A Systematic Review and Meta-Analysis Investigating the Relationship between Exposures to Chemical and Non-Chemical Stressors during Prenatal Development and Childhood Externalizing Behaviors
Abstract
1. Introduction
2. Materials and Methods
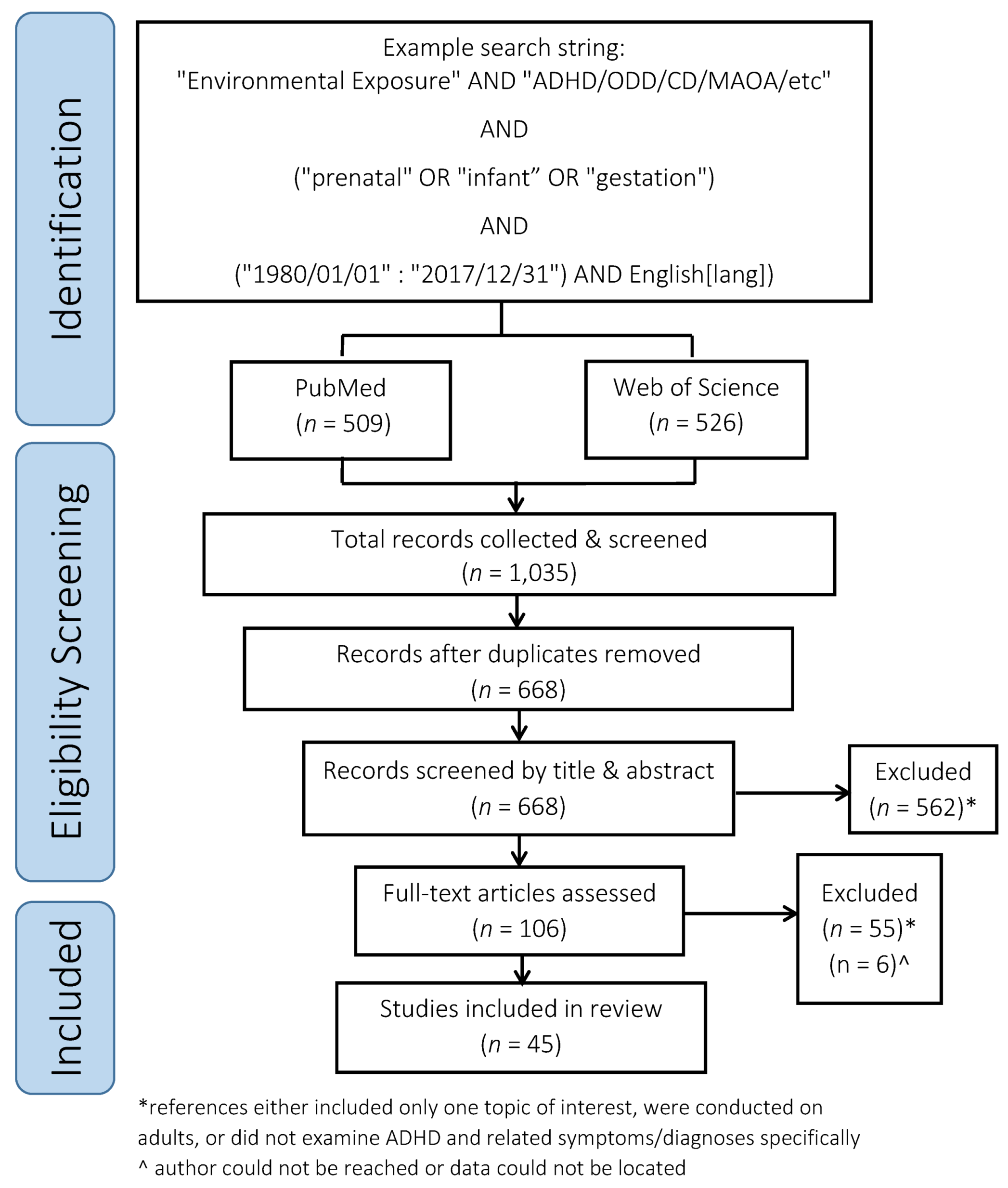
2.1. Search Details
2.2. Reference Screening Process
- Does the study include MAOA, childhood externalizing behaviors, and prenatal exposures to chemical and/or non-chemical stressors?
- Does the paper report a relationship between the topics?
- Was an externalizing behavioral health outcome the focus of this study?
2.3. Data Extraction and Analysis
2.4. Quality Assessment
2.4.1. Precision of Evidence
2.4.2. Bias
2.5. Statistical Analysis
2.5.1. Data Grouping
2.5.2. Meta-Analysis
- 0% to 40%: heterogeneity might not be important
- 30% to 60%: may represent moderate heterogeneity
- 50% to 90%: may represent substantial heterogeneity
- 75% to 100%: considerable heterogeneity [73].
2.5.3. Meta-Regression
2.5.4. Sensitivity Analysis
3. Results
3.1. Summary of Exposures to Chemical Stressors and Externalizing Behaviors Findings
3.2. Prenatal Exposures to Recreational Drugs
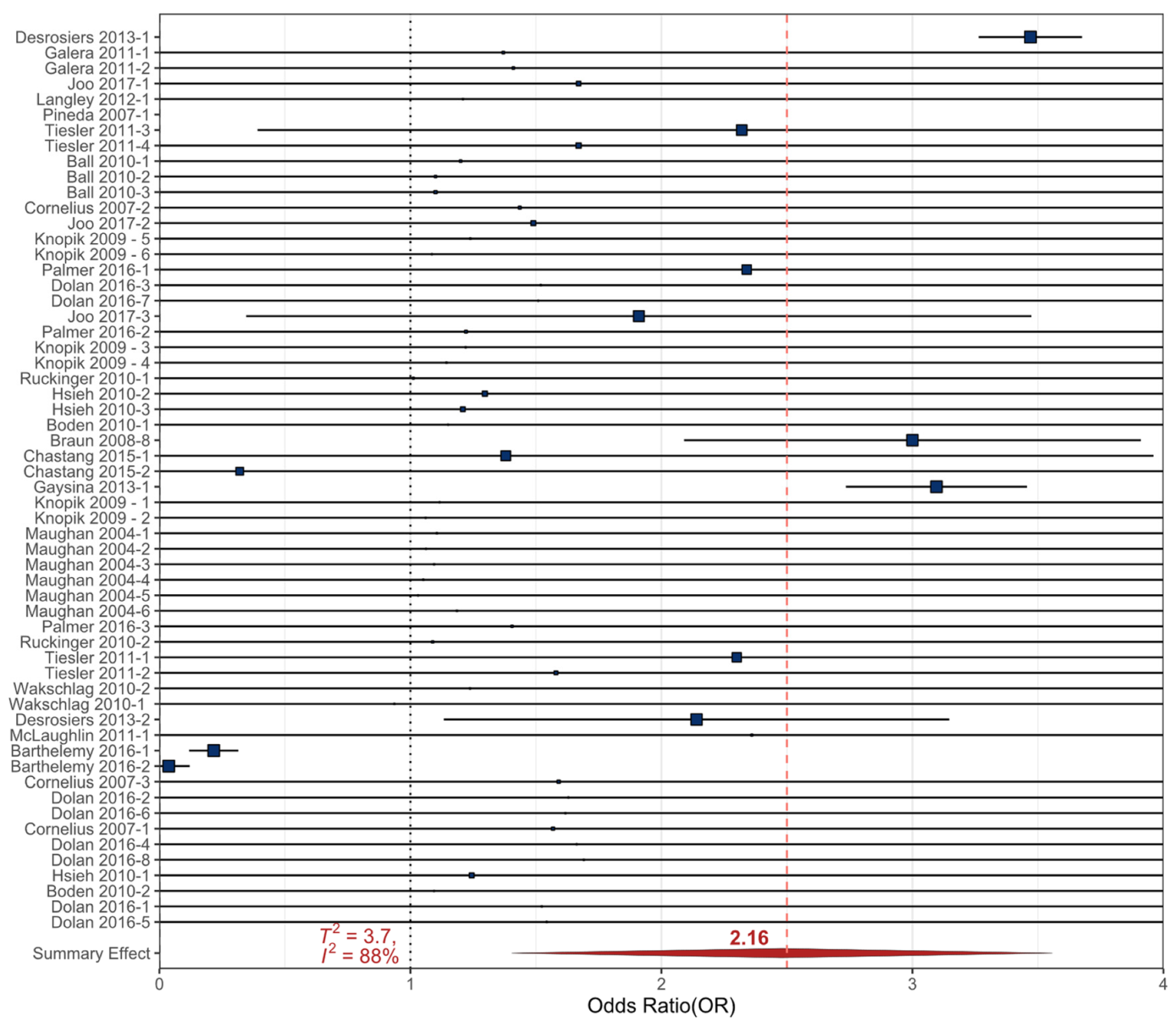
3.2.1. Cigarette Smoke
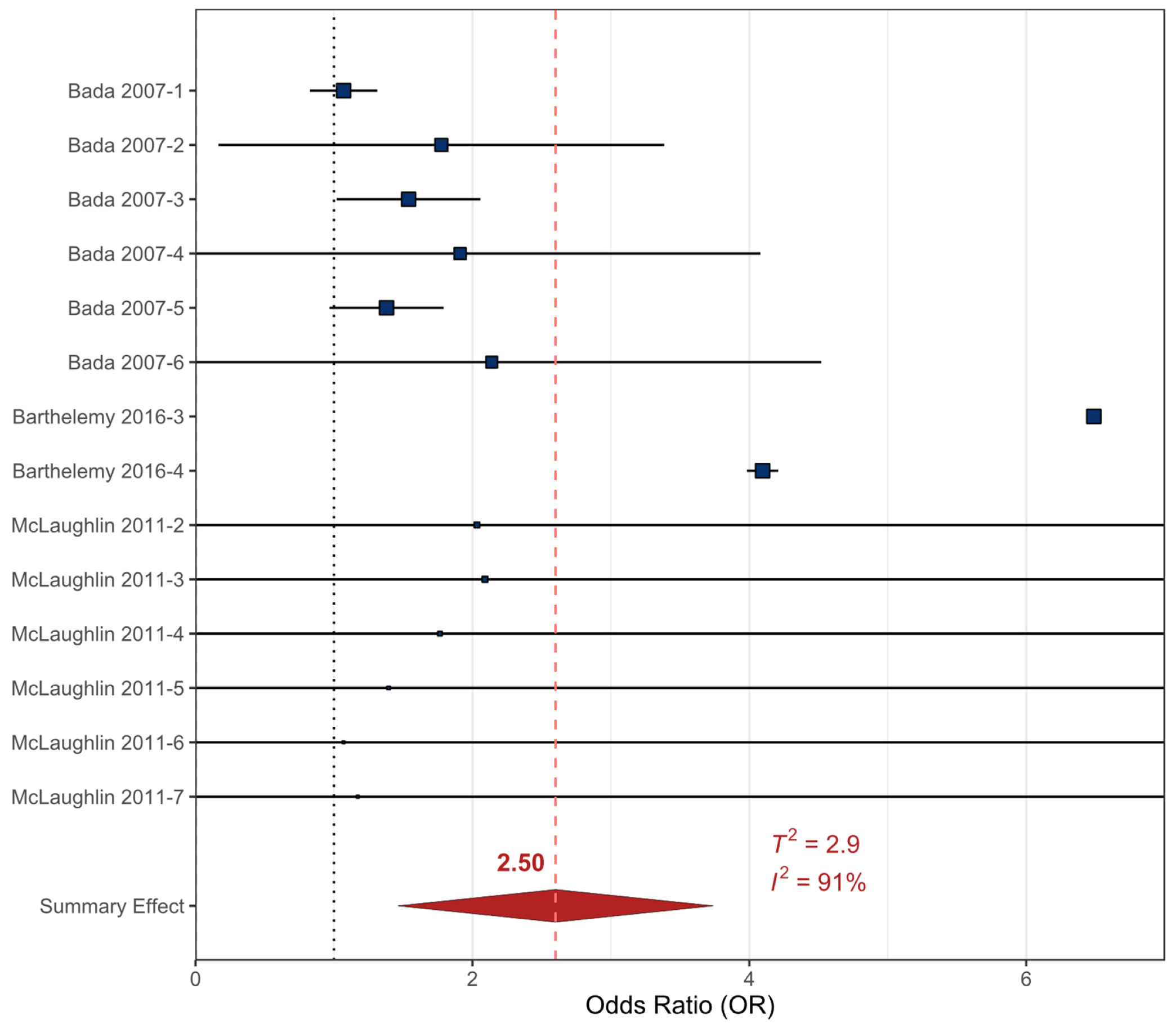
3.2.2. Cocaine
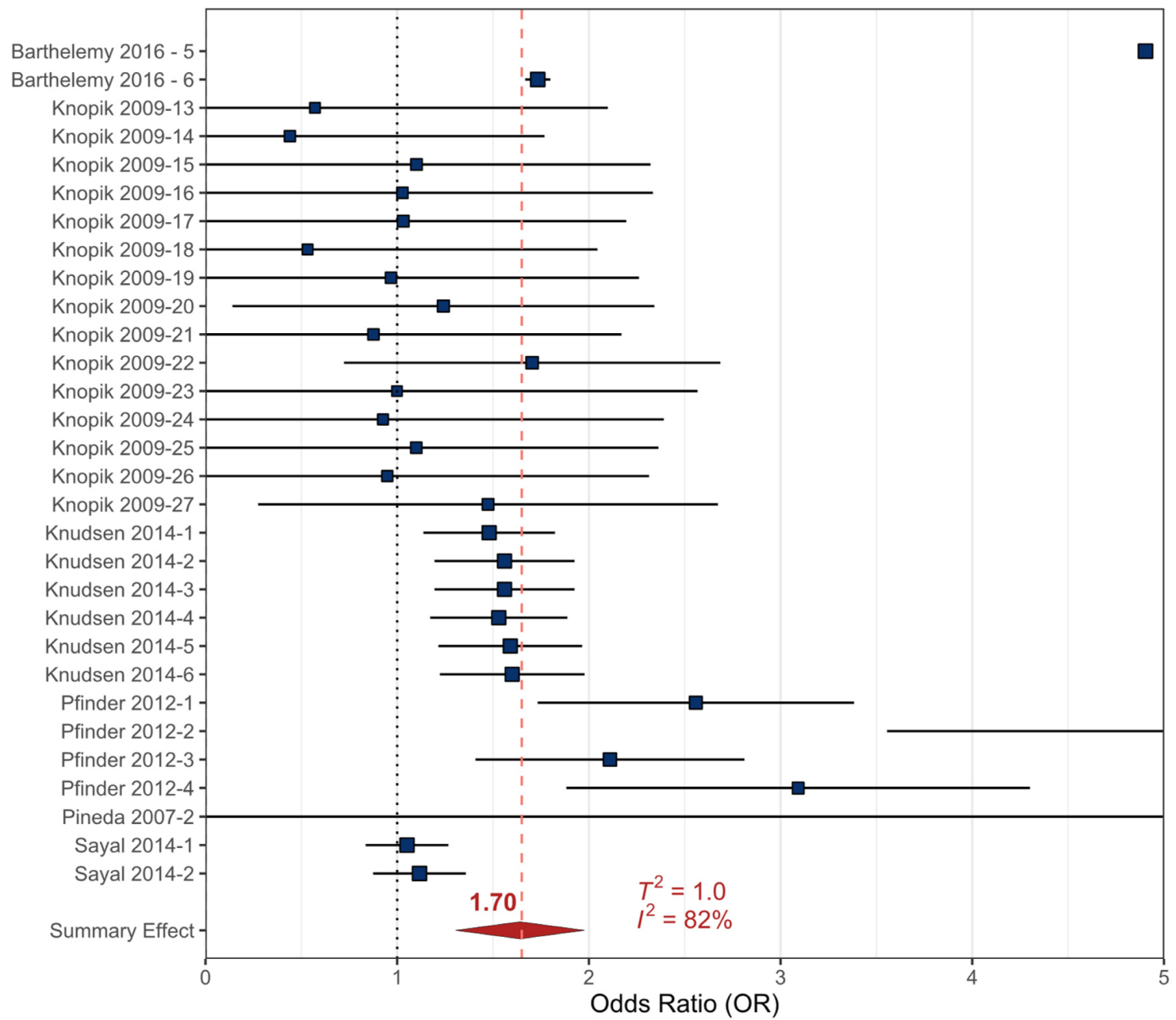
3.2.3. Alcohol
3.2.4. Marijuana, Methamphetamine, and Pharmaceuticals
3.3. Environmental Chemical Exposures
3.3.1. Phthalates/Plasticizers (PhPl)
3.3.2. Organic Contaminants (OCs)
3.3.3. Mercury (Hg)
3.3.4. Lead (Pb)
3.4. Non-Chemical Stressor Data
3.4.1. Maternal Stress during Pregnancy
3.4.2. Exposure to Prenatal Stress
3.5. MAOA Data
4. Discussion
4.1. Exposures to Chemical Stressors
4.1.1. Exposure to Recreational Drugs
4.1.2. Exposure to Environmental Contaminants
4.2. MAOA
4.3. Exposure to Non-Chemical Stressors
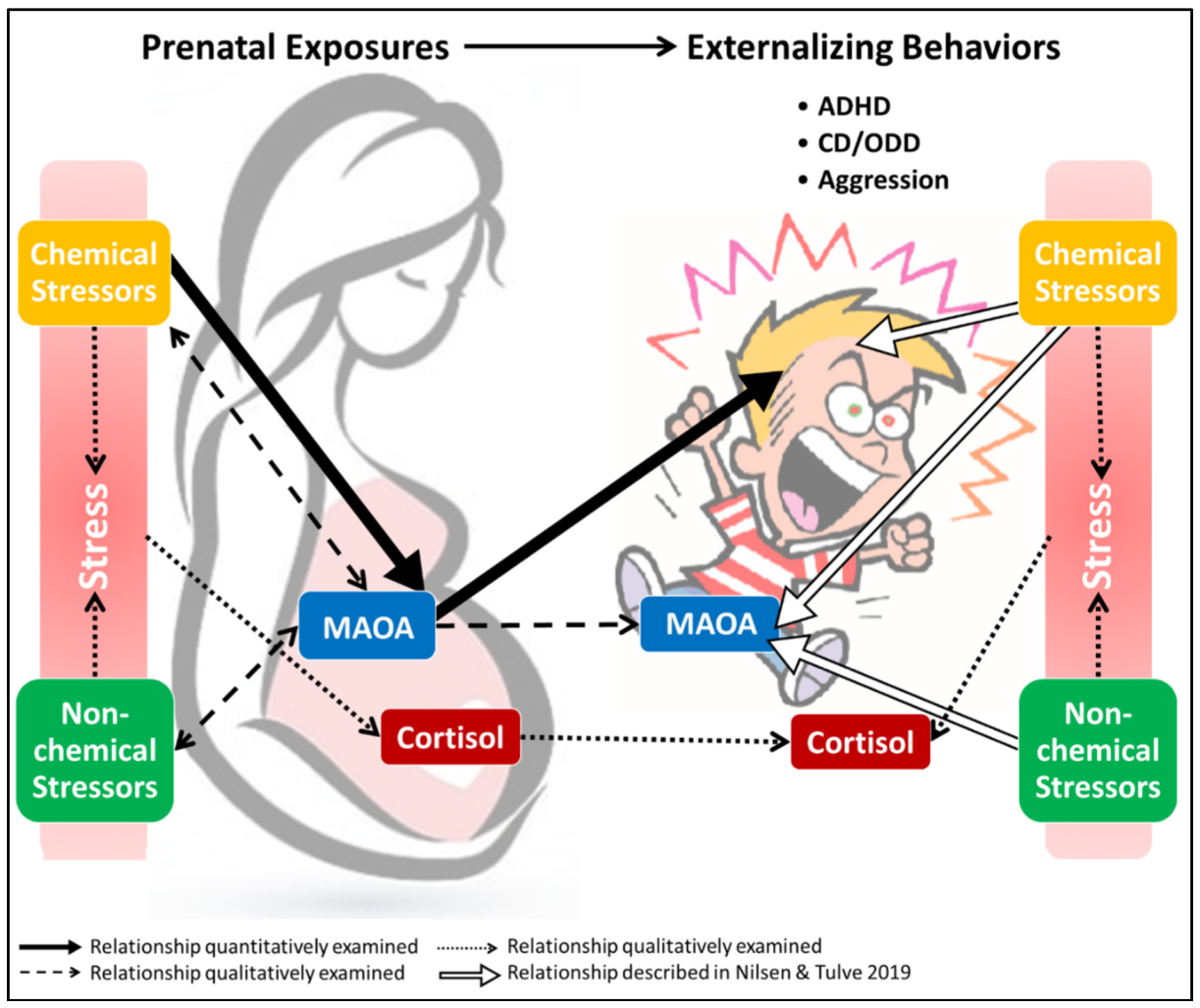
4.4. Linking Chemical and Non-Chemical Stressors with Inherent Biological Characteristics
4.4.1. Non-Chemical Stressors and Cortisol
4.4.2. Maternal Stress Transfer
4.4.3. Cortisol, Drug Use, and MAOA
4.4.4. Cortisol and Non-Chemical Stressor Evaluation
4.5. Prenatal vs. Childhood Exposures
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Search Terms: |
| PubMed: |
|
| Web of Science: |
|
| R Studio Syntax: |
Random-effects meta-analysis:
|
Forest Plot:
|
Meta-Regression:
|
Bubble Plot:
|
Sensitivity Analysis:
|
Publication Bias
|
References
- Shaw, M.; De Jong, M. Child abuse and neglect: A major public health issue and the role of child and adolescent mental health services. Psychiatrist 2012, 36, 321–325. [Google Scholar] [CrossRef]
- Swanson, J.D.; Wadhwa, P.M. Developmental origins of child mental health disorders. J. Child Psychol. Psychiatry 2008, 49, 1009–1019. [Google Scholar] [CrossRef]
- Greenberg, M.T.; Domitrovich, C.; Bumbarger, B. The prevention of mental disorders in school-aged children: Current state of the field. Prev. Treat. 2001, 4, 1a. [Google Scholar] [CrossRef]
- Lewis, A.J.; Galbally, M.; Gannon, T.; Symeonides, C. Early life programming as a target for prevention of child and adolescent mental disorders. BMC Med. 2014, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Osmond, C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986, 327, 1077–1081. [Google Scholar] [CrossRef]
- Barker, D.J. The fetal and infant origins of adult disease. BMJ Br. Med. J. 1990, 301, 1111. [Google Scholar] [CrossRef]
- O’Donnell, K.J.; Meaney, M.J. Fetal origins of mental health: The developmental origins of health and disease hypothesis. Am. J. Psychiatry 2016, 174, 319–328. [Google Scholar] [CrossRef]
- Schlotz, W.; Phillips, D.I. Fetal origins of mental health: Evidence and mechanisms. Brain Behav. Immun. 2009, 23, 905–916. [Google Scholar] [CrossRef]
- Davis, E.P.; Sandman, C.A. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010, 81, 131–148. [Google Scholar] [CrossRef]
- Mulder, E.J.; De Medina, P.R.; Huizink, A.C.; Van den Bergh, B.R.; Buitelaar, J.K.; Visser, G.H. Prenatal maternal stress: Effects on pregnancy and the (unborn) child. Early Hum. Dev. 2002, 70, 3–14. [Google Scholar] [CrossRef]
