Prevention and Control of Multidrug-Resistant Bacteria in The Netherlands and Germany—The Impact of Healthcare Structures
Abstract
1. Introduction
2. Multidrug-Resistant Microorganisms, Antibiotic Use and Healthcare-Associated Infections in The Netherlands and Germany
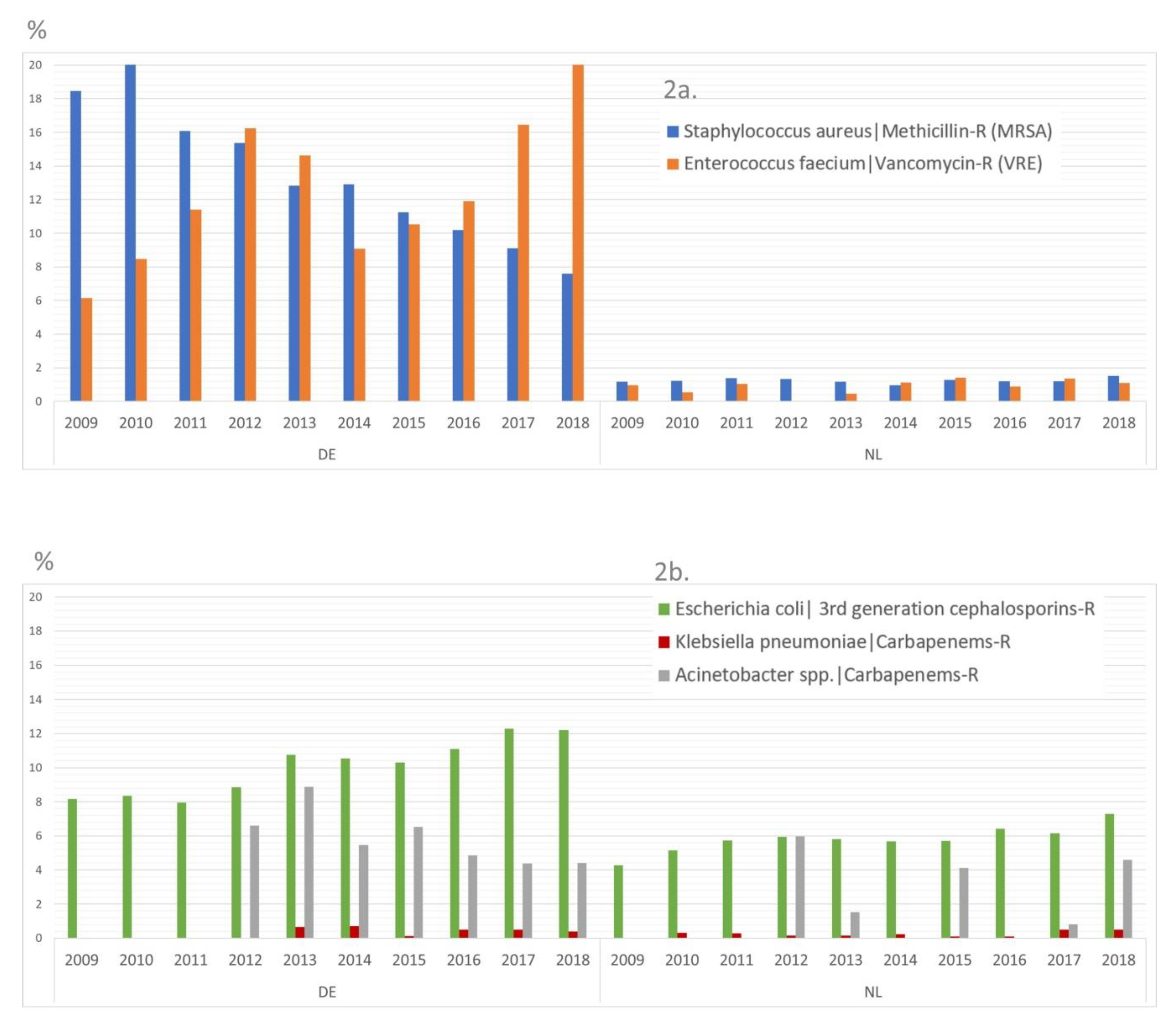
2.1. Data about the Occurrence of MDRO
2.2. Data about HAI
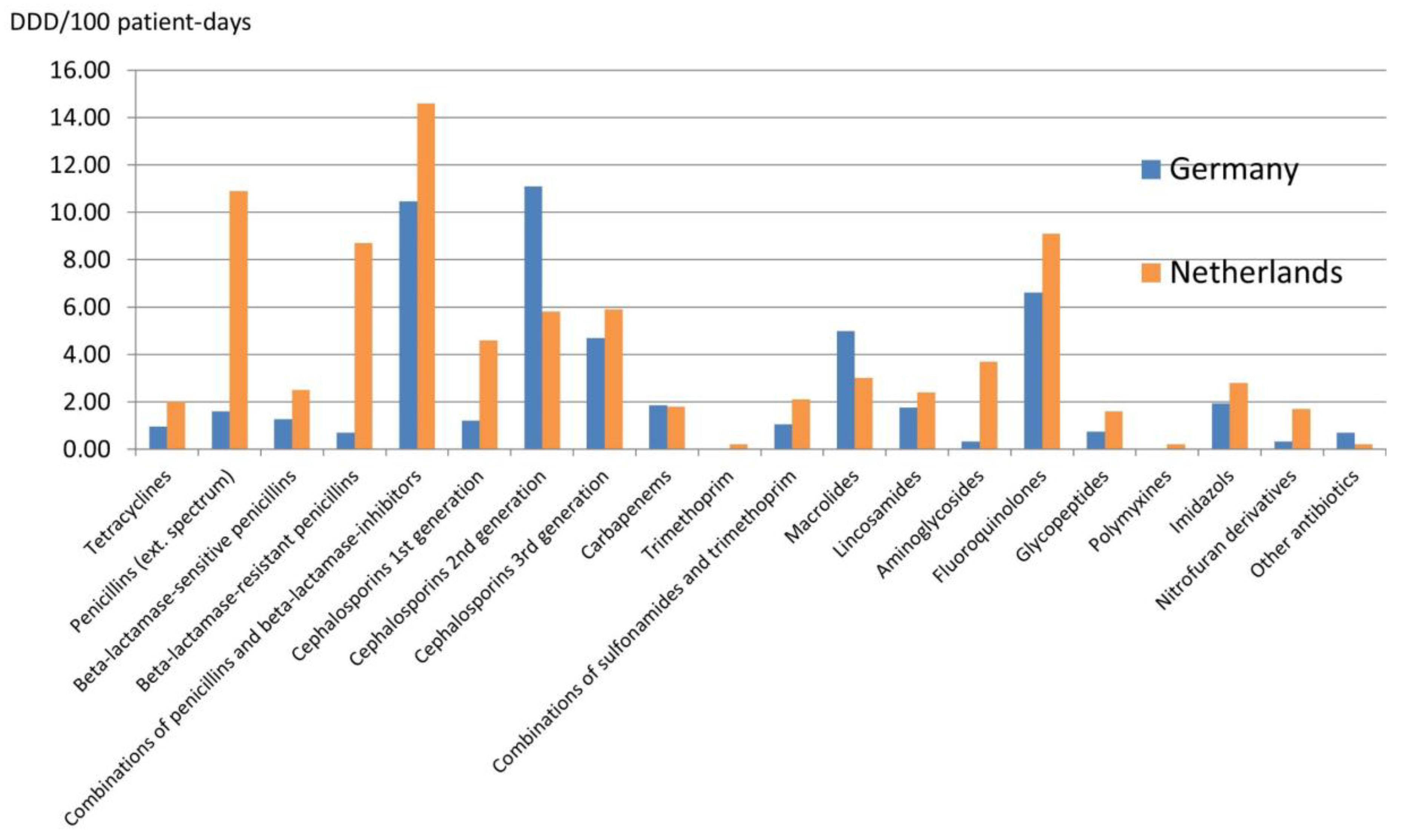
