Ocular Cell Lines and Genotoxicity Assessment
Abstract
1. Introduction
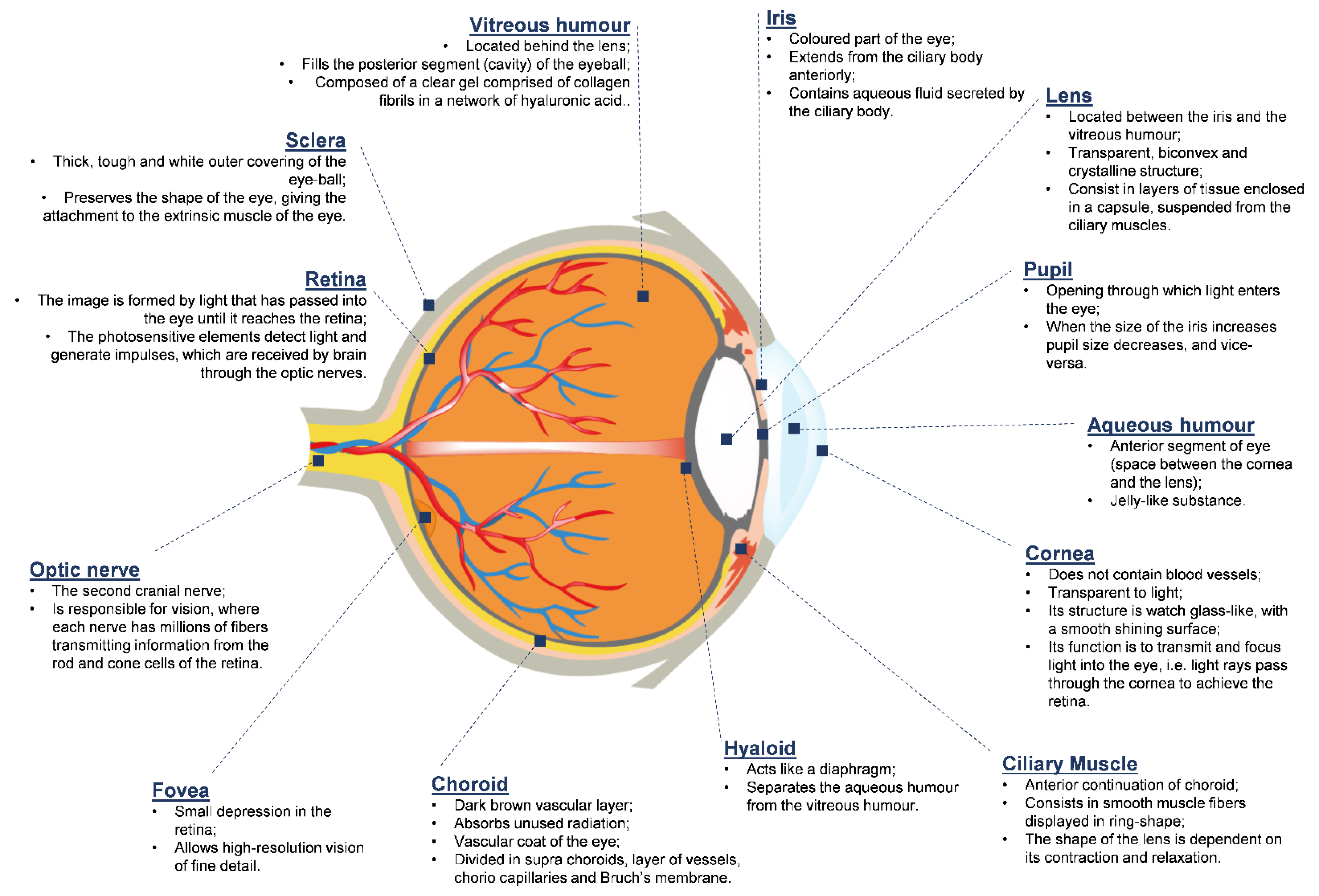
2. The Anatomy and Physiology of the Human Eye
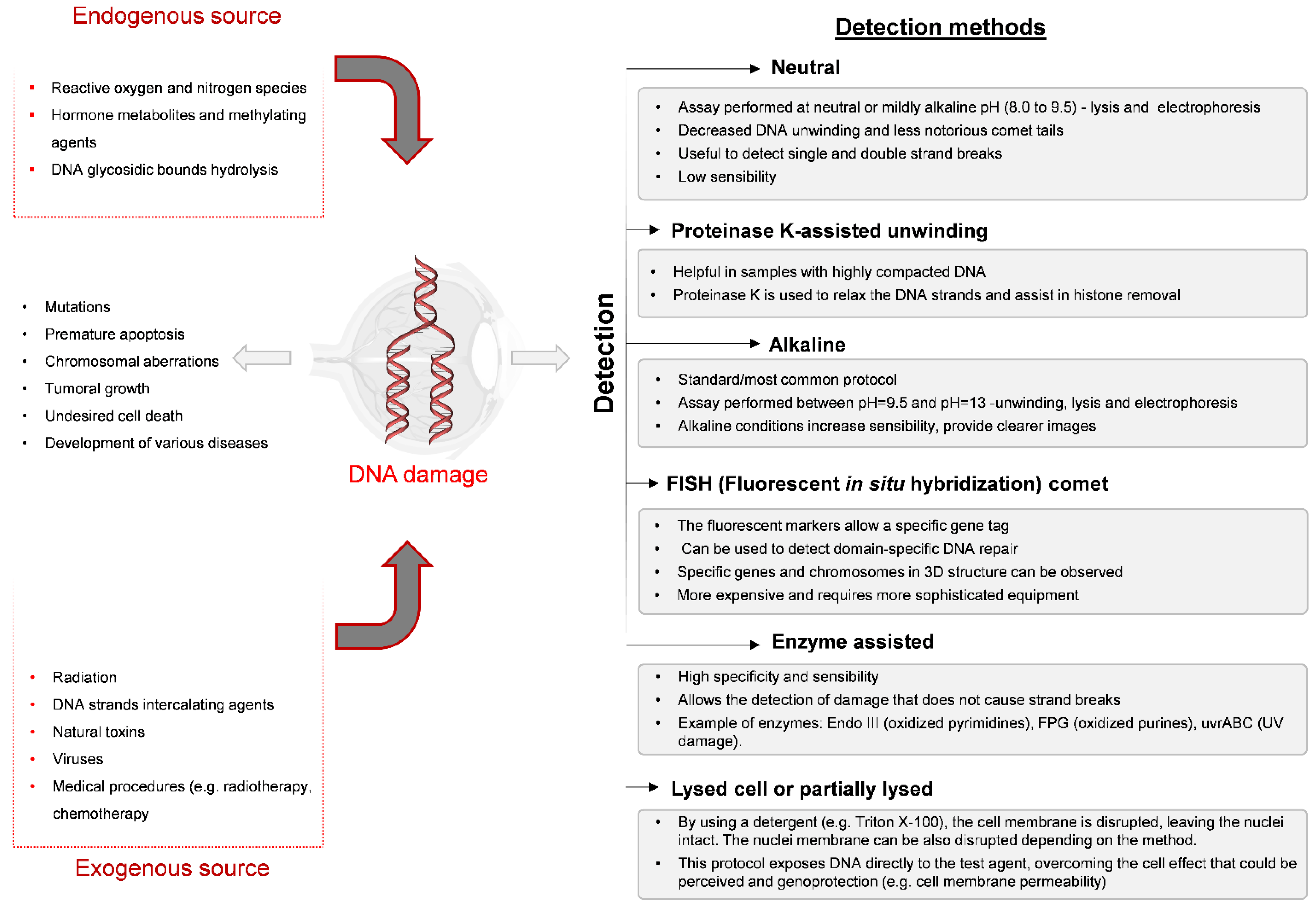
3. Comet Assay
3.1. Lens Epithelial Cells
3.2. Corneal Cells
3.3. Exfoliated Tear Duct Cells
| Cell Type | Previous Treatment | Lysis Solution | Electrophoresis Conditions | Neutralization Solution | Staining | Ref. |
|---|---|---|---|---|---|---|
| Corneal (porcine) | Cells, suspended in 1% Type VII low gelling point agarose, were transferred to a precoated slide with 1% standard agarose | pH = 10 Triton X-100 2.5 M NaCl 0.1 M EDTA 10 mM Tris | Alkaline pH (not specified) 300 mM NaOH 1 mM EDTA 25 V/300 mA 20 min | pH = 7.5 0.5 M Tris | Ethidium bromide | [66] |
| Cornea (human) | Cells, suspended in 0.65% low melting point agarose, were transferred to a slide coated with 0.65% normal melting point agarose | pH = 10 2.5 M NaCl 100 mM EDTA 10 mM Tris 1% Triton X-100 10% DMSO | pH > 13 300 mM NaOH 1 mM EDTA pH > 13 20 V/300 mA 20 min | pH = 7.5 0.4 M Tris | Ethidium bromide | [67] |
| Cornea and retina (rat) | Cells, suspended in 0.5% low melting point agarose, were transferred to a precoated slide with 1% agarose (type I) | pH = 10 2.5 M NaCl 100 mM EDTA 10 mM Tris 1% N-laurylsarcosin 10% DMSO 1% Triton X-100 | pH > 13 300 mM NaOH 1 mM EDTA 0.78 V/cm 40–60 min | pH = 7.5 0.4 M Tris | Propidium iodide | [68] |
| Cornea (human) | Cells, suspended in 0.65% low melting point agarose, were transferred to a slide coated with 0.65% normal melting point agarose | pH = 10 2.5 M NaCl 100 mM EDTA 10 mM Tris 1% Triton X-100 10% DMSO | pH>13 300 mM NaOH 1 mM EDTA 20 V/300 mA 20 min | pH = 7.5 0.4 M Tris | Ethidium bromide | [69] |
| Lens (human) | Cells, suspended in 0.6% low melting point agarose, were transferred to a slide coated with normal melting point agarose | pH = 10 2.5 M NaCl 10 mM Tris 100 mM Na2 EDTA 1% Triton X-100 | Alkaline pH (not specified) 300 mM NaOH 1 mM EDTA 20 V 20 min | pH = 7.5 0.4 M Tris | Ethidium bromide | [70] |
| Lens (human) | Cells, suspended in phosphate buffer saline and 1% low melting point agarose, were transferred to a slide precoated with agarose (not specified) | pH = 10 2.5 M NaCl 10 mM Tris 100 mM EDTA 1% Triton X-100 | pH = 13 300 mM NaOH 1 mM EDTA 1.4 V/cm / 300 mA 40-60 min | Phosphate buffer saline | SYBR Gold | [71] |
| Between lysis and electrophoresis, samples were treated with three enzymes ((i) DNA glycosy-lase (FPG); (ii) endonuclease III (endoIII); (iii) T4 endonuclease V (T4endoV)) | ||||||
| Tear duct | Cells, suspended in 0.5% low melting point agarose, were transferred to a slide precoated with normal melting point agarose | pH = 10 2.5 M NaCl 10 mM Tris 100 mM Na2EDTA 1% Triton X-100 10% DMSO | pH = 13 300 mM NaOH 1 mM EDTA 20 V/300 mA 20 min | pH = 7.5 0.4 M Tris | Ethidium bromide | [72] |
| Cell Type | Procedure | Ref. |
|---|---|---|
| Corneal Epithelium (porcine) | Trypsin treatment (0.25% trypsin/EDTA): dissected cornea was immersed in trypsin/EDTA, incubated at 37 °C (5% CO2 atmosphere) and released cells from corneal epithelium were collected after 30, 60 or 90 min, depending on the layer needed for each assay. Cell membrane integrity was assessed with the trypan blue exclusion test. | [66] |