- Hohmann, C.F.; Hodges, A.; Beard, N.; Aneni, J. Effects of brief stress exposure during early postnatal development in balb/CByJ mice: I. Behavioral characterization. Dev. Psychobiol. 2013, 55, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; King, K.; Weitzman, M. Prenatal tobacco smoke and postnatal secondhand smoke exposure and child neurodevelopment. Curr. Opin. Pediatrics 2008, 20, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Goodlad, J.K.; Marcus, D.K.; Fulton, J.J. Lead and attention-deficit/hyperactivity disorder (ADHD) symptoms: A meta-analysis. Clin. Psychol. Rev. 2013, 33, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Lanphear, B.P.; Bellinger, D.; Axelrad, D.A.; McPartland, J.; Sutton, P.; Davidson, L.; Daniels, N.; Sen, S.; Woodruff, T.J. Developmental PBDE exposure and IQ/ADHD in childhood: A systematic review and meta-analysis. Environ. Health Perspect. 2017, 125, 086001. [Google Scholar] [CrossRef] [PubMed]
- Heilbrun, L.P.; Palmer, R.F.; Jaen, C.R.; Svoboda, M.D.; Perkins, J.; Miller, C.S. Maternal chemical and drug intolerances: Potential risk factors for autism and attention deficit hyperactivity disorder (ADHD). J. Am. Board Fam. Med. 2015, 28, 461–470. [Google Scholar] [CrossRef]
- Dolan, C.V.; Geels, L.; Vink, J.M.; van Beijsterveldt, C.E.M.; Neale, M.C.; Bartels, M.; Boomsma, D.I. Testing Causal Effects of Maternal Smoking During Pregnancy on Offspring’s Externalizing and Internalizing Behavior. Behav. Genet. 2016, 46, 378–388. [Google Scholar] [CrossRef]
- Sioen, I.; Den Hond, E.; Nelen, V.; Van de Mieroop, E.; Croes, K.; Van Larebeke, N.; Nawrot, T.S.; Schoeters, G. Prenatal exposure to environmental contaminants and behavioural problems at age 7–8 years. Environ. Int. 2013, 59, 225–231. [Google Scholar] [CrossRef]
- Perera, F.; Herbstman, J. Prenatal environmental exposures, epigenetics, and disease. Reprod. Toxicol. 2011, 31, 363–373. [Google Scholar] [CrossRef]
- Hay-Schmidt, A.; Finkielman, O.T.E.; Jensen, B.A.H.; Hogsbro, C.F.; Holm, J.B.; Johansen, K.H.; Jensen, T.K.; Andrade, A.M.; Swan, S.H.; Bornehag, C.G.; et al. Prenatal exposure to paracetamol/acetaminophen and precursor aniline impairs masculinisation of male brain and behaviour. Reproduction 2017, 154, 145–152. [Google Scholar] [CrossRef]
- Engel, S.M.; Miodovnik, A.; Canfield, R.L.; Zhu, C.B.; Silva, M.J.; Calafat, A.M.; Wolff, M.S. Prenatal Phthalate Exposure Is Associated with Childhood Behavior and Executive Functioning. Environ. Health Perspect. 2010, 118, 565–571. [Google Scholar] [CrossRef]
- Nair, P.; Black, M.M.; Ackerman, J.R.; Schuler, M.E.; Keane, V.A. Children’s cognitive-behavioral functioning at age 6 and 7: Prenatal drug exposure and caregiving environment. Ambul. Pediatrics 2008, 8, 154–162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Evans, S.F.; Kobrosly, R.W.; Barrett, E.S.; Thurston, S.W.; Calafat, A.M.; Weiss, B.; Stahlhut, R.; Yolton, K.; Swan, S.H. Prenatal bisphenol A exposure and maternally reported behavior in boys and girls. NeuroToxicol. 2014, 45, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Harley, K.G.; Gunier, R.B.; Kogut, K.; Johnson, C.; Bradman, A.; Calafat, A.M.; Eskenazi, B. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ. Res. 2013, 126, 43–50. [Google Scholar] [CrossRef]
- Shaw, W. The Unique Vulnerability of the Human Brain to Toxic Chemical Exposure and the Importance of Toxic Chemical Evaluation and Treatment in Orthomolecutar Psychiatry. J. Orthomol. Med. 2010, 25, 125–134. [Google Scholar]
- Oades, R.D.; Lasky-Su, J.; Christiansen, H.; Faraone, S.V.; Sonuga-Barke, E.J.; Banaschewski, T.; Chen, W.; Anney, R.J.; Buitelaar, J.K.; Ebstein, R.P. The influence of serotonin-and other genes on impulsive behavioral aggression and cognitive impulsivity in children with attention-deficit/hyperactivity disorder (ADHD): Findings from a family-based association test (FBAT) analysis. Behav. Brain Funct. 2008, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Oades, R.D. Role of the serotonin system in ADHD: Treatment implications. Expert Rev. Neurother. 2007, 7, 1357–1374. [Google Scholar] [CrossRef]
- Aziz, S.A.; Knowles, C.O. Inhibition of monoamine oxidase by the pesticide chlordimeform and related compounds. Nature 1973, 242, 417–418. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, M.F.; Bellinger, D.C.; Wright, R.O.; Weisskopf, M.G. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics 2010, 125, e1270–e1277. [Google Scholar] [CrossRef]