2.3. Data about Antibiotic Use
3. Data about Healthcare Structures in The Netherlands and Germany
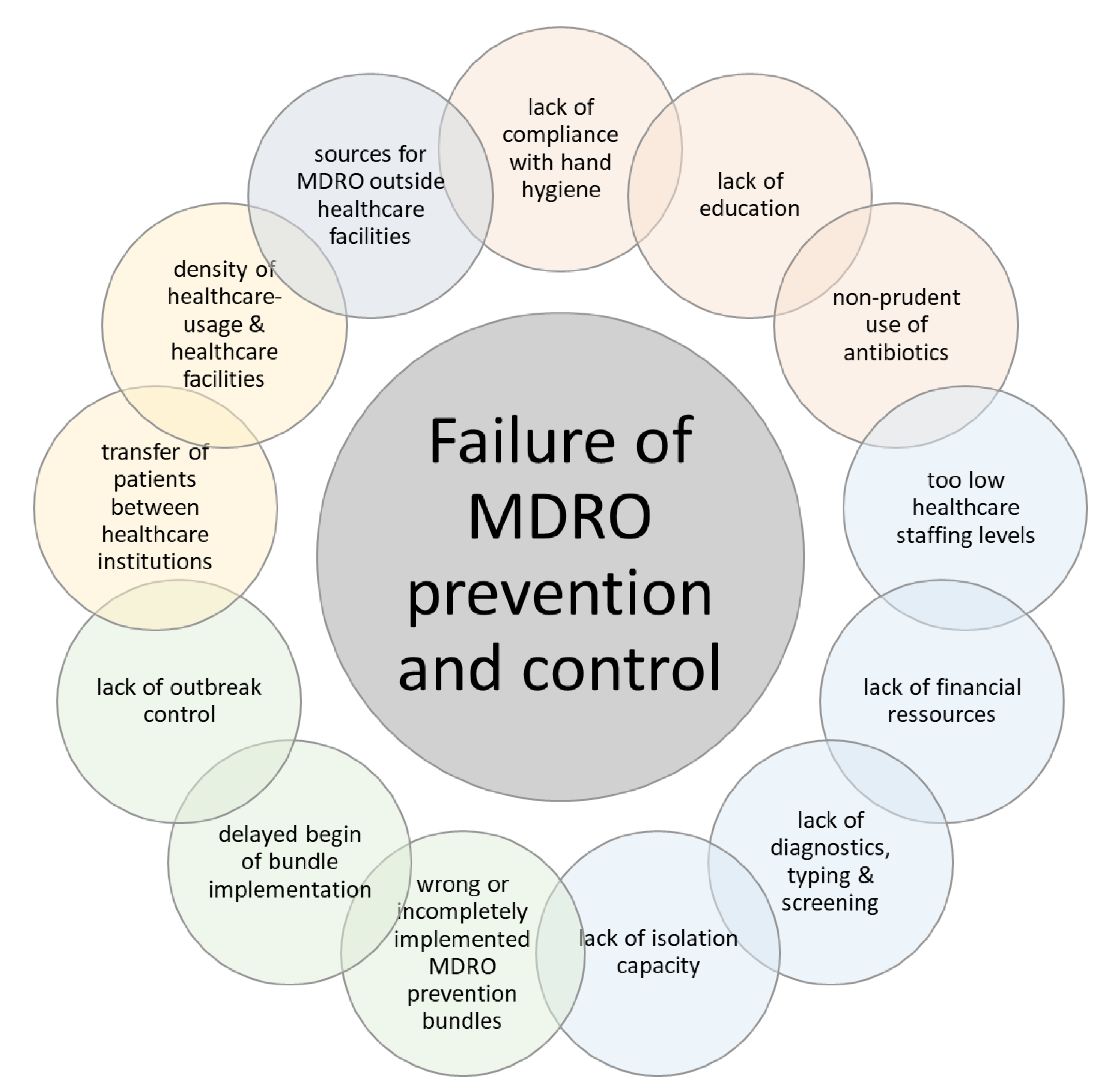
4. Discussion
- Hospitalization (number of cases, length of stay) per se increases the risk of MDRO transmission (from patient-to-patient, via healthcare workers or via surfaces). Moreover, due to the higher number of hospitals in Germany, many hospitals are less specialized. Consequently, there is more patient traffic between different hospitals (as patients are sent from basic care to more specialized hospitals). In this setting, each admission bears the possibility of inter-institutional transmission, facilitating regional dissemination of MRDO. Some studies have demonstrated that this led to a clonal expansion of some MDRO [13,14].