| Corneal(rabbit) | The eyes were enucleated and immersed in PBS. Cells were obtained after enzymatic treatment with collagenase. The cornea was dissected in half, and the anterior section was treated with collagenase to separate the stroma from epithelial cells. With this process, the intact epithelium can be peeled off from the stroma. The epithelial piece was washed with serum-free culture media, and after mincing, was treated with 0.25% trypsin in calcium-free buffer solution. | [73] |
| Cornea and Retina (Wistar rats) | Dissected tissues were immersed in HBSS buffer (4 °C). A cell suspension was obtained by mincing cornea and retina tissues, in the same buffer, with a tweezer. | [68] |
| Lens (human) | Cell suspension of single cells was obtained by mechanical disruption. Lenses were removed by an experienced surgeon after corneal incision and were kept in DMEM⁄F12 with 15% FBS and antibiotics at 4 °C. The lenses were then subjected to several rounds of pipetting and the cells were easily obtained. | [71] |
| Tear Duct | Cells were obtained from tears via collection with a capillary, which contains the exfoliated epithelial cells. | [72] |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sanchez-Lopez, E.; Espina, M.; Doktorovova, S.; Souto, E.B.; Garcia, M.L. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye—Part II—Ocular drug-loaded lipid nanoparticles. Eur. J. Pharm. Biopharm. 2017, 110, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Espina, M.; Doktorovova, S.; Souto, E.B.; Garcia, M.L. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye—Part I—Barriers and determining factors in ocular delivery. Eur. J. Pharm. Biopharm. 2017, 110, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, J.F.; Veiga, F.; Silva, A.M.; Souto, E.B. Ocular Drug Delivery—New Strategies for Targeting Anterior and Posterior Segments of the Eye. Curr. Pharm. Des. 2016, 22, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Kondalkar, G.; Dev, A.; Rane, A. Current Aspects in Ocular Polymeric in-situ Gelling System. Eur. J. Biomed. Pharm. Sci. 2016, 3, 144–154. [Google Scholar]
- Kaushik, A.A.C.V. Recent Trends in Novel Ophthalmic Drug Delivery System—A Short Review. Am. J. Appl. Sci. 2016, 3, 189–194. [Google Scholar]
- Lee, R.F.; Steinert, S. Use of the single cell gel electrophoresis/comet assay for detecting DNA damage in aquatic (marine and freshwater) animals. Mutat. Res. Rev. Mutat. Res. 2003, 544, 43–64. [Google Scholar] [CrossRef]
- Kirkland, D.; Reeve, L.; Gatehouse, D.; Vanparys, P. A core in vitro genotoxicity battery comprising the Ames test plus the in vitro micronucleus test is sufficient to detect rodent carcinogens and in vivo genotoxins. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2011, 721, 27–73. [Google Scholar] [CrossRef]
- Chang, H.Y.; Koh, V.C.Y.; Md Nasir, N.D.; Ng, C.C.Y.; Guan, P.; Thike, A.A.; Teh, B.T.; Tan, P.H. MED12, TERT and RARA in fibroepithelial tumours of the breast. J. Clin. Pathol. 2020, 73, 51–56. [Google Scholar] [CrossRef]
- Sawant, S.G.; Fielden, M.R.; Black, K.A. Evaluation of genotoxicity testing of FDA approved large molecule therapeutics. Regul. Toxicol. Pharmacol. 2014, 70, 87–97. [Google Scholar] [CrossRef]
- Thybaud, V.E.A. Strategies in case of positive in vivo results in genotoxicity testing. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2011, 723, 121–128. [Google Scholar] [CrossRef]