- Hollingworth, R.; Leister, J.; Ghali, G. Mode of action of formamidine pesticides: An evaluation of mono amine oxidase as the target. Chem. Biol. Interact. 1979, 24, 35–49. [Google Scholar] [CrossRef]
- Nilsen, F.M.; Tulve, N.S. A systematic review and meta-analysis examining the interrelationships between chemical and non-chemical stressors and inherent characteristics in children with ADHD. Environ. Res. 2020, 180, 108884. [Google Scholar] [CrossRef]
- Brummett, B.H.; Boyle, S.H.; Siegler, I.C.; Kuhn, C.M.; Surwit, R.S.; Garrett, M.E.; Collins, A.; Ashley-Koch, A.; Williams, R.B. HPA axis function in male caregivers: Effect of the monoamine oxidase-A gene promoter (MAOA-uVNTR). Biol. Psychol. 2008, 79, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Caspi, A.; McClay, J.; Moffitt, T.E.; Mill, J.; Martin, J.; Craig, I.W.; Taylor, A.; Poulton, R. Role of genotype in the cycle of violence in maltreated children. Science 2002, 297, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Kim-Cohen, J.; Caspi, A.; Taylor, A.; Williams, B.; Newcombe, R.; Craig, I.W.; Moffitt, T.E. MAOA, maltreatment, and gene–environment interaction predicting children’s mental health: New evidence and a meta-analysis. Mol. Psychiatry 2006, 11, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Widom, C.S.; Brzustowicz, L.M. MAOA and the Cycle of Violence: Childhood Abuse and Neglect, MAOA Genotype, and Risk for Violent and Antisocial Behavior. Biol. Psychiatry 2006, 60, 684–689. [Google Scholar] [CrossRef]
- de Boer, S.F.; Caramaschi, D.; Natarajan, D.; Koolhaas, J.M. The vicious cycle towards violence: Focus on the negative feedback mechanisms of brain serotonin neurotransmission. Front. Behav. Neurosci. 2009, 3, 52. [Google Scholar] [CrossRef]
- Meyer-Lindenberg, A.; Buckholtz, J.W.; Kolachana, B.; Hariri, A.R.; Pezawas, L.; Blasi, G.; Wabnitz, A.; Honea, R.; Verchinski, B.; Callicott, J.H. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 6269–6274. [Google Scholar] [CrossRef]
- Ducci, F.; Enoch, M.; Hodgkinson, C.; Xu, K.; Catena, M.; Robin, R.; Goldman, D. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Mol. Psychiatry 2008, 13, 334–347. [Google Scholar] [CrossRef][Green Version]
- Weder, N.; Yang, B.Z.; Douglas-Palumberi, H.; Massey, J.; Krystal, J.H.; Gelernter, J.; Kaufman, J. MAOA genotype, maltreatment, and aggressive behavior: The changing impact of genotype at varying levels of trauma. Biol. Psychiatry 2009, 65, 417–424. [Google Scholar] [CrossRef]
- Cook, A.; Spinazzola, J.; Ford, J.; Lanktree, C.; Blaustein, M.; Cloitre, M.; DeRosa, R.; Hubbard, R.; Kagan, R.; Liautaud, J. Complex trauma in children and adolescents. Psychiatr. Ann. 2017, 35, 390–398. [Google Scholar] [CrossRef]
- Arseneault, L.; Cannon, M.; Fisher, H.L.; Polanczyk, G.; Moffitt, T.E.; Caspi, A. Childhood trauma and children’s emerging psychotic symptoms: A genetically sensitive longitudinal cohort study. Am. J. Psychiatry 2011, 168, 65–72. [Google Scholar] [CrossRef]
- Schore, A.N. The effects of early relational trauma on right brain development, affect regulation, and infant mental health. Infant Ment. Health J. 2001, 22, 201–269. [Google Scholar] [CrossRef]
- Frazzetto, G.; Di Lorenzo, G.; Carola, V.; Proietti, L.; Sokolowska, E.; Siracusano, A.; Gross, C.; Troisi, A. Early trauma and increased risk for physical aggression during adulthood: The moderating role of MAOA genotype. PLoS ONE 2007, 2, e486. [Google Scholar] [CrossRef] [PubMed]
- Evans-Campbell, T.; Lindhorst, T.; Huang, B.; Walters, K.L. Interpersonal violence in the lives of urban American Indian and Alaska Native women: Implications for health, mental health, and help-seeking. Am. J. Public Health 2006, 96, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Tulve, N. Development of a Conceptual Framework Depicting a Childs Total (Built, Natural, Social) Environment in Order to Optimize Health and Well-Being. Ommega Int. 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Clow, A.; Hucklebridge, F.; Stalder, T.; Evans, P.; Thorn, L. The cortisol awakening response: More than a measure of HPA axis function. Neurosci. Biobehav. Rev. 2010, 35, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Gaab, J.; Rohleder, N.; Nater, U.M.; Ehlert, U. Psychological determinants of the cortisol stress response: The role of anticipatory cognitive appraisal. Psychoneuroendocrinology 2005, 30, 599–610. [Google Scholar] [CrossRef]
