Atmospheric Pollution Exposure Increases Disease Activity of Systemic Lupus Erythematosus
Abstract
1. Introduction
2. Material and Methods
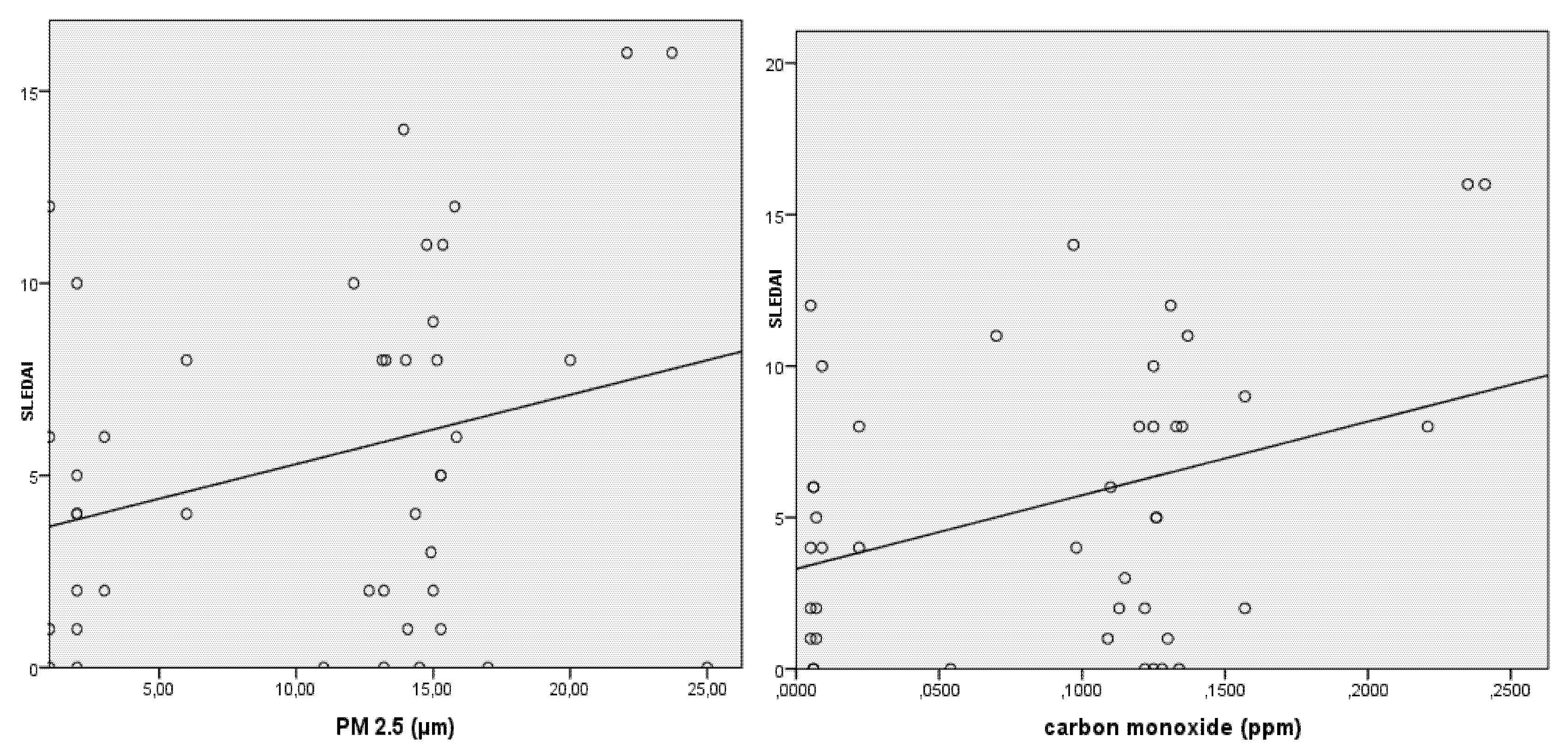
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Silva, C.A.; Avcin, T.; Brunner, H.I. Taxonomy for systemic lUpus erythematosus with on set before adulthood. Arthritis Care Res. (Hoboken) 2012, 64, 1787–1793. [Google Scholar] [CrossRef]
- Borba, E.F.; Latorre, L.C.; Brenol, J.C.T.; Kayser, C.; Silva, N.A.D.; Zimmermann, A.F.; Pádua, P.M.D.; Costallat, L.T.L.; Bonfá, E.; Sato, E.I. Consenso de Lúpus Eritematoso Sistêmico. Bras. Rheumatol. Julho/Agosto 2008, 48, 196–207. [Google Scholar] [CrossRef][Green Version]
- Cavalcante, E.G.; Aikawa, N.E.; Lozano, R.G.; Lolito, A.P.; Jesus, A.A.; Silva, C.A. Chronic polyarthritis as the first manifestation of juvenile systemic lupus erythematosus patients. Lupus 2011, 20, 960–964. [Google Scholar] [CrossRef]
- Vidotto, J.P.; Pereira, L.A.; Braga, A.L.; Silva, C.A.; Sallum, A.M.; Campos, L.M.; Martins, L.C.; Fahat, S.C. Atmospheric pollution, influence on hospital admissions in paediatric rhematic diseases. Lupus 2012, 21, 526–533. [Google Scholar] [CrossRef]
- Campos, L.M.; Silva, C.A.; Aikawa, N.E.; Jesus, A.A.; Moraes, J.B.C.; Miraglia, J.; Ishida, M.A.; Bueno, C.; Pereira, R.M.R.; Bonfa, E. Acute pancreatitis in juvenile sys temic lupus erythematosus: a manifestation of macrophage activation syndrome? Arthritis Care Res. 2013, 65, 1121–1127. [Google Scholar] [CrossRef]
- Ritz, A.S. Air pollution as a potential contributor to the ‘epidemic’ of autoimune disease. Med. Hypotheses 2010, 74, 110–117. [Google Scholar] [CrossRef]
- ACR. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lúpus syndromes. Arthritis Rheum 1999, 42, 599–608. [Google Scholar] [CrossRef]
- Farhat, S.C.; Silva, C.A.; Orione, M.A.; Campos, L.M.; Sallum, A.M.; Braga, A.L. Air pollution in autoimune rheumatic diseases: A review. Autoimmun. Rev. 2011, 11, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.C.; Silva, C.A.; Braga, A.L.; Sallum, A.M.; Campos, L.M.; Farhat, S.C. Exposure to air pollutants and disease activity in juvenile-onset systemic lupus erythematosus patients. Arthritis Care Res. 2015, 67, 1609–1614. [Google Scholar] [CrossRef]
- Tramuto, F.; Cusimano, R.; Cerame, G.; Vultaggio, M.; Calamusa, G.; Maida, C.M.; Vitale, F. Urbana ir pollution and emergency room admissions for respiratory symptoms, a case-crossover study in Palarmo, Italy. Environ. Health 2011, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Makar, M.; Antonelli, J.; Di, Q.; Cutler, D.; Schwartz, J.; Dominic, F. Estimating the casual effect of low levels of fine particulate matter on hospitalization. Epidemiology 2017, 28, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Zeft, A.S.; Spalding, S.J. Autoinflammatory syndromes: fever is not always a sign of infection. Cleve Clin. J. Med. 2012, 79, 569–581. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nascimento, L.F.C.; Medeiros, A.P.P. Internações por pneumonias e queimadas: Uma abordagem espacial. J. Pediatr. 2012, 88, 177–183. [Google Scholar]