- Abenavoli, L.; Izzo, A.A.; Milic, N.; Cicala, C.; Santini, A.; Capasso, R. Milk thistle (Silybum marianum): A concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother. Res. 2018, 32, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Daliu, P.; Santini, A.; Novellino, E. A decade of nutraceutical patents: Where are we now in 2018? Expert Opin. Ther. Pat. 2018, 28, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Daliu, P.; Santini, A.; Novellino, E. From pharmaceuticals to nutraceuticals: Bridging disease prevention and management. Expert Rev. Clin. Pharmacol. 2019, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A. Extractable and Non-extractable polyphenols: An overview. In Non-Extractable Polyphenols and Carotenoids: Importance in Human Nutrition and Health; Saura-Calixto, F., Pérez-Jiménez, J., Eds.; Royal Society of Chemistry: London, UK, 2018; pp. 1–37. [Google Scholar]
- Durazzo, A.; D’Addezio, L.; Camilli, E.; Piccinelli, R.; Turrini, A.; Marletta, L.; Marconi, S.; Lucarini, M.; Lisciani, S.; Gabrielli, P.; et al. From Plant Compounds to Botanicals and Back: A Current Snapshot. Molecules 2018, 23, 1844. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Armstrong, L.; Rescigno, A.; Yeskaliyeva, B.; Seitimova, G.; Beyatli, A.; Sharmeen, J.; Mahomoodally, M.F.; Sharopov, F.; Durazzo, A.; et al. Lamium Plants-A Comprehensive Review on Health Benefits and Biological Activities. Molecules 2019, 24, 1913. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.A. Current shot and re-thinking of antioxidant research strategy. Braz. J. Anal. Chem. 2018, 5, 9–11. [Google Scholar] [CrossRef]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 2018, 84, 659–672. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals: Beyond the diet before the drugs. Curr. Bioact. Comp. 2014, 10, 1–12. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. To Nutraceuticals and Back: Rethinking a Concept. Foods 2017, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Novellino, E. Nutraceuticals—Shedding light on the grey area between pharmaceuticals and food. Expert Rev. Clin. Pharmacol. 2018, 11, 545–547. [Google Scholar] [CrossRef]
- Rigon, R.B.; Fachinetti, N.; Severino, P.; Durazzo, A.; Lucarini, M.; Atanasov, A.G.; El Mamouni, S.; Chorilli, M.; Santini, A.; Souto, E.B. Quantification of Trans-Resveratrol-Loaded Solid Lipid Nanoparticles by a Validated Reverse-Phase HPLC Photodiode Array. Appl. Sci. 2019, 9, 4961. [Google Scholar] [CrossRef]
- Souto, E.B.; Souto, S.B.; Zielinska, A.; Durazzo, A.; Lucarini, M.; Santini, A.; Horbańczuk, O.K.; Atanasov, A.G.; Marques, C.; Andrade, L.N.; et al. Perillaldehyde 1,2-epoxide loaded SLN-tailored mAb: Production, physicochemical characterization and in vitro cytotoxicity profile in MCF-7 cell lines. Pharmaceutics 2020, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Zielinska, A.; Souto, S.B.; Durazzo, A.; Lucarini, M.; Santini, A.; Silva, A.M.; Atanasov, A.G.; Marques, C.; Andrade, L.N.; et al. (+)-Limonene 1,2-epoxide-loaded SLN: Evaluation of drug release, antioxidant activity and cytotoxicity in HaCaT cell line. Int. J. Mol. Sci. 2020. submitted. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.; Severino, P.; Nalone, L.A.; Souto, S.B.; Silva, A.M.; Lucarini, M.; Durazzo, A.; Santini, A.; Souto, E.B. Sucupira Oil-Loaded Nanostructured Lipid Carriers (NLC): Lipid Screening, Factorial Design, Release Profile, and Cytotoxicity. Molecules 2020, 25, 685. [Google Scholar] [CrossRef] [PubMed]
- Belpaeme, K.; Cooreman, K.; Kirsch-Volders, M. Development and validation of the in vivo alkaline comet assay for detecting genomic damage in marine flatfish. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 1998, 415, 167–184. [Google Scholar] [CrossRef]