- Oquendo, M.; Echavarria, G.; Galfalvy, H.; Grunebaum, M.; Burke, A.; Barrera, A.; Cooper, T.; Malone, K.; Mann, J.J. Lower cortisol levels in depressed patients with comorbid post-traumatic stress disorder. Neuropsychopharmacology 2003, 28, 591. [Google Scholar] [CrossRef]
- Meewisse, M.-L.; Reitsma, J.B.; De Vries, G.-J.; Gersons, B.P.; Olff, M. Cortisol and post-traumatic stress disorder in adults: Systematic review and meta-analysis. Br. J. Psychiatry 2007, 191, 387–392. [Google Scholar] [CrossRef]
- Buss, C.; Davis, E.P.; Shahbaba, B.; Pruessner, J.C.; Head, K.; Sandman, C.A. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc. Natl. Acad. Sci. USA 2012, 109, E1312–E1319. [Google Scholar] [CrossRef]
- Kalra, S.; Einarson, A.; Karaskov, T.; Van Uum, S.; Koren, G. The relationship between stress and hair cortisol in healthy pregnant women. Clin. Investig. Med. 2007, 30, E103–E107. [Google Scholar] [CrossRef]
- Elzinga, B.M.; Schmahl, C.G.; Vermetten, E.; van Dyck, R.; Bremner, J.D. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology 2003, 28, 1656. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- AAP. American Academy of Pediatrics Mental Health Screening and Assessment Tools for Primary Care. In Addressing Mental Health Concerns in Primary Care: A Clinicians Toolkit; American Academy of Pediatrics: Itasca, IL, USA, 2019. [Google Scholar]
- Ghosh, S.; Sinha, M. ADHD, ODD, and CD: Do They Belong to a Common Psychopathological Spectrum? A Case Series. Case Rep. Psychiatry 2012, 2012, 520689. [Google Scholar] [CrossRef] [PubMed]
- Connor, D.F.; Doerfler, L.A. ADHD with comorbid oppositional defiant disorder or conduct disorder: Discrete or nondistinct disruptive behavior disorders? J. Atten. Disord. 2008, 12, 126–134. [Google Scholar] [CrossRef]
- Kossmeier, M.; Tran, U.S.; Voracek, M. Metaviz: Forest Plots, Funnel Plots, and Visual Funnel Plot Inference for Meta-Analysis. R Package Version 0.3.0. Available online: https://CRAN.R-project.org/package=metaviz (accessed on 30 March 2020).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Wickham, H. Tidyverse: Easily Install and Load the Tidyverse. R package Version 1.2.1. Available online: https://CRAN.R-project.org/package=tidyverse (accessed on 30 March 2020).
- Guyatt, G.H.; Oxman, A.D.; Schünemann, H.J.; Tugwell, P.; Knottnerus, A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011, 64, 380–382. [Google Scholar] [CrossRef]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A.E. GRADE handbook for grading quality of evidence and strength of recommendations. In Available from Guidelinedevelopment.org/Handbook. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 30 March 2020).
- GRADEpro_GDT, GRADEpro Guideline Development Tool [Software]; Developed by Evidence Prime, Inc.; McMaster University: Hamilton, ON, Canada, 2015.
- Sutton, A.J.; Duval, S.J.; Tweedie, R.L.; Abrams, K.R.; Jones, D.R. Empirical assessment of effect of publication bias on meta-analyses. BMJ 2000, 320, 1574–1577. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006, 295, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Macaskill, P.; Walter, S.D.; Irwig, L. A comparison of methods to detect publication bias in meta-analysis. Stat. Med. 2001, 20, 641–654. [Google Scholar] [CrossRef]
- Whiting, P.; Savović, J.; Higgins, J.P.; Caldwell, D.M.; Reeves, B.C.; Shea, B.; Davies, P.; Kleijnen, J.; Churchill, R. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J. Clin. Epidemiol. 2016, 69, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.; Rothstein, H. Meta-Analysis: Fixed Effect vs Random Effects. Available online: www.meta-analysis.com (accessed on 5 August 2007).
- Vasdekis, V.G.; Vlachonikolis, I.G. On the difference between ML and REML estimators in the modelling of multivariate longitudinal data. J. Stat. Plan. Inference 2005, 134, 194–205. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Columbia University, Mailman School of Public Health. Population Health Models: Meta-Regression. Available online: https://www.mailman.columbia.edu/research/population-health-methods/meta-regression/ (accessed on 5 August 2019).