- Hand hygiene must be performed more rigorously in German hospitals. Due to fewer personnel and more patients, there are more contacts per individual healthcare worker. In addition, one healthcare worker has contact with more patients. If it is assumed that the compliance level with hand hygiene is similar in The Netherlands and Germany, these underlying structures might facilitate the transmission of MDRO. Moreover, a high awareness about the AMR problem among healthcare workers has been recently shown for both countries [15].
- Although the density of hospital beds is higher in Germany, the bed occupancy rate is also higher than in The Netherlands. While in German hospitals occupancy rates are regularly above 80%, they are usually around 60%-70% in Dutch hospitals (Table 3). This has an impact on the availability of single rooms for isolation. Besides the rather low scientific evidence for the isolation of patients with MDRO and the lack of randomized trials regarding this issue [16], as well as the potential disadvantages of “isolated care” in single-rooms (e.g., stigmatization and less awareness), many clinicians and hospital administrations in Germany refer to a lack of isolation beds and financial penalties due to blocked beds, when arguing against single-room isolation. In The Netherlands, due to the structural difference, single-room isolation is easier to organize and rather undisputed for most MDROs including methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, multidrug-resistant Gram-negative bacteria (including even extended-spectrum betalactamase-producing, carbapenem-susceptible Enterbacteriaceae). For risk patients, the concept of pre-emptive isolation at admission as long as screening results are pending, is also a key component of Dutch IPC recommendations. In Germany, pre-emptive isolation is performed for patients with a high risk of carriage of carbapenem-resistant Enterobacteriaceae or Acinetobacter baumannii or for patients with a very high risk of MRSA carriage (e.g., known carriage from previous hospitalizations). However, for MRSA, the fact that >30% of patients at admission are risk patients in Germany, prevents the ability to use pre-emptive isolation for all patients at risk [17].
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals 2011–2012. Available online: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/healthcare-associated-infections-antimicrobial-use-PPS.pdf (accessed on 13 July 2019).
- National Institute for Public Health and the Environment. Referentiecijfers 2014 t/m 2018: Prevalentieonderzoek Ziekenhuizen. 2019. Available online: https://www.rivm.nl/documenten/prezies-referentiecijfers-po-tm-2018 (accessed on 26 January 2020).
- Nationales Referenzzentrum für Surveillance von Nosokomialen Infektionen. CDAD KISS Referenzdaten 2018. Available online: https://www.nrz-hygiene.de/fileadmin/nrz/module/cdad/201801_201812_CDAD_Ref.pdf (accessed on 13 July 2019).
- National Reference Laboratory for Clostridioides difficile. Thirteenth Annual Report of the National Reference Laboratory for Clostridioides difficile and Results of the Sentinel Surveillance. 2019. Available online: https://www.rivm.nl/sites/default/files/2019-09/Annual%20report%20C.%20difficile%20reference%20laboratory%20may%202018-may%202019.pdf (accessed on 26 January 2020).
- Idelevich, E.A.; Seifert, H.; Sundqvist, M.; Scudeller, L.; Amit, S.; Balode, A.; Drevinek, P.; Kocak Tufan, Z.; Koraqi, A.; Lamy, B.; et al. Microbiological diagnostics of bloodstream infections in Europe-an ESGBIES survey. Clin. Microbiol. Infect. 2019, 25, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Antimicrobial Consumption—Annual Epidemiological Report for 2017. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-consumption-annual-epidemiological-report-2017 (accessed on 13 July 2019).
- National Institute for Public Health and the Environment. NethMap 2018. Available online: https://www.rivm.nl/bibliotheek/rapporten/2018-0046.pdf (accessed on 13 July 2019).
