in HT patients treated with pinilol. However CG salivary flow were higher than experimental group. | ||||
| 0.31 ± 0.11 | 0.27 ± 0.2 | 0.36 ± 0.15 | |||||||||
| Van Hoof et al. 1983 The Netherlands [27] | Intravenous injection of propranolol | 1 mg | 5 mg | NT: USW: 0.85 ± 0.08 * BHT: USW: 0.51 ± 0.04 * | UWS flow rate significantly decreased in NT patients treatment with propranolol and phentolamine. Salivary UWS flow rate was significantly lower in BHT patients no treatment than normotensive patients. | ||||||
| NT: UWS: 0.88 ± 0.15 | 0.72 ± 0.12** | 0.77 ± 0.12 | |||||||||
| BHT: UWS: 0.34 ± 0.04 | 0.32 ± 0.05 | 0.34 ± 0.04 | |||||||||
| Intravenous injection of phentolamine | 1 mg | 5 mg | |||||||||
| NT: UWS: 0.88 ± 0.15 | 0.74 ± 0.14** | 0.77 ± 0.11 | |||||||||
| BHT: UWS: 0.54 ± 0.14 | 0.47 ± 0.10 | 0.53 ± 0.10 | |||||||||
| Nederfors et al. 1995 Sweden [28] | ACE inhibitors (Captopril) | UWS:0.59 ± 0.24 SWS:1.67 ± 0.57 SPS:1.41 ± 0.77 SSS:1.39 ± 0.51 | Day 1 | Day 7 | - | UWS: 0.64 ± 0.57 SWS: 1.84 ± 0.60 SPS: 1.61 ± 0.82 SSS: 1.35 ± 0.63 | Day 1 | Day 7 | SPS is significantly higher in patients treated with captopril | ||
| UWS: 0.65 ± 0.27 SWS: 1.79 ± 0.47 SSP: 1.44 ± 0.84 ** SSS: 1.38 ± 0.71 | UWS: 0.69 ± 0.69 SWS: 1.85 ± 0.46 SSP: 1.86 ± 0.91** SSS: 1.41 ± 0.62 | UWS: 0.65 ± 0.29 SWS: 1.95 ± 0.72 SPS: 1.62 ± 0.70 SSS: 1.57 ± 0.64 | UWS: 0.62 ± 0.28 SWS: 1.81 ± 0.68 SPS: 1.56 ± 0.87 SSS: 1.57 ± 0.74 | ||||||||
| Nederfors et al. 2004 Sweden [20] | Diuretic (Thiazide, furosemide) | Day 7 | Day 7 | SSS was significantly affected, statistically (P < 0.05) decreased in the morning during chronic treatment with both drugs. The percentage reduction in SSS was 26 and 24% for bendroflumethiazide and furosemide, respectively. | |||||||
| Furosemide UWS: 0.31 ± 0.12 SWS: 1.37 ± 0.54 SPS: 0.81 ± 0.44 SSS: 1.41 ± 0.57 | Bendroflumethiazide UWS: 0.34 ± 0.14 SWS: 1.34 ± 0.39 SPS: 0.76 ± 0.44 SSS: 1.27 ± 0.54 | Furosemide UWS: 0.29 ± 0.09 SWS: 1.34 ± 0.42 SPS: 0.83 ± 0.53 SSS: 1.05 ± 0.46** | Bendroflumethiazide UWS: 0.30 ± 0.14 SWS: 1.29 ± 0.49 SPS: 0.76 ± 0.37 SSS: 0.96 ± 0.57** | UWS: 0.31 ± 0.12 SWS: 1.40 ± 0.39 SPS: 0.67 ± 0.12 SSS: 1.18 ± 0.56 | UWS: 0.37 ± 0.20 SWS: 1.36 ± 0.37 SPS: 0.75 ± 0.42 SSS: 1.12 ± 0.49 | ||||||
| Tahrir et al. 2006 Irak [30] | β-Adrenergic blockers: Atenolol | UWS 0.24 ± 0.14 | 24 h | 1 week | 4 weeks | UWS: 0.38 ± 0.18 | The UWS flow rate increased, not significantly, in HT patients treated with atenolol | ||||
| 0.26 ± 0.11 | 0.28 ± 0.2 | 0.33 ± 0.15 | |||||||||
| Author/Year | Antihypertensive Medications | UWS (mL/min) | SWS (mL/min) | SPS (mL/min) | Hyposalivation Salivary Flow Rate | Hyposalivation (%) | Xerostomia (%) | Level of Xerostomia (cm) | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Streckfus et al., 1994b, USA [24] | Diuretic (HCTZ) | - | - | CG: 0.695 ± 0.44 HT: 0.685 ± 0.39 HCTZ: 0.422 ± 0.24 | Not available | - | - | - | 0.02 |