- Gong, H.; Linn, W.S.; Clark, K.W.; Anderson, K.R.; Sioutas, C.; Alexis, N.E.; Cascio, W.E.; Devlin, R.B. Exposures of healthy and a sinthomatic volunteers to concentrated ambiente ultrafine particles in Los Angeles. Inhal. Toxicol. 2008, 20, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Zeft, A.S.; Prahalad, S.; Lefreve, S.; Clifford, B.; McNally, B.; Bohnsack, J.F.; Pope, C.A. Juvenile idiopathic arthritis and exposure to fine particulate air pollution. Clin. Exp. Rheumatol. 2009, 27, 877–884. [Google Scholar] [PubMed]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Exposure Air to Pollution: A Major Public Health Concern; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- BRASIL. Infographics; Brazilian Institute of Geography and Statistics (IBGE): Rio de Janeiro, Brazil, 2016. [Google Scholar]
- BRASIL. Municipalities of the Legal Amazon; Brazilian Institute of Geography and Statistics (IBGE): Rio de Janeiro, Brazil, 2014. [Google Scholar]
- Hochberg, M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus (letter). Arthritis Rheum 1997, 40, 1725. [Google Scholar] [CrossRef]
- Longo, K.S.R.; Freitas, A.; Setzer, E.; Prins, P.; Andreae Artaxo, M. The Coupled Aerosol and Tracer Transport model to the Brazilian developments on the Regional Atmospheric Modeling System (CATT-BRAMS) Part 2: Model sensivity to the biomass burning inventories. Atmos. Chem. Phys. Discuss 2007, 7, 8571–8595. [Google Scholar] [CrossRef]
- Roper, C.; Chubb, L.G.; Cambal, L.; Tunno, B.; Clougherty, J.E.; Mischler, S.E. Characterization of ambiente and extrated PM2.5 colleted on filters for toxicology applications. Inhal. Toxicol. 2015, 27, 673–681. [Google Scholar] [CrossRef]
- Guimarães, L.S.; Hirakata, V.N. Uso do modelo de equacões de estimativas generalizadas na análise de dados longitudinais. Rev. HCPA 2012, 32, 501–511. [Google Scholar]
- Zeger, S.L.; Liang, K.Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986, 42, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.Y.; Zeger, S.L. Longitudinal data analysis using generalizedlinear models. Biometrika 1986, 73, 13–22. [Google Scholar] [CrossRef]
- Bermatsky, S.; Founier, M.; Pineau, C.A.; Clarke, A.E.; Vinet, E.; Smargiassi, A. Association between ambiente fine particulate levels and disease activity in patients with systemic lúpus erythematosus (SLE). Environ. Health Perspect 2011, 119, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Seaton, A.; MacNee, W.; Donald, K.; Godden, D. Particulate air pollution and acute health effects. Lancet 1999, 345, 176–178. [Google Scholar] [CrossRef]