- Thabane, L.; Mbuagbaw, L.; Zhang, S.; Samaan, Z.; Marcucci, M.; Ye, C.; Thabane, M.; Giangregorio, L.; Dennis, B.; Kosa, D.; et al. A tutorial on sensitivity analyses in clinical trials: The what, why, when and how. BMC Med. Res. Methodol. 2013, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.W.; Gilman, S.E.; Mick, E.; Fitzmaurice, G.; Ganz, M.L.; Seidman, L.J.; Buka, S.L. Revisiting the association between maternal smoking during pregnancy and ADHD. J. Psychiatr. Res. 2010, 44, 1058–1062. [Google Scholar] [CrossRef]
- Barthelemy, O.J.; Richardson, M.A.; Rose-Jacobs, R.; Forman, L.S.; Cabral, H.J.; Frank, D.A. Effects of intrauterine substance and postnatal violence exposure on aggression in children. Aggress. Behav. 2016, 42, 209–221. [Google Scholar] [CrossRef]
- Boden, J.M.; Fergusson, D.M.; Horwood, L.J. Risk Factors for Conduct Disorder and Oppositional/Defiant Disorder: Evidence from a New Zealand Birth Cohort. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 1125–1133. [Google Scholar] [CrossRef]
- Braun, J.M.; Froehlich, T.E.; Daniels, J.L.; Dietrich, K.N.; Hornung, R.; Auinger, P.; Lanphear, B.P. Association of environmental toxicants and conduct disorder in U.S. children: NHANES 2001-2004. Environ. Health Perspect. 2008, 116, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Chastang, J.; Baiz, N.; Cadwalladder, J.S.; Robert, S.; Dywer, J.; Charpin, D.A.; Caillaud, D.; de Blay, F.; Raherison, C.; Lavaud, F.; et al. Postnatal Environmental Tobacco Smoke Exposure Related to Behavioral Problems in Children. PLoS ONE 2015, 10, e0133604. [Google Scholar]
- Cornelius, M.D.; Goldschmidt, L.; DeGenna, N.; Day, N.L. Smoking during teenage pregnancies: Effects on behavioral problems in offspring. Nicotine Tob. Res. 2007, 9, 739–750. [Google Scholar] [CrossRef]
- Desrosiers, C.; Boucher, O.; Forget-Dubois, N.; Dewailly, E.; Ayotte, P.; Jacobson, S.W.; Jacobson, J.L.; Muckle, G. Associations between prenatal cigarette smoke exposure and externalized behaviors at school age among Inuit children exposed to environmental contaminants. Neurotoxicol. Teratol. 2013, 39, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Gaysina, D.; Fergusson, D.M.; Leve, L.D.; Horwood, J.; Reiss, D.; Shaw, D.S.; Elam, K.K.; Natsuaki, M.N.; Neiderhiser, J.M.; Harold, G.T. Maternal Smoking During Pregnancy and Offspring Conduct Problems Evidence From 3 Independent Genetically Sensitive Research Designs. JAMA Psychiatry 2013, 70, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.; Lim, M.H.; Ha, M.; Kwon, H.J.; Yoo, S.J.; Choi, K.H.; Paik, K.C. Secondhand Smoke Exposure and Low Blood Lead Levels in Association With Attention-Deficit Hyperactivity Disorder and Its Symptom Domain in Children: A Community-Based Case-Control Study. Nicotine Tob. Res. 2017, 19, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Knopik, V.S.; Heath, A.C.; Bucholz, K.K.; Madden, P.A.F.; Waldron, M. Genetic and environmental influences on externalizing behavior and alcohol problems in adolescence: A female twin study. Pharmacol. Biochem. Behav. 2009, 93, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Langley, K.; Heron, J.; Smith, G.D.; Thapar, A. Maternal and paternal smoking during pregnancy and risk of ADHD symptoms in offspring: Testing for intrauterine effects. Am. J. Epidemiol. 2012, 176, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Maughan, B.; Taylor, A.; Caspi, A.; Moffitt, T.E. Prenatal smoking and early childhood conduct problems—Testing genetic and environmental explanations of the association. Arch. Gen. Psychiatry 2004, 61, 836–843. [Google Scholar] [CrossRef]
- McLaughlin, A.A.; Minnes, S.; Singer, L.T.; Min, M.Y.; Short, E.J.; Scott, T.L.; Satayathum, S. Caregiver and self-report of mental health symptoms in 9-year old children with prenatal cocaine exposure. Neurotoxicol. Teratol. 2011, 33, 582–591. [Google Scholar] [CrossRef]
- Palmer, R.H.C.; Bidwell, L.C.; Heath, A.C.; Brick, L.A.; Madden, P.A.F.; Knopik, V.S. Effects of Maternal Smoking during Pregnancy on Offspring Externalizing Problems: Contextual Effects in a Sample of Female Twins. Behav. Genet. 2016, 46, 403–415. [Google Scholar] [CrossRef]
- Wakschlag, L.S.; Kistner, E.O.; Pine, D.S.; Biesecker, G.; Pickett, K.E.; Skol, A.D.; Dukic, V.; Blair, R.J.R.; Leventhal, B.L.; Cox, N.J.; et al. Interaction of prenatal exposure to cigarettes and MAOA genotype in pathways to youth antisocial behavior. Mol. Psychiatry 2010, 15, 928–937. [Google Scholar] [CrossRef]
- Galera, C.; Cote, S.M.; Bouvard, M.P.; Pingault, J.B.; Melchior, M.; Michel, G.; Boivin, M.; Tremblay, R.E. Early Risk Factors for Hyperactivity-Impulsivity and Inattention Trajectories From Age 17 Months to 8 Years. Arch. Gen. Psychiatry 2011, 68, 1267–1275. [Google Scholar] [CrossRef]
- Ruckinger, S.; Rzehak, P.; Chen, C.M.; Sausenthaler, S.; Koletzko, S.; Bauer, C.P.; Hoffmann, U.; Kramer, U.; Berdel, D.; von Berg, A.; et al. Prenatal and Postnatal Tobacco Exposure and Behavioral Problems in 10-Year-Old Children: Results from the GINI-plus Prospective Birth Cohort Study. Environ. Health Perspect. 2010, 118, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Pineda, D.A.; Palacio, L.G.; Puerta, I.C.; Merchan, V.; Arango, C.P.; Galvis, A.Y.; Gomez, M.; Aguirre, D.C.; Lopera, F.; Arcos-Burgos, M. Environmental influences that affect attention deficit/hyperactivity disorder: Study of a genetic isolate. Eur. Child Adolesc. Psychiatry 2007, 16, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.J.; Jeng, S.F.; Su, Y.N.; Liao, H.F.; Hsieh, W.S.; Wu, K.Y.; Chen, P.C. CYP1A1 Modifies the Effect of Maternal Exposure to Environmental Tobacco Smoke on Child Behavior. Nicotine Tob. Res. 2010, 12, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Tiesler, C.M.T.; Chen, C.M.; Sausenthaler, S.; Herbarth, O.; Lehmann, I.; Schaaf, B.; Kramer, U.; von Berg, A.; von Kries, R.; Wichmann, H.E.; et al. Passive smoking and behavioural problems in children: Results from the LISAplus prospective birth cohort study. Environ. Res. 2011, 111, 1173–1179. [Google Scholar] [CrossRef]
- Hobel, C.J.; Goldstein, A.; Barrett, E.S. Psychosocial stress and pregnancy outcome. Clin. Obstet. Gynecol. 2008, 51, 333–348. [Google Scholar] [CrossRef]
- Maconochie, N.; Doyle, P.; Prior, S.; Simmons, R. Risk factors for first trimester miscarriage—Results from a UK-population-based case–control study. BJOG Int. J. Obstet. Gynaecol. 2007, 114, 170–186. [Google Scholar] [CrossRef]
- Bada, H.S.; Das, A.; Bauer, C.R.; Shankaran, S.; Lester, B.; LaGasse, L.; Hammond, J.; Wright, L.L.; Higgins, R. Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics 2007, 119, E348–E359. [Google Scholar] [CrossRef]