- Doktorovova, S.; Silva, A.M.; Gaivao, I.; Souto, E.B.; Teixeira, J.P.; Martins-Lopes, P. Comet assay reveals no genotoxicity risk of cationic solid lipid nanoparticles. J. Appl. Toxicol. 2014, 34, 395–403. [Google Scholar] [CrossRef]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers—A systematic review of in vitro data. Eur. J. Pharm. Biopharm. 2014, 87, 1–18. [Google Scholar] [CrossRef]
- Kurashige, T.; Shimamura, M.; Nagayama, Y. Differences in quantification of DNA double-strand breaks assessed by 53BP1/γH2AX focus formation assays and the comet assay in mammalian cells treated with irradiation and N-acetyl-L-cysteine. J. Radiat. Res. 2016, 57, 312–317. [Google Scholar] [CrossRef][Green Version]
- Ostling, O.; Johanson, K.J. Microelectrophoretic Study of Radiation-Induced DNA Damages in Individual Mammalian Cells. Biochem. Biophys. Res. Commun. 1984, 123, 291–298. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Collins, A.R. The comet assay for DNA damage and repair. Mol. Biotechnol. 2004, 26, 249. [Google Scholar] [CrossRef]
- Tice, R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y. Single cell gel/Comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Speit, G.A.H.A. The Comet Assay (Single-Cell Gel Test). In Methods in Molecular Biology. DNA Repair Protocols; Henderson, D.S., Ed.; Humana Press Inc.: Totowa, New Jersey, USA, 1999; Volume 113. [Google Scholar]
- Sheetu, W.; Rishi, P.; Shivani Rai, P.; Vyas, S.P. Nanocarriers in Ocular Drug Delivery: An Update Review. Curr. Pharm. Des. 2009, 15, 2724–2750. [Google Scholar] [CrossRef]
- Rojas, E.; Lorenzo, Y.; Haug, K.; Nicolaissen, B.; Valverde, M. Epithelial cells as alternative human biomatrices for comet assay. Front. Genet. 2014, 5, 386. [Google Scholar] [CrossRef] [PubMed]
- Nabi, I.R.; Mathews, A.P.; Cohen-Gould, L.; Gundersen, D.; Rodriguez-Boulan, E. Immortalization of polarized rat retinal pigment epithelium. J. Cell Sci. 1993, 104, 37–49. [Google Scholar]
- Lou, D.-A.; Hu, F. Specific antigen and organelle expression of a long-term rhesus endothelial cell line. In Vitro Cell. Dev. Biol. 1987, 23, 75–85. [Google Scholar] [CrossRef]
- Gospodarowicz, D.; Mescher, A.L.; Birdwell, C.R. Stimulation of corneal endothelial cell proliferation in vitro by fibroblast and epidermal growth factors. Exp. Eye Res. 1977, 25, 75–89. [Google Scholar] [CrossRef]
- Bethea, C.L.; Kozak, S.L. Effect of extracellular matrix on PC 12 cell shape and dopamine processing. Mol. Cell. Endocrinol. 1984, 37, 319–329. [Google Scholar] [CrossRef]
- McFall, R.C.; Sery, T.W.; Makadon, M. Characterization of a New Continuous Cell Line Derived from a Human Retinoblastoma. Cancer Res. 1977, 37, 1003–1010. [Google Scholar]
- McFall, R.C.; Nagy, R.M.; Nagle, B.T.; McGreevy, L.M. Scanning Electron Microscopic Observation of Two Retinoblastoma Cell Lines. Cancer Res. 1978, 38, 2827–2835. [Google Scholar]
- Rostomily, R.C.; Bermingham-McDonogh, O.; Berger, M.S.; Tapscott, S.J.; Reh, T.A.; Olson, J.M. Expression of Neurogenic Basic Helix-Loop-Helix Genes in Primitive Neuroectodermal Tumors. Cancer Res. 1997, 57, 3526–3531. [Google Scholar]
- Andley, U.P.; Rhim, J.S.; Chylack, L.T., Jr.; Fleming, T.P. Propagation and immortalization of human lens epithelial cells in culture. Invest. Ophthalmol. Vis. Sci. 1994, 35, 3094–3102. [Google Scholar] [PubMed]
- Fleming, T.; Song, Z.; Andle, U. Expression of Growth Control and Differentiation Genes in Human Lens Epithelial Cells with Extended Life Span. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1387–1398. [Google Scholar]