- Quinn, A.K.; Ae-Ngibise, K.A.; Enuameh, Y.; Mujtaba, M.N.; Chillrud, S.N.; Blair, J. Association of Carbon Monoxide exposure with blood pressure among pregnant women in rural Ghana: Evidence from GAPHS. Int. J. Hyg. Environ. Health 2016, 219, 176–183. [Google Scholar] [CrossRef]
- Gong, H.; Linn, W.S.; Terrel, S.L.; Anderson, K.R.; Clark, K.W.; Sioutas, C.; Cascio, W.E.; Alexis, N.; Devlin, R.B. Exposures of elderly volunteers with and without chronic obstructive pulmonar disease (COPD) to concentrated ambiente fine particulate pollution. Inhal. Toxicol. 2004, 16, 731–744. [Google Scholar] [CrossRef]
- Wang, F.F.; Geng, C.M.; Hao, W.D.; Zhao, Y.D.; Li, Q.; Wang, H.M.; Qian, Y. The cellular toxicity of PM2.5 emitted from alcool combustion in human umbilical vein endotelial cells. Biomed. Environ. Sci. 2016, 29, 107–116. [Google Scholar]
- Calderón-Garcidueñas, L.; Macías-Parra, M.; Hoffman, H.J.; Valencia-Salazar, G.; Henríquez-Roldán, C.; Osnaya, N. Immunotoxicit and environment: Immunodysregulation and systemic inflammation in children. Toxicol. Pathol. 2009, 37, 161–169. [Google Scholar] [CrossRef]
- Bernstein, J.A.; Alex, N.; Barnes, C.; Bernstein, J.A.; Nel, A.; Peden, D.; Diaz-Sanchez, D.; Tarlo, S.M.; Williams, P.B. Health effects ofd air pollution. J. Allergy Clin. Immunol. 2004, 114, 1116–1123. [Google Scholar] [CrossRef]
- Carmo, C.N.; Hacon, S.; Longo, K.M.; Freitas, S.; Ignotti, E.; Ponce de Leon, A.; Artaxo, P. Association between burning particulate matter and respiratory diseases in the southern region of the Brazilian Amazon. Rev Panam. Salud. Publica. 2010, 27, 10–16. [Google Scholar]
- Rodrigues, P.C.O.; Santos, E.S.; Ignotti, E.; Hacon, S. Space-Time analysis to identify areas at risk of mortality from cardiovascular disease. BioMed Res. Int. 2015, 2015, 841645. [Google Scholar] [CrossRef] [PubMed]
- Castro, H.A.C.; Cunha, M.F.; Mendonça, C.A.S.; Junger, W.L.; Cunha-Cruz, J.; Leon, A.P. Efeitos da poluição do ar na função respiratória de escolares, Rio de Janeiro. RJ Revista Saúde Pública São Paulo 2009, 43, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Stewart, M.D.; Peterson, J.E.; Baretta, E.D.; Bachand, R.T.; Hosko, M.J.; Herrmann, A.A. Experimental human exposure to carbono monoxide. Arch. Environ. Health 2013, 21, 154–164. [Google Scholar]
- World Health Organization. Air Quality Guidelines: Global Update 2005, Particula Tematter Ozone, Nitrogen, Dioxide and Sulfur Dioxide; WHO: Geneva, Switzerland; Available online: http://www.curo.who.int/_data/assets/pdf_file/0005/78638/E90038.pdf?ua=1 (accessed on 18 December 2017).