- Knudsen, A.K.; Skogen, J.C.; Ystrom, E.; Sivertsen, B.; Tell, G.S.; Torgersen, L. Maternal pre-pregnancy risk drinking and toddler behavior problems: The Norwegian Mother and Child Cohort Study. Eur. Child Adolesc. Psychiatry 2014, 23, 901–911. [Google Scholar] [CrossRef]
- Sayal, K.; Heron, J.; Draper, E.; Alati, R.; Lewis, S.J.; Fraser, R.; Barrow, M.; Golding, J.; Emond, A.; Davey Smith, G.; et al. Prenatal exposure to binge pattern of alcohol consumption: Mental health and learning outcomes at age 11. Eur. Child Adolesc. Psychiatry 2014, 23, 891–899. [Google Scholar] [CrossRef]
- Pfinder, M.; Liebig, S.; Feldmann, R. Explanation of social inequalities in hyperactivity/inattention in children with prenatal alcohol exposure. Klin. Padiatr. 2012, 224, 303–308. [Google Scholar] [CrossRef]
- Diaz, S.D.; Smith, L.M.; LaGasse, L.L.; Derauf, C.; Newman, E.; Shah, R.; Arria, A.; Huestis, M.A.; Della Grotta, S.; Dansereau, L.M.; et al. Effects of prenatal methamphetamine exposure on behavioral and cognitive findings at 7.5 years of age. J. Pediatrics 2014, 164, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Brandlistuen, R.E.; Ystrom, E.; Eberhard-Gran, M.; Nulman, I.; Koren, G.; Nordeng, H. Behavioural effects of fetal antidepressant exposure in a Norwegian cohort of discordant siblings. Int. J. Epidemiol. 2015, 44, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Kobrosly, R.W.; Evans, S.; Miodovnik, A.; Barrett, E.S.; Thurston, S.W.; Calafat, A.M.; Swan, S.H. Prenatal Phthalate Exposures and Neurobehavioral Development Scores in Boys and Girls at 6–10 Years of Age. Environ. Health Perspect. 2014, 122, 521–528. [Google Scholar] [CrossRef]
- Perera, F.P.; Chang, H.W.; Tang, D.; Roen, E.L.; Herbstman, J.; Margolis, A.; Huang, T.J.; Miller, R.L.; Wang, S.; Rauh, V. Early-life exposure to polycyclic aromatic hydrocarbons and ADHD behavior problems. PLoS ONE 2014, 9, e111670. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Fito, N.; Torrent, M.; Carrizo, D.; Julvez, J.; Grimalt, J.O.; Sunyer, J. Exposure to hexachlorobenzene during pregnancy and children’s social behavior at 4 years of age. Environ. Health Perspect. 2007, 115, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Ode, A.; Kallen, K.; Gustafsson, P.; Rylander, L.; Jonsson, B.A.; Olofsson, P.; Ivarsson, S.A.; Lindh, C.H.; Rignell-Hydbom, A. Fetal exposure to perfluorinated compounds and attention deficit hyperactivity disorder in childhood. PLoS ONE 2014, 9, e95891. [Google Scholar] [CrossRef]
- Caspersen, I.H.; Aase, H.; Biele, G.; Brantsaeter, A.L.; Haugen, M.; Kvalem, H.E.; Skogan, A.H.; Zeiner, P.; Alexander, J.; Meltzer, H.M.; et al. The influence of maternal dietary exposure to dioxins and PCBs during pregnancy on ADHD symptoms and cognitive functions in Norwegian preschool children. Environ. Int. 2016, 94, 649–660. [Google Scholar] [CrossRef]
- Furlong, M.A.; Barr, D.B.; Wolff, M.S.; Engel, S.M. Prenatal exposure to pyrethroid pesticides and childhood behavior and executive functioning. Neurotoxicology 2017, 62, 231–238. [Google Scholar] [CrossRef]
- Boucher, O.; Jacobson, S.W.; Plusquellec, P.; Dewailly, E.; Ayotte, P.; Forget-Dubois, N.; Jacobson, J.L.; Muckle, G. Prenatal Methylmercury, Postnatal Lead Exposure, and Evidence of Attention Deficit/Hyperactivity Disorder among Inuit Children in Arctic Quebec. Environ. Health Perspect. 2012, 120, 1456–1461. [Google Scholar] [CrossRef]
- Sagiv, S.K.; Thurston, S.W.; Bellinger, D.C.; Tolbert, P.E.; Altshul, L.M.; Korrick, S.A. Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am. J. Epidemiol. 2010, 171, 593–601. [Google Scholar] [CrossRef]
- Marks, A.R.; Harley, K.; Bradman, A.; Kogut, K.; Barr, D.B.; Johnson, C.; Calderon, N.; Eskenazi, B. Organophosphate pesticide exposure and attention in young Mexican-American children: The CHAMACOS study. Environ. Health Perspect. 2010, 118, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, S.K.; Thurston, S.W.; Bellinger, D.C.; Amarasiriwardena, C.; Korrick, S.A. Prenatal exposure to mercury and fish consumption during pregnancy and attention-deficit/hyperactivity disorder-related behavior in children. Arch. Pediatrics Adolesc. Med. 2012, 166, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, M.D.; De Genna, N.M.; Leech, S.L.; Willford, J.A.; Goldschmidt, L.; Day, N.L. Effects of prenatal cigarette smoke exposure on neurobehavioral outcomes in 10-year-old children of adolescent mothers. Neurotoxicol. Teratol. 2011, 33, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Min, M.O.; Minnes, S.; Yoon, S.; Short, E.J.; Singer, L.T. Self-Reported Adolescent Behavioral Adjustment: Effects of Prenatal Cocaine Exposure. J. Adolesc. Health 2014, 55, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Thapar, A.; Fowler, T.; Rice, F.; Scourfield, J.; van den Bree, M.; Thomas, H.; Harold, G.; Hay, D. Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. Am. J. Psychiatry 2003, 160, 1985–1989. [Google Scholar] [CrossRef]
- Abdelouahab, N.; Huel, G.; Suvorov, A.; Foliguet, B.; Goua, V.; Debotte, G.; Sahuquillo, J.; Charles, M.A.; Takser, L. Monoamine oxidase activity in placenta in relation to manganese, cadmium, lead, and mercury at delivery. Neurotoxicol. Teratol. 2010, 32, 256–261. [Google Scholar] [CrossRef]
- Chasnoff, I.J.; Landress, H.J.; Barrett, M.E. The prevalence of illicit-drug or alcohol use during pregnancy and discrepancies in mandatory reporting in Pinellas County, Florida. N. Engl. J. Med. 1990, 322, 1202–1206. [Google Scholar] [CrossRef]
- Ruiz, J.D.C.; Quackenboss, J.J.; Tulve, N.S. Contributions of a child’s built, natural, and social environments to their general cognitive ability: A systematic scoping review. PLoS ONE 2016, 11, e0147741. [Google Scholar] [CrossRef]
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, J. Phthalates: Toxicology and exposure. Int. J. Hyg. Environ. Health 2007, 210, 623–634. [Google Scholar] [CrossRef]
- Huang, T.; Saxena, A.R.; Isganaitis, E.; James-Todd, T. Gender and racial/ethnic differences in the associations of urinary phthalate metabolites with markers of diabetes risk: National Health and Nutrition Examination Survey 2001–2008. Environ. Health 2014, 13, 6. [Google Scholar] [CrossRef]
- Della Seta, D.; Farabollini, F.; Dessi-Fulgheri, F.; Fusani, L. Environmental-Like Exposure to Low Levels of Estrogen Affects Sexual Behavior and Physiology of Female Rats. Endocrinology 2008, 149, 5592–5598. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, A.G.; Bloom, M.S.; Butts, C.D.; Wineland, R.J.; Brock, J.W.; Cruze, L.; Unal, E.R.; Kucklick, J.R.; Somerville, S.E.; Newman, R.B. Influence of race on prenatal phthalate exposure and anogenital measurements among boys and girls. Environ. Int. 2018, 110, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.W. Neurotoxic effects of mercury—A review. Environ. Res. 1977, 14, 329–373. [Google Scholar] [CrossRef]