- Amirouchene-Angelozzi, N.; Nemati, F.; Gentien, D.; Nicolas, A.; Dumont, A.; Carita, G.; Camonis, J.; Desjardins, L.; Cassoux, N.; Piperno-Neumann, S.; et al. Establishment of novel cell lines recapitulating the genetic landscape of uveal melanoma and preclinical validation of mTOR as a therapeutic target. Mol. Oncol. 2014, 8, 1508–1520. [Google Scholar] [CrossRef]
- Kahn, C.R.; Young, E.; Lee, I.H.; Rhim, J.S. Human corneal epithelial primary cultures and cell lines with extended life span: In vitro model for ocular studies. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3429–3441. [Google Scholar]
- Farris, A.D.; Koelsch, G.; Pruijn, G.J.; van Venrooij, W.J.; Harley, J.B. Conserved features of Y RNAs revealed by automated phylogenetic secondary structure analysis. Nucleic Acids Res. 1999, 27, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Samuel Bradford, C.; Miller, A.E.; Toumadje, A.; Nishiyama, K.; Shirahata, S.; Barnes, D.W. Characterization of cell cultures derived from Fugu, Japanese pufferfish. Mol. Mar. Biol. Biotechnol. 1998, 6, 279–288. [Google Scholar]
- Dunn, K.C.; Aotaki-Keen, A.E.; Putkey, F.R.; Hjelmeland, L.M. ARPE-19, A Human Retinal Pigment Epithelial Cell Line with Differentiated Properties. Exp. Eye Res. 1996, 62, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Kruszewski, F.H.; Walker, T.L.; DiPasquale, L.C. Evaluation of a Human Corneal Epithelial Cell Line as anin VitroModel for Assessing Ocular Irritation. Fundam. Appl. Toxicol. 1997, 36, 130–140. [Google Scholar] [CrossRef]
- Janssen, J.J.; Kuhlmann, E.D.; van Vugt, A.H.; Winkens, H.J.; Janssen, B.P.; Deutman, A.F.; Driessen, C.A. Retinoic acid delays transcription of human retinal pigment neuroepithelium marker genes in ARPE-19 cells. Neuroreport 2000, 11, 1571–1579. [Google Scholar] [CrossRef]
- Silva, A.M.; Martins-Gomes, C.; Fangueiro, J.F.; Andreani, T.; Souto, E.B. Comparison of antiproliferative effect of epigallocatechin gallate when loaded into cationic solid lipid nanoparticles against different cell lines. Pharm. Dev. Technol. 2019, 24, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Alvarado, H.L.; Abrego, G.; Martins-Gomes, C.; Garduno-Ramirez, M.L.; Garcia, M.L.; Calpena, A.C.; Souto, E.B. In Vitro Cytotoxicity of Oleanolic/Ursolic Acids-Loaded in PLGA Nanoparticles in Different Cell Lines. Pharmaceutics 2019, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Canadas, C.; Alvarado, H.; Calpena, A.C.; Silva, A.M.; Souto, E.B.; Garcia, M.L.; Abrego, G. In vitro, ex vivo and in vivo characterization of PLGA nanoparticles loading pranoprofen for ocular administration. Int. J. Pharm. 2016, 511, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, J.F.; Andreani, T.; Egea, M.A.; Garcia, M.L.; Souto, S.B.; Silva, A.M.; Souto, E.B. Design of cationic lipid nanoparticles for ocular delivery: Development, characterization and cytotoxicity. Int. J. Pharm. 2014, 461, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Kruse, F.E. Stem cells and corneal epithelial regeneration. Eye 1994, 8, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.H.; Pawlina, W. Chapter 5. Epithelial tissue. In Histology: A Text and Atlas with Correlated Cell and Molecular Biology, 7th ed.; Taylor, K.H.S.C., Ajello, J.P., Clow, R., Eds.; Wolters Kluwer Health: Philadelphia, PA, USA, 2015; pp. 105–154. [Google Scholar]
- Chiego, D.J. Chapter 2. Structure and function of cells tissues and organs. In Essentials of Oral Histology and Embryology: A Clinical Approach, 5th ed.; Duncan, K.F.L., Hebberd, K., Dumas, J., Eddy, J., Waters, J., Pauls, K., Eds.; Elsevier: St. Louis, Missouri, USA, 2018; pp. 18–35. [Google Scholar]