| Nonzee et al., 2012, Thailand [25] | β-Adrenergic blockers (Propanolol, atenolol) Diuretic (HCTZ) ACE inhibitor (Enalapril) Calcium channel blocker (Amlodipine) | - | CG: 1.31 ± 0.34 HT: 0.73 ± 0.30 | - | SWS hyposalivation was diagnosed if the color moved 25 mm at 3 min according to Fontana et al. | CG: 5% HT: 57% | CG: 25.5% HT: 50% | CG: 1.53 ± 1.89 HT: 3.32 ± 2.72 | 0.05 |
| Muñoz et al., 2012, Chile [21] | Diuretics | CG: 1.92 ± 0.40 HT: 0.57 ± 0.29 | - | - | Not available | - | - | - | 0.13 |
| De la luz et al., 2013, Mexico [17] | Not available | CG: 0.31 ± 0.17 HT: 0.27 ± 0.17 | CG: 1.33 ± 0.70 HT: 1.12 ± 0.62 | - | UWS < 0.15 mL/min SWS < 0.5 mL/min | - | CG: 12.7% HT: 23.6% | UWS: 0.023 SWS: 0.001 Xerostomia 0.001 | |
| Kagawa et al., 2013, Japan [26] | Not available | CG: 0.32 (0.19–0.51) HT: 0.35 (0.23–0.57) | CG: 1.66 (1.18–2.39) HT: 1.53 (1.01–2.07) | - | Not available | - | - | - | UWS: 0.85 SSS: 0.39 |
| Prasanthi et al., 2014 India [11] | Diuretics | CG: 2.16 ± 0.72 HT: 0.88 ± 0.41 | CG: 7.90 ± 1.87 HT: 2.71 ± 1.08 | - | UWS < 0.3 mL/min SWS < 0.7 mL/min | - | - | - | 0.001 |
| Ivanovski et al., 2015, Republic of Macedonia, [22] | Diuretics β-Adrenergic blockers α-Adrenergic blocker Angiotensin converting enzyme inhibitors Calcium channel blocker Heart glycosides Antihypertensives drugs with central effect | CG: 0.6 ± 0.1 HT: 0.3 ± 0.2 | - | - | USW < 0.2 mL/min | - | - | - | 0.000 |
| Nimma et al., 2016, India [23] | Not available | CG: 2.73 ± 0.68 HT: 2.58 ± 0.37 | CG: 3.30 ± 0.70 HT: 3.63 ± 0.65 | - | - | - | - | UWS: 0.13 SWS: 0.39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez Martínez-Acitores, L.; Hernández Ruiz de Azcárate, F.; Casañas, E.; Serrano, J.; Hernández, G.; López-Pintor, R.M. Xerostomia and Salivary Flow in Patients Taking Antihypertensive Drugs. Int. J. Environ. Res. Public Health 2020, 17, 2478. https://doi.org/10.3390/ijerph17072478
Ramírez Martínez-Acitores L, Hernández Ruiz de Azcárate F, Casañas E, Serrano J, Hernández G, López-Pintor RM. Xerostomia and Salivary Flow in Patients Taking Antihypertensive Drugs. International Journal of Environmental Research and Public Health. 2020; 17(7):2478. https://doi.org/10.3390/ijerph17072478
Chicago/Turabian StyleRamírez Martínez-Acitores, Lucía, Fernando Hernández Ruiz de Azcárate, Elisabeth Casañas, Julia Serrano, Gonzalo Hernández, and Rosa María López-Pintor. 2020. "Xerostomia and Salivary Flow in Patients Taking Antihypertensive Drugs" International Journal of Environmental Research and Public Health 17, no. 7: 2478. https://doi.org/10.3390/ijerph17072478
APA StyleRamírez Martínez-Acitores, L., Hernández Ruiz de Azcárate, F., Casañas, E., Serrano, J., Hernández, G., & López-Pintor, R. M. (2020). Xerostomia and Salivary Flow in Patients Taking Antihypertensive Drugs. International Journal of Environmental Research and Public Health, 17(7), 2478. https://doi.org/10.3390/ijerph17072478