- Ely, J. Mercury induced Alzheimer’s disease: Accelerating incidence? Bull. Environ. Contam. Toxicol. 2001, 67, 800–806. [Google Scholar] [CrossRef]
- Genuis, S.J. Toxic causes of mental illness are overlooked. Neurotoxicology 2008, 29, 1147–1149. [Google Scholar] [CrossRef]
- Moses, S.K.; Whiting, A.V.; Bratton, G.R.; Taylor, R.J.; O’Hara, T.M. Inorganic nutrients and contaminants in subsistence species of Alaska: Linking wildlife and human health. Int. J. Circumpolar Health 2009, 68, 53–74. [Google Scholar] [CrossRef]
- Dewailly, E.; Ayotte, P.; Bruneau, S.; Lebel, G.; Levallois, P.; Weber, J.P. Exposure of the Inuit population of Nunavik (Arctic Quebec) to lead and mercury. Arch. Environ. Health Int. J. 2001, 56, 350–357. [Google Scholar] [CrossRef]
- Hoekstra, P.; O’hara, T.; Backus, S.; Hanns, C.; Muir, D. Concentrations of persistent organochlorine contaminants in bowhead whale tissues and other biota from northern Alaska: Implications for human exposure from a subsistence diet. Environ. Res. 2005, 98, 329–340. [Google Scholar] [CrossRef]
- Schaefer, S.E.; Erber, E.; Trzaskos, J.P.; Roache, C.; Osborne, G.; Sharma, S. Sources of food affect dietary adequacy of Inuit women of childbearing age in Arctic Canada. J. Health Popul. Nutr. 2011, 29, 454. [Google Scholar] [CrossRef][Green Version]
- Barros, N.; Tulve, N.S.; Heggem, D.T.; Bailey, K. Review of built and natural environment stressors impacting American-Indian/Alaska-Native children. Rev. Environ. Health 2018, 33, 349–381. [Google Scholar] [CrossRef]
- Chandler, M.J.; Lalonde, C. Cultural continuity as a hedge against suicide in Canada’s First Nations. Transcult. Psychiatry 1998, 35, 191–219. [Google Scholar] [CrossRef]
- Ehlers, C.L.; Gizer, I.R. Evidence for a genetic component for substance dependence in Native Americans. Am. J. Psychiatry 2013, 170, 154–164. [Google Scholar] [CrossRef]
- Grandbois, D. Stigma of mental illness among American Indian and Alaska Native nations: Historical and contemporary perspectives. Issues Ment. Health Nurs. 2005, 26, 1001–1024. [Google Scholar] [CrossRef]
- Voltas, N.; Aparicio, E.; Arija, V.; Canals, J. Association study of monoamine oxidase-A gene promoter polymorphism (MAOA-uVNTR) with self-reported anxiety and other psychopathological symptoms in a community sample of early adolescents. J. Anxiety Disord. 2015, 31, 65–72. [Google Scholar] [CrossRef]
- Beaver, K.M.; Barnes, J.C.; Boutwell, B.B. The 2-repeat allele of the MAOA gene confers an increased risk for shooting and stabbing behaviors. Psychiatr. Q. 2014, 85, 257–265. [Google Scholar] [CrossRef]
- Ng, J.W.; Barrett, L.M.; Wong, A.; Kuh, D.; Smith, G.D.; Relton, C.L. The role of longitudinal cohort studies in epigenetic epidemiology: Challenges and opportunities. Genome Biol. 2012, 13, 246. [Google Scholar] [CrossRef]
- Ziegler, C.; Richter, J.; Mahr, M.; Gajewska, A.; Schiele, M.A.; Gehrmann, A.; Schmidt, B.; Lesch, K.-P.; Lang, T.; Helbig-Lang, S. MAOA gene hypomethylation in panic disorder—Reversibility of an epigenetic risk pattern by psychotherapy. Transl. Psychiatry 2016, 6, e773. [Google Scholar] [CrossRef]
- Melas, P.A.; Wei, Y.; Wong, C.C.; Sjöholm, L.K.; Åberg, E.; Mill, J.; Schalling, M.; Forsell, Y.; Lavebratt, C. Genetic and epigenetic associations of MAOA and NR3C1 with depression and childhood adversities. Int. J. Neuropsychopharmacol. 2013, 16, 1513–1528. [Google Scholar] [CrossRef]
- Gillespie, N.A.; Whitfield, J.B.; Williams, B.; Heath, A.C.; Martin, N.G. The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychol. Med. 2005, 35, 101–111. [Google Scholar] [CrossRef]
- Holz, N.; Boecker, R.; Buchmann, A.F.; Blomeyer, D.; Baumeister, S.; Hohmann, S.; Jennen-Steinmetz, C.; Wolf, I.; Rietschel, M.; Witt, S.H.; et al. Evidence for a Sex-Dependent MAOAx Childhood Stress Interaction in the Neural Circuitry of Aggression. Cereb. Cortex 2016, 26, 904–914. [Google Scholar] [CrossRef]
- Chen, H.; Pine, D.S.; Ernst, M.; Gorodetsky, E.; Kasen, S.; Gordon, K.; Goldman, D.; Cohen, P. The MAOA gene predicts happiness in women. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2013, 40, 122–125. [Google Scholar] [CrossRef]
- Carrel, L.; Willard, H.F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 2005, 434, 400. [Google Scholar] [CrossRef]
- Prom-Wormley, E.; Eaves, L.J.; Foley, D.; Gardner, C.; Archer, K.; Wormley, B.; Maes, H.; Riley, B.; Silberg, J. Monoamine oxidase A and childhood adversity as risk factors for conduct disorder in females. Psychol. Med. 2009, 39, 579–590. [Google Scholar] [CrossRef]
- Fowler, J.S.; Alia-Klein, N.; Kriplani, A.; Logan, J.; Williams, B.; Zhu, W.; Craig, I.W.; Telang, F.; Goldstein, R.; Volkow, N.D. Evidence that brain MAO A activity does not correspond to MAO A genotype in healthy male subjects. Biol. Psychiatry 2007, 62, 355–358. [Google Scholar] [CrossRef]
- Guimaraes, A.P.; Zeni, C.; Polanczyk, G.; Genro, J.P.; Roman, T.; Rohde, L.A.; Hutz, M.H. MAOA is associated with methylphenidate improvement of oppositional symptoms in boys with attention deficit hyperactivity disorder. Int. J. Neuropsychopharmacol. 2009, 12, 709–714. [Google Scholar] [CrossRef]
- Woods, S.M.; Melville, J.L.; Guo, Y.; Fan, M.-Y.; Gavin, A. Psychosocial stress during pregnancy. Am. J. Obstet. Gynecol. 2010, 202, e1–e7. [Google Scholar] [CrossRef]
- Lichtveld, K.; Thomas, K.; Tulve, N.S. Chemical and non-chemical stressors affecting childhood obesity: A systematic scoping review. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 1. [Google Scholar] [CrossRef]
- Hucklebridge, F.; Hussain, T.; Evans, P.; Clow, A. The diurnal patterns of the adrenal steroids cortisol and dehydroepiandrosterone (DHEA) in relation to awakening. Psychoneuroendocrinology 2005, 30, 51–57. [Google Scholar] [CrossRef]
- Pico-Alfonso, M.A.; Garcia-Linares, M.I.; Celda-Navarro, N.; Herbert, J.; Martinez, M. Changes in cortisol and dehydroepiandrosterone in women victims of physical and psychological intimate partner violence. Biol. Psychiatry 2004, 56, 233–240. [Google Scholar] [CrossRef]
- Cokkinides, V.E.; Coker, A.L.; Sanderson, M.; Addy, C.; Bethea, L. Physical violence during pregnancy: Maternal complications and birth outcomes. Obstet. Gynecol. 1999, 93, 661–666. [Google Scholar] [CrossRef]
- Braithwaite, E.C.; Pickles, A.; Sharp, H.; Glover, V.; O’Donnell, K.J.; Tibu, F.; Hill, J. Maternal prenatal cortisol predicts infant negative emotionality in a sex-dependent manner. Physiol. Behav. 2017, 175, 31–36. [Google Scholar] [CrossRef]