- Sorte, K.; Sune, P.; Bhake, A.; Shivkumar, V.B.; Gangane, N.; Basak, A. Quantitative assessment of DNA damage directly in lens epithelial cells from senile cataract patients. Mol. Vis. 2011, 17, 1–6. [Google Scholar]
- Zhang, J.; Wu, J.; Yang, L.; Zhu, R.; Yang, M.; Qin, B.; Shi, H.; Guan, H. DNA Damage in Lens Epithelial Cells and Peripheral Lymphocytes from Age-Related Cataract Patients. Ophthalmic Res. 2014, 51, 124–128. [Google Scholar] [CrossRef]
- Ringen, O.; Oscoz, A.; Haug, K.; Moe, M.; Zetterstrom, C.; Collins, A.; Nicolaissen, B. Human Lens Epithelium in Cataract: Qualitative and Quantitative Evaluation of DNA Damage, Apoptosis and Evaluation of a System for Intervention Assays. Invest. Ophthalmol. Vis. Sci. 2010, 51, 2622. [Google Scholar]
- Haug, K.; Azqueta, A.; Johnsen-Soriano, S.; Shahdadfar, A.; Drolsum, L.K.; Moe, M.C.; Røger, M.T.; Romero, F.J.; Collins, A.R.; Nicolaissen, B. Donor cornea transfer from Optisol GS to organ culture storage: A two-step procedure to increase donor tissue lifespan. Acta Ophthalmol. 2013, 91, 219–225. [Google Scholar] [CrossRef]
- Lorenzo, Y.; Berg, H.; Ustgaard-Andersen, K.; Johnsen, O.; Ringvold, A. Trypsin for dissociation of Limbal Cells for Engineering of Grafts May Induce DNA Strand Breaks in the Harvested Cells. J. Ocular Biol. 2013, 1, 6. [Google Scholar]
- Azqueta, A.; Shaposhnikov, S.; Collins, A.R. DNA oxidation: Investigating its key role in environmental mutagenesis with the comet assay. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2009, 674, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Choy, C.K.M.; Benzie, I.F.F.; Cho, P. UV-Mediated DNA Strand Breaks in Corneal Epithelial Cells Assessed Using the Comet Assay Procedure. Photochem. Photobiol. 2005, 81, 493–497. [Google Scholar] [CrossRef]
- Ye, J.; Wu, H.; Zhang, H.; Wu, Y.; Yang, J.; Jin, X.; Shi, X. Role of benzalkonium chloride in DNA strand breaks in human corneal epithelial cells. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 1681. [Google Scholar] [CrossRef] [PubMed]
- Struwe, M.; Greulich, K.-O.; Junker, U.; Jean, C.; Zimmer, D.; Suter, W.; Plappert-Helbig, U. Detection of photogenotoxicity in skin and eye in rat with the photo comet assay. Photochem. Photobiol. Sci. 2008, 7, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, H.; Wang, C.; Wu, Y.; Xie, J.; Jin, X.; Yang, J.; Ye, J. Genoprotective effect of hyaluronic acid against benzalkonium chloride-induced DNA damage in human corneal epithelial cells. Mol. Vis. 2011, 17, 3364–3370. [Google Scholar] [PubMed]
- Pierscionek, B.K.; Li, Y.; Yasseen, A.A.; Colhoun, L.M.; Schachar, R.A.; Chen, W. Nanoceria have no genotoxic effect on human lens epithelial cells. Nanotechnology 2009, 21, 035102. [Google Scholar] [CrossRef]
- Øsnes-Ringen, O.; Azqueta, A.O.; Moe, M.C.; Zetterström, C.; Røger, M.; Nicolaissen, B.; Collins, A.R. DNA damage in lens epithelium of cataract patients in vivo and ex vivo. Acta Ophthalmol. 2013, 91, 652–656. [Google Scholar] [CrossRef]
- Rojas, E.; Valverde, M.; Lopez, M.C.; Naufal, I.; Sanchez, I.; Bizarro, P.; Lopez, I.; Fortoul, T.I.; Ostrosky-Wegman, P. Evaluation of DNA damage in exfoliated tear duct epithelial cells from individuals exposed to air pollution assessed by single cell gel electrophoresis assay. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2000, 468, 11–17. [Google Scholar] [CrossRef]