- Robert Koch-Institut. Antibiotika Verbrauchs Surveillance (AVS.RKI). Available online: https://avs.rki.de/ (accessed on 13 July 2019).
- Europäische Kommission. Eurostat. Available online: https://ec.europa.eu/eurostat/de/home (accessed on 13 July 2019).
- OECD. Health at a Glance: Europe 2018—The State of Health in the EU Cycle. Available online: https://read.oecd-ilibrary.org/social-issues-migration-health/health-at-a-glance-europe-2018_health_glance_eur-2018-en#page1 (accessed on 8 September 2019).
- de With, K.; Wilke, K.; Kern, W.; Strauß, R.; Kramme, E.; Friedrichs, A. Strategien zur Sicherung Rationaler Antibiotika-Anwendung im Krankenhaus. 2018. Available online: https://www.awmf.org/leitlinien/detail/ll/092-001.html (accessed on 14 July 2019).
- Ciccolini, M.; Donker, T.; Grundmann, H.; Bonten, M.J.M.; Woolhouse, M.E.J. Efficient surveillance for healthcare-associated infections spreading between hospitals. Proc. Natl. Acad. Sci. USA 2014, 111, 2271–2276. [Google Scholar] [CrossRef] [PubMed]
- Ciccolini, M.; Donker, T.; Köck, R.; Mielke, M.; Hendrix, R.; Jurke, A.; Rahamat-Langendoen, J.; Becker, K. Infection prevention in a connected world: The case for a regional approach. Int. J. Med. Microbiol. 2013, 303, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Keizer, J.; Braakman-Jansen, L.M.A.; Kampmeier, S.; Köck, R.; Al Naiemi, N.; Te Riet-Warning, R.; Beerlage-De Jong, N.; Becker, K.; Van Gemert-Pijnen, J.E.W.C. Cross-border comparison of antimicrobial resistance (AMR) and AMR prevention measures: The healthcare workers’ perspective. Antimicrob. Resist. Infect. Control 2019, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Köck, R.; Becker, K.; Cookson, B.; van Gemert-Pijnen, J.E.; Harbarth, S.; Kluytmans, J.; Mielke, M.; Peters, G.; Skov, R.L.; Struelens, M.J. Systematic literature analysis and review of targeted preventive measures to limit healthcare-associated infections by meticillin-resistant Staphylococcus aureus. Eurosurveillance 2014, 19. Available online: https://edoc.rki.de/handle/176904/1927 (accessed on 13 July 2019).
- Jurke, A.; Daniels-Haardt, I.; Silvis, W.; Berends, M.S.; Glasner, C.; Becker, K.; Köck, R.; Friedrich, A.W. Changing epidemiology of meticillin-resistant Staphylococcus aureus in 42 hospitals in the Dutch-German border region, 2012 to 2016: Results of the search-and-follow-policy. Eurosurveillance 2019, 24, 1800244. [Google Scholar] [CrossRef] [PubMed]
- Gaygısız, Ü.; Lajunen, T.; Gaygısız, E. Socio-economic factors, cultural values, national personality and antibiotics use: A cross-cultural study among European countries. J. Infect. Public Health 2017, 10, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Schmiege, D.; Evers, M.; Kistemann, T.; Falkenberg, T. What drives antibiotic use in the community? A systematic review of determinants in the human outpatient sector. Int. J. Hyg. Environ. Health 2020, 226, 113497. [Google Scholar] [CrossRef] [PubMed]
- Dik, J.-W.H.; Sinha, B.; Friedrich, A.W.; Lo-Ten-Foe, J.R.; Hendrix, R.; Köck, R.; Bijker, B.; Postma, M.J.; Freitag, M.H.; Glaeske, G.; et al. Cross-border comparison of antibiotic prescriptions among children and adolescents between the north of The Netherlands and the north-west of Germany. Antimicrob. Resist. Infect. Control 2016, 5, 14. [Google Scholar]
- Nationales Referenzzentrum für Surveillance von nosokomialen Infektionen. ITS-KISS Referenzdaten 2018. 2019. Available online: https://www.nrz-hygiene.de/fileadmin/nrz/module/its/201701_201812_ALLE_ITSRef.pdf (accessed on 15 March 2019).