- Yehuda, R.; Halligan, S.L.; Bierer, L.M. Cortisol levels in adult offspring of Holocaust survivors: Relation to PTSD symptom severity in the parent and child. Psychoneuroendocrinology 2002, 27, 171–180. [Google Scholar] [CrossRef]
- Lehrner, A.; Bierer, L.M.; Passarelli, V.; Pratchett, L.C.; Flory, J.D.; Bader, H.N.; Harris, I.R.; Bedi, A.; Daskalakis, N.P.; Makotkine, I. Maternal PTSD associates with greater glucocorticoid sensitivity in offspring of Holocaust survivors. Psychoneuroendocrinology 2014, 40, 213–220. [Google Scholar] [CrossRef]
- Bowers, M.E.; Yehuda, R. Intergenerational transmission of stress in humans. Neuropsychopharmacology 2016, 41, 232. [Google Scholar] [CrossRef]
- Perroud, N.; Rutembesa, E.; Paoloni-Giacobino, A.; Mutabaruka, J.; Mutesa, L.; Stenz, L.; Malafosse, A.; Karege, F. The Tutsi genocide and transgenerational transmission of maternal stress: Epigenetics and biology of the HPA axis. World J. Biol. Psychiatry 2014, 15, 334–345. [Google Scholar] [CrossRef]
- Sturge-Apple, M.L.; Davies, P.T.; Cicchetti, D.; Manning, L.G. Interparental violence, maternal emotional unavailability and children’s cortisol functioning in family contexts. Dev. Psychol. 2012, 48, 237. [Google Scholar] [CrossRef]
- Class, Q.A.; Abel, K.M.; Khashan, A.S.; Rickert, M.E.; Dalman, C.; Larsson, H.; Hultman, C.M.; Langstrom, N.; Lichtenstein, P.; D’Onofrio, B.M. Offspring psychopathology following preconception, prenatal and postnatal maternal bereavement stress. Psychol. Med. 2014, 44, 71–84. [Google Scholar] [CrossRef]
- Barker, E.D.; Copeland, W.; Maughan, B.; Jaffee, S.R.; Uher, R. Relative impact of maternal depression and associated risk factors on offspring psychopathology. Br. J. Psychiatry 2012, 200, 124–129. [Google Scholar] [CrossRef]
- Lester, B.M.; Lagasse, L.L.; Shankaran, S.; Bada, H.S.; Bauer, C.R.; Lin, R.; Das, A.; Higgins, R. Prenatal cocaine exposure related to cortisol stress reactivity in 11-year-old children. J. Pediatrics 2010, 157, 288–295. [Google Scholar] [CrossRef]
- Ouellet-Morin, I.; Dionne, G.; Lupien, S.J.; Muckle, G.; Cote, S.; Perusse, D.; Tremblay, R.E.; Boivin, M. Prenatal alcohol exposure and cortisol activity in 19-month-old toddlers: An investigation of the moderating effects of sex and testosterone. Psychopharmacology 2011, 214, 297–307. [Google Scholar] [CrossRef]
- Hill, J.; Breen, G.; Quinn, J.; Tibu, F.; Sharp, H.; Pickles, A. Evidence for interplay between genes and maternal stress in utero: Monoamine oxidase A polymorphism moderates effects of life events during pregnancy on infant negative emotionality at 5weeks. Genes Brain Behav. 2013, 12, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.; Forns, J.; Martínez, D.; Avella-García, C.; Valvi, D.; Ballesteros-Gómez, A.; Luque, N.; Rubio, S.; Julvez, J.; Sunyer, J.; et al. Exposure to bisphenol A during pregnancy and child neuropsychological development in the INMA-Sabadell cohort. Environ. Res. 2015, 142, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Youssef, N.; Lockwood, L.; Su, S.; Hao, G.; Rutten, B. The effects of trauma, with or without PTSD, on the transgenerational DNA methylation alterations in human offsprings. Brain Sci. 2018, 8, 83. [Google Scholar] [CrossRef]
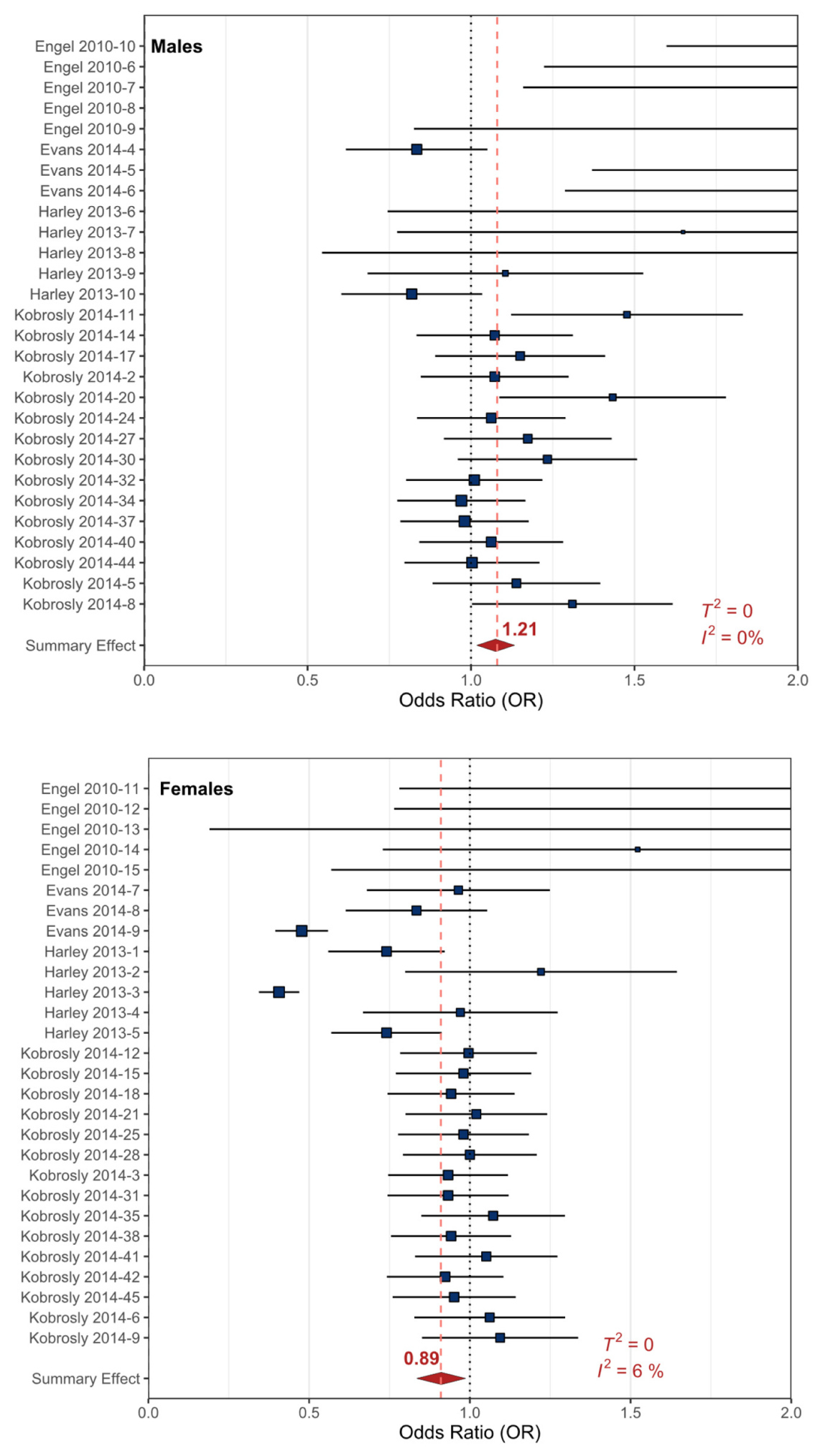
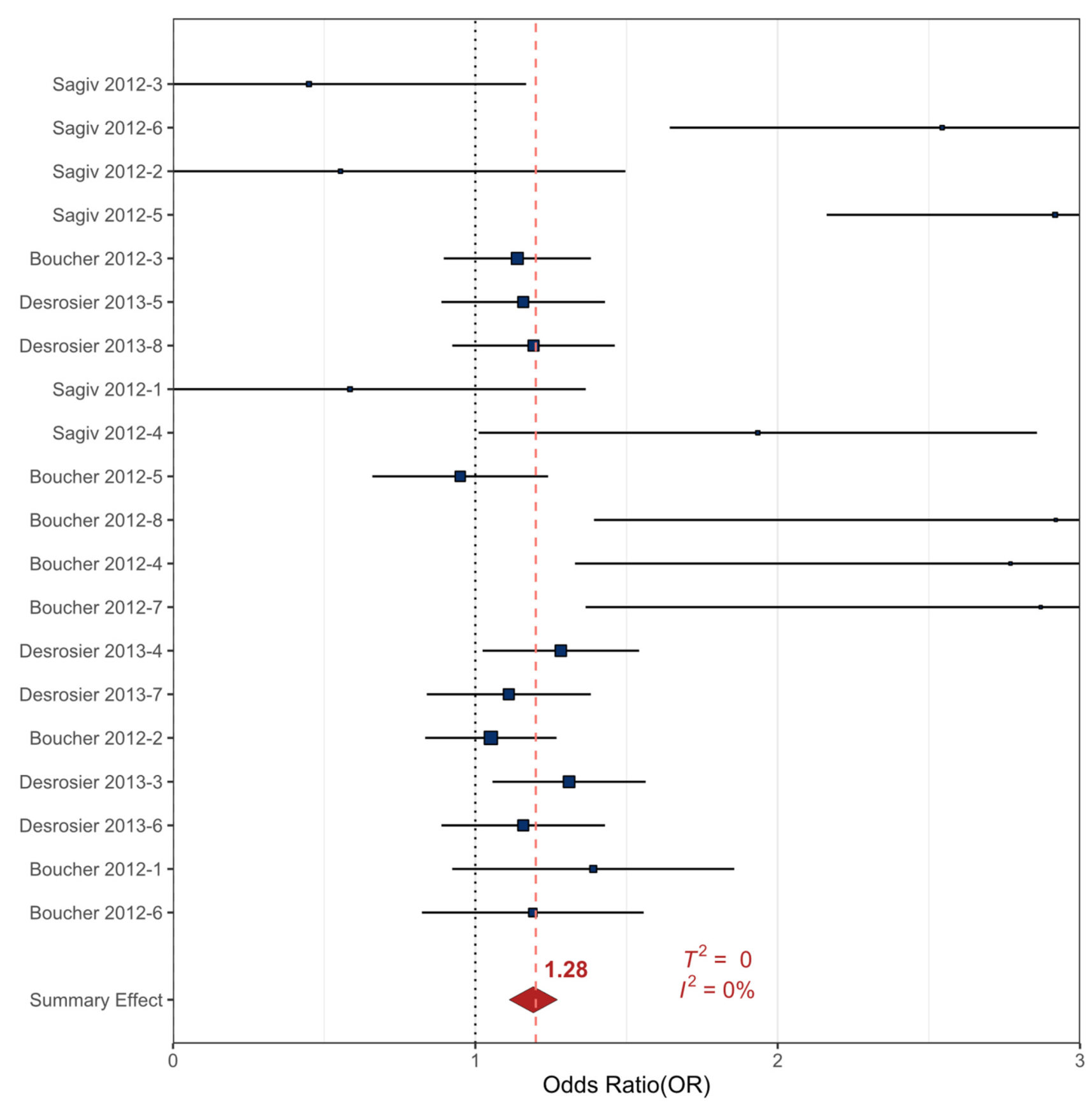
| Outcomes | Anticipated Absolute Effects | Relative Effect (90% CI) | № of Participants (Observational Studies) | Precision of the Evidence (GRADE) | |
|---|---|---|---|---|---|
| U.S. Population Risk with DSM Criteria | Risk with Chemical Stressor Exposures | ||||
| Cigarette Smoke Exposures | |||||
| CD/ODD | 61 per 1,000 | 145 per 1,000 | OR 2.61 * (2.08 to 3.14) | 56835 (13) | ⨁⨁⨁◯ MODERATE |
| ADHD | 110 per 1,000 | 254 per 1,000 | OR 2.76 * (1.44 to 4.07) | 61706 (13) | ⨁⨁⨁◯ MODERATE |
| All Externalizing | 74 per 1,000 | 148 per 1,000 | OR 2.17 * (1.36 to 2.96) | 180001 (21) | ⨁⨁⨁◯ MODERATE |
| Cocaine Exposures | |||||
| All Externalizing | 74 per 1,000 | 166 per 1,000 | OR 2.49 * (1.52 to 3.48) | 5694 (3) | ⨁⨁⨁◯ MODERATE |
| Alcohol Exposures | |||||
| All Externalizing | 74 per 1,000 | 119 per 1,000 | OR 1.69 * (1.33 to 2.07) | 367471 (5) | ⨁⨁⨁◯ MODERATE |
| Phthalate/Plasticizer Exposures | |||||
| All Externalizing (males) | 101 per 1,000 | 120 per 1,000 | OR 1.21 * (1.06 to 1.35) | 2813 (4) | ⨁⨁⨁◯ MODERATE |
| All Externalizing (females) | 45 per 1,000 | 40 per 1,000 | OR 0.88 * (0.77 to 1.00) | 2886 (4) | ⨁⨁⨁◯ MODERATE |
| All Externalizing (both sexes) | 74 per 1,000 | 84 per 1,000 | OR 1.13 * (0.98 to 1.27) | 3513 (3) | ⨁⨁◯◯ LOW |
| Organic Contaminant Exposures | |||||
| ADHD | 74 per 1,000 | 73per 1,000 | OR 0.99 * (0.87 to 1.11) | 17410 (7) | ⨁⨁◯◯ LOW |
| All Externalizing | 110 per 1,000 | 101per 1,000 | OR 0.91 * (0.78 to 1.04) | 18164 (8) | ⨁⨁◯◯ LOW |
| Mercury Exposures | |||||
| ADHD | 110 per 1,000 | 155 per 1,000 | OR 1.49 * (1.04 to 1.93) | 3422 (3) | ⨁⨁⨁◯ MODERATE |
| All Externalizing | 74 per 1,000 | 93 per 1,000 | OR 1.28 * (1.06 to 1.50) | 4574 (3) | ⨁⨁⨁◯ MODERATE |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. Nilsen, F.; Frank, J.; S. Tulve, N. A Systematic Review and Meta-Analysis Investigating the Relationship between Exposures to Chemical and Non-Chemical Stressors during Prenatal Development and Childhood Externalizing Behaviors. Int. J. Environ. Res. Public Health 2020, 17, 2361. https://doi.org/10.3390/ijerph17072361
M. Nilsen F, Frank J, S. Tulve N. A Systematic Review and Meta-Analysis Investigating the Relationship between Exposures to Chemical and Non-Chemical Stressors during Prenatal Development and Childhood Externalizing Behaviors. International Journal of Environmental Research and Public Health. 2020; 17(7):2361. https://doi.org/10.3390/ijerph17072361
Chicago/Turabian StyleM. Nilsen, Frances, Jessica Frank, and Nicolle S. Tulve. 2020. "A Systematic Review and Meta-Analysis Investigating the Relationship between Exposures to Chemical and Non-Chemical Stressors during Prenatal Development and Childhood Externalizing Behaviors" International Journal of Environmental Research and Public Health 17, no. 7: 2361. https://doi.org/10.3390/ijerph17072361
APA StyleM. Nilsen, F., Frank, J., & S. Tulve, N. (2020). A Systematic Review and Meta-Analysis Investigating the Relationship between Exposures to Chemical and Non-Chemical Stressors during Prenatal Development and Childhood Externalizing Behaviors. International Journal of Environmental Research and Public Health, 17(7), 2361. https://doi.org/10.3390/ijerph17072361