- Chan, K.Y.; Haschke, R.H. Isolation and culture of corneal cells and their interactions with dissociated trigeminal neurons. Exp. Eye Res. 1982, 35, 137–156. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Lopez-Machado, A.L.; Bonilla, V.L.; Pizarro, P.G.; Silva, A.M.; Souto, E.B. Lipid nanoparticles as carriers for the treatment of neurodegeneration associated with Alzheimer’s disease and glaucoma: present and future challenges. Curr. Pharm. Des. 2020, 26, 1. [Google Scholar] [CrossRef]
- Guo, D.; Bi, H.; Wu, Q.; Wang, D.; Cui, Y. Zinc oxide nanoparticles induce rat retinal ganglion cell damage through bcl-2, caspase-9 and caspase-12 pathways. J. Nanosci. Nanotechnol. 2013, 13, 3769–3777. [Google Scholar] [CrossRef] [PubMed]
- Coelho, F.; do Vale Braido, G.V.; Cavicchioli, M.; Mendes, L.S.; Specian, S.S.; Franchi, L.P.; Lima Ribeiro, S.J.; Messaddeq, Y.; Scarel-Caminaga, R.M.; TS, O.C. Toxicity of therapeutic contact lenses based on bacterial cellulose with coatings to provide transparency. Cont. Lens. Anterior. Eye 2019, 42, 512–519. [Google Scholar] [CrossRef] [PubMed]
| Ocular Cell Line | Characteristics | Refs |
|---|---|---|
| RPE-J | RPE-J is a retinal pigment epithelial (RPE) cell line, obtained from the primary cultures of RPE cells obtained on 7-day-old Long–Evans rats. | [37] |
| RF/6A | RF/6A—monkey endothelial cell line—is spontaneously transformed at an early age and had been passaged over 540 times. | [38] |
| BCE C/D 1-b | BCE C/D-1b—cow endothelial cell line—is established from explants of normal adult bovine corneas. | [39,40] |
| WERI-Rb-1 | WERI-Rb-1 is a human retinoblastoma tumorigenic cell line used in cell differentiation and biomedical studies, and in animal models of tumor therapy. | [41,42,43] |
| B-3 | B-3 cell line was removed from a human lens obtained from a 5–12-month-old patient who was treated for premature retinopathy. This cell line was infected with Ad12-SV40 at 60% confluence and passage 3. | [44,45] |
| MP65 | MP65 cell line-primary tumor of uveal melanoma of the eye in adults—is part of a panel that imitates the genetic alterations and mutations of this disease. | [46] |
| 2.040 pRSV-T | 2.040 pRSV-T cell line is a primary culture of normal corneal epithelium, it is immortalized by transfection with the plasmid pRSV-T using lipofectamine. pRSV-T incorporates the SV40 early region genes and the Rous Sarcoma virus long terminal repeat. | [47] |
| HCE-2 (50.B1) | HCE-2 cell line is a primary culture of normal corneal epithelium, which by incubation was immortalized with the Ad12-SV40 hybrid virus. | [47] |
| MP46 | MP46 is a cell line from the primary tumor of uveal melanoma of the eye in adults with the same characteristics of the MP65 cell line. | [46] |
| SIRC | Statens Seruminstitut Rabbit Cornea is a cell line from the cornea of rabbits. The early appearance of distinct cytopathic changes makes it suitable for both the propagation and quantification of the rubella virus, making this cell line appropriate for primary isolation of the rubella virus. | [48] |
| Fugu Eye | Fugu eye cell line was created from normal eye tissue. These cells maintain a compact genome size with small introns, that are telomerase positive. The cell line can be used for in vitro vertebrate genome research and for marine fish or aquaculture studies. | [49] |