- Köck, R.; Schaumburg, F.; Mellmann, A.; Köksal, M.; Jurke, A.; Becker, K.; Friedrich, A.W. Livestock-associated methicillin-resistant Staphylococcus aureus (MRSA) as causes of human infection and colonization in Germany. PLoS ONE 2013, 8, e55040. [Google Scholar] [CrossRef] [PubMed]
- van Alen, S.; Ballhausen, B.; Peters, G.; Friedrich, A.W.; Mellmann, A.; Köck, R.; Becker, K. In the centre of an epidemic: Fifteen years of LA-MRSA CC398 at the University Hospital Münster. Vet. Microbiol. 2017, 200, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Madec, J.-Y.; Haenni, M.; Nordmann, P.; Poirel, L. Extended-spectrum β-lactamase/AmpC- and carbapenemase-producing Enterobacteriaceae in animals: A threat for humans? Clin. Microbiol. Infect. 2017, 23, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, D.; Kant, A.; van Essen-Zandbergen, A.; Dierikx, C.; Hordijk, J.; Wit, B.; Mevius, D.J.; Veldman, K.T. Diversity of plasmids and genes encoding resistance to extended spectrum cephalosporins in commensal Escherichia coli from Dutch livestock in 2007–2017. Front. Microbiol. 2019, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Bundesministerium für Ernährung und Landwirtschaft (BMEL), Bundesamt für Verbraucherschutz und Lebensmittelsicherheit (BVL), Bundesinstitut für Risikobewertung (BfR). Lagebild zum Antibiotikaeinsatz bei Tieren in Deutschland. 2018. Available online: https://www.bmel.de/SharedDocs/Downloads/Tier/Tiergesundheit/Tierarzneimittel/Lagebild%20Antibiotikaeinsatz%20bei%20Tieren%20Juli%202018.pdf?__blob=publicationFile (accessed on 19 June 2019).
- Friedrich, A.W.; Daniels-Haardt, I.; Köck, R.; Verhoeven, F.; Mellmann, A.; Harmsen, D.; van Gemert-Pijnen, J.E.; Becker, K.; Hendrix, M.G.R. EUREGIO MRSA-net Twente/Münsterland—A Dutch-German cross-border network for the prevention and control of infections caused by methicillin-resistant Staphylococcus aureus. Eurosurveillance 2008, 13, 18965. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jurke, A.; Köck, R.; Becker, K.; Thole, S.; Hendrix, R.; Rossen, J.; Daniels-Haardt, I.; Friedrich, A.W. Reduction of the nosocomial meticillin-resistant Staphylococcus aureus incidence density by a region-wide search and follow-strategy in forty German hospitals of the EUREGIO, 2009 to 2011. Eurosurveillance 2013, 18, 20579. [Google Scholar] [CrossRef] [PubMed]
| Pathogen * | Germany | The Netherlands | ||
|---|---|---|---|---|
| Cases (Incidence) | Deaths (Mortality) | Cases (Incidence) | Deaths (Mortality) | |
| CRAB | 278 (0.34) | 24 (0.03) | 14 (0.08) | 1 (0.01) |
| VRE | 3089 (3.80) | 206 (0.25) | 63 (0.37) | 4 (0.02) |
| CRKP | 125 (0.15) | 3 (0) | 0 (0) | 0 (0) |
| MRSA | 13,684 (16.85) | 653 (0.80) | 249 (1.47) | 12 (0.07) |
| 3rd generation-cephalosporin-res. E. coli | 28,392 (34.97) | 868 (1.07) | 3503 (20.73) | 107 (0.63) |
| all assessed MDROs | 54,509 (67.13) | 2363 (2.91) | 4982 (29.48) | 206 (1.22) |
| Type of Antibiotic | EU-Mean | Germany | The Netherlands |
|---|---|---|---|
| Total use | 21.8 | 13.7 | 10.1 |
| Penicillins | 11.5 | 5.0 | 4.0 |
| Other betalactams | 2.0 | 2.8 | 0 |
| Sulfonamide/trimethoprim | 0.6 | 0.5 | 0.4 |
| Macrolides | 2.9 | 2.1 | 1.4 |