| ARPE-19 HPV-16 | ARPE/HPV-16 transformed cell line was derived from the ARPE-19 cell line by transfection with DH5-HPV-16. ARPE-19 is a spontaneously arising retinal pigment epithelium (RPE) cell line that derived from the normal eyes of a 19-year-old male who died from head trauma in a motor vehicle accident. The cells express the RPE-specific markers CRALBP and RPE-65. | [50] |
| MP38 | MP38 is a cell line from the primary tumor of uveal melanoma of the eye in adults. It belongs to a unique and first panel of 6 uveal melanoma cell lines from either patient tumors or patient-derived tumor xenografts (PDXs). All these cell lines display GNAQ or GNA11 activating mutations. Four of them present BAP1 (BRCA1 associated protein-1) deficiency, which is known to be a characteristic of aggressive disease. | [46] |
| 10.014 pRSV-T | 10.014 pRSV-T cell line—an epithelial cell—was transfected with a plasmid that has the SV40 early region primary culture of normal corneal epithelium, using lipofectamine to immortalize the transfection with plasmid pRSV-T. This plasmid has the SV40 early region genes as well as the Rous Sarcoma virus long terminal repeat. | [51] |
| ARPE-19 | ARPE-19 cell line—a transfection host—is a spontaneously arising retinal pigment epithelium (RPE). These cells form stable monolayers, exhibiting morphological and functional polarity. ARPE-19 expresses the RPE-specific markers CRALBP and RPE-65. These cells are diploid and can be carried for over 30 passages. | [50,52] |
| MP41 | MP41 is also a cell line from the primary tumor of uveal melanoma of the eye in adults and has the same characteristics as MP65, MP46 and MP38 cells lines. | [46] |
| Y79 | Y79 cell line was isolated after the enucleation of a primary tumor from the right eye, creating a culture. It is known that the donor had a family history of retinoblastoma. After the development of this culture, it was possible to observe analogous ultrastructural characteristics as the original tumor (mainly nuclear membrane infoldings, triple membrane structures, microtubules, large coated vesicles, basal bodies and centrioles, and annulate lamellae). | [43,53,54,55,56] |
| MM28 | MM28 is also part of a panel of cell lines from the primary tumor of uveal melanoma from the adult eye, from either patient tumors or patient-derived tumor xenografts, also showing GNAQ or GNA11 activating mutations and BAP1 deficiency. | [46] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souto, E.B.; Campos, J.R.; Da Ana, R.; Martins-Gomes, C.; Silva, A.M.; Souto, S.B.; Lucarini, M.; Durazzo, A.; Santini, A. Ocular Cell Lines and Genotoxicity Assessment. Int. J. Environ. Res. Public Health 2020, 17, 2046. https://doi.org/10.3390/ijerph17062046
Souto EB, Campos JR, Da Ana R, Martins-Gomes C, Silva AM, Souto SB, Lucarini M, Durazzo A, Santini A. Ocular Cell Lines and Genotoxicity Assessment. International Journal of Environmental Research and Public Health. 2020; 17(6):2046. https://doi.org/10.3390/ijerph17062046
Chicago/Turabian StyleSouto, Eliana B., Joana R. Campos, Raquel Da Ana, Carlos Martins-Gomes, Amélia M. Silva, Selma B. Souto, Massimo Lucarini, Alessandra Durazzo, and Antonello Santini. 2020. "Ocular Cell Lines and Genotoxicity Assessment" International Journal of Environmental Research and Public Health 17, no. 6: 2046. https://doi.org/10.3390/ijerph17062046
APA StyleSouto, E. B., Campos, J. R., Da Ana, R., Martins-Gomes, C., Silva, A. M., Souto, S. B., Lucarini, M., Durazzo, A., & Santini, A. (2020). Ocular Cell Lines and Genotoxicity Assessment. International Journal of Environmental Research and Public Health, 17(6), 2046. https://doi.org/10.3390/ijerph17062046