| Tetracyclines | 2.2 | 1.8 | 2.0 |
| Quinolones | 1.6 | 1.1 | 0.7 |
| Other antibiotics | 1.1 | 0.5 | 1.5 |
| Indicator | The Netherlands | Germany | |
|---|---|---|---|
| Population | 16,979,140 | 82,175,684 | |
| Health expenditures in Mio. € | 72,788.63 | 351,701.00 | |
| Pro inhabitant in € | 3,885 | 4,160 | |
| Life expectancy at birth (years) | 81.7 | 81.0 | |
| Length of hospital stay (curative care) in days | 5.0 | 7.5 | |
| Hospital days per year | 8,268165 | 146,048,193 | |
| per 1,000 inhabitants | 486.96 | 1777.27 | |
| Hospital beds total (curative, rehabilitative, long-term care) | 61,767 | 663,941 | |
| Curative | 51,176 | 498,718 | |
| rehabilitative | 1946 | 165,223 | |
| per 1000 inhabitants | 3.64 | 8.08 | |
| Hospital beds/100,000 inhabitants curative care | 301 | 606 | |
| Bed occupancy rate (curative care) | 66% | 80% | |
| Hospital discharges | 1,649,905 | 19,480,503 | |
| Per 1000 inhabitants | 97.17 | 237.06 | |
| Hospital personnel (full-time equivalents) | 198,670 | 988,000 | |
| per 100 patient-days | 2.40 | 0.68 | |
| Physicians | 21,808 | 166,000 | |
| per 100 patient-days | 0.26 | 0.11 | |
| Qualified nurses/midwives | 58,489 | 341,000 | |
| per 100 patient-days | 0.71 | 0.23 | |
| Nursing associates | 11,563 | 34,000 | |
| per 100 patient-days | 0.14 | 0.02 | |
| Antibiotic Stewardship Teams | |||
| 1 team * per hospital mandatorily | 1 FTE per 500 beds recommended * | ||
| Selected interventions per 100,000 inhabitants | |||
| cataract-surgery | 1014 | 1041 | |
| appendectomies | 96 | 155 | |
| Transluminal coronary angioplasty (PTCA) | 234 | 406 | |
| % of 1-day interventions (day-patients, outpatients) | |||
| cataract-surgery | 99.6% | 82.5% | |
| tonsillectomies | 68.4% | 4.0% | |
| Inguinal hernia | 80.2% | 0.3% | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köck, R.; Becker, K.; Idelevich, E.A.; Jurke, A.; Glasner, C.; Hendrix, R.; Friedrich, A.W. Prevention and Control of Multidrug-Resistant Bacteria in The Netherlands and Germany—The Impact of Healthcare Structures. Int. J. Environ. Res. Public Health 2020, 17, 2337. https://doi.org/10.3390/ijerph17072337
Köck R, Becker K, Idelevich EA, Jurke A, Glasner C, Hendrix R, Friedrich AW. Prevention and Control of Multidrug-Resistant Bacteria in The Netherlands and Germany—The Impact of Healthcare Structures. International Journal of Environmental Research and Public Health. 2020; 17(7):2337. https://doi.org/10.3390/ijerph17072337
Chicago/Turabian StyleKöck, Robin, Karsten Becker, Evgeny A. Idelevich, Annette Jurke, Corinna Glasner, Ron Hendrix, and Alexander W. Friedrich. 2020. "Prevention and Control of Multidrug-Resistant Bacteria in The Netherlands and Germany—The Impact of Healthcare Structures" International Journal of Environmental Research and Public Health 17, no. 7: 2337. https://doi.org/10.3390/ijerph17072337
APA StyleKöck, R., Becker, K., Idelevich, E. A., Jurke, A., Glasner, C., Hendrix, R., & Friedrich, A. W. (2020). Prevention and Control of Multidrug-Resistant Bacteria in The Netherlands and Germany—The Impact of Healthcare Structures. International Journal of Environmental Research and Public Health, 17(7), 2337. https://doi.org/10.3390/ijerph17072337