- Cançado, J.E.; Braga, A.; Pereira, L.A.; Arbex, M.A.; Saldiva, P.H.; Santos Ude, P. Clinical repercussions of exposure to athmosferic pollution. J. Bras. Pneumol. 2006, 32, S5–S11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fernandes, E.G.C. Avaliação da Influência da Exposição à Poluição Atmosférica Sobre o Escore de Atividade do Lúpus Eritematoso Sistêmico (SLEDAI-2K) em Crianças e Adolescentes. Ph.D. Thesis, Faculdade de Medicina, Universidade de São, Paulo, São Paulo, Brazil, 2015. [Google Scholar]
- Reddington, C.L.; Liu, D.; Alfarra, M.R.; Reyes-Villegas, E.; Spracklen, D.V.; Kong, S.; Williams, P.I.; Ting, Y.C.; Haslett, S.; Taylor, J.W.; et al. Black-carbon absorption enhancement in the atmosphere determined by particle mixing state. Nat. Geosci. 2017, 10, 184–188. [Google Scholar]
- Arbex, M.A.; Santos, U.D.P.; Martins, L.C.; Saldiva, P.H.N.; Pereira, L.A.A.; Braga, A.L.F. A poluição do ar e o sistema respiratório. J. Bras. Pneumol. 2012, 38, 643–655. [Google Scholar] [CrossRef]
- Rodrigues, P.C.O.; Ignotti, E.; Hacon, S.S. Distribuição espaço-temporal das queimadas e internações por doenças respiratórias em menores de cinco anos de idade em Rondônia, 2001 a 2010. Epidemiol. Serv. Saúde. 2013. [Google Scholar] [CrossRef]
- Botelho, C.; Correia, A.L.; Silva, A.M.C.; Macedo, A.G.; Silva, C.O.S. Fatores ambientais e hospitalizações em crianças menores de cinco anos com infecção respiratória aguda. Cad. Saúde. Publica. 2003, 19, 1771–1780. [Google Scholar] [CrossRef]
- BRASIL. Instituto Nacional de Pesquisas Espaciais (INPE). Projeto PRODES. Monitoramento de Floresta Amazônica Brasileira por Satélite. Available online: http://www.obt.inpe.br/prodes/index.html (accessed on 25 April 2017).
- MATO GROSSO Secretaria de Estado do Meio Ambiente (SEMA). Relatório de Monitoramento da Qualidade do ar e Agravos à Saúde Relacionados com a Poluição Atmosférica; SEMA: Mato Grosso, Brazil, 2010. [Google Scholar]
- Silva, E.C.S.; Sena, W.M.S.; Cavalcante, Y.V.N. Mecanismos Imunobiológicos do Lúpus Eritematoso Sistêmico. In Proceedings of the XIII Jornada de Ensino, Pesquisa e Extensão-Jepex UFRPE, Recife, Pernambuco, Brazil, 9–13 December 2013. [Google Scholar]
| SLEDAI | N | % | Mean | Median | SD | Variance | Min. | Max. |
|---|---|---|---|---|---|---|---|---|
| 1st appointment | 69 | 71.9 | 6.63 | 5.00 | 4.61 | 21.27 | 0.00 | 16.00 |
| 2nd appointment | 15 | 15.6 | 6.16 | 5.50 | 5.07 | 25.75 | 0.00 | 17.00 |
| 3rd appointment | 12 | 12.5 | 5.97 | 4.00 | 4.80 | 23.03 | 0.00 | 16.00 |
| Mean | Median | Standard Deviation | Variance | Minimum | Maximum | |
|---|---|---|---|---|---|---|
| CO (PPM) | ||||||
| 1st appointment | 0.06 | 0.01 | 0.07 | 0.01 | 0.01 | 0.25 |
| 2nd appointment | 0.08 | 0.10 | 0.06 | 0.00 | 0.01 | 0.24 |
| 3rd appointment | 0.13 | 0.13 | 0.07 | 0.00 | 0.01 | 0.25 |
| PM2.5 (µm) | ||||||
| 1st appointment | 7.38 | 2.50 | 6.92 | 47.83 | 1.00 | 22.00 |
| 2nd appointment | 10.93 | 13.92 | 7.46 | 55.70 | 1.00 | 25.00 |
| 3rd appointment | 14.12 | 14.68 | 5.04 | 25.40 | 1.00 | 23.71 |
| Temperature (°C) | ||||||
| 1st appointment | 28.38 | 28.50 | 1.56 | 2.44 | 23.90 | 30.80 |
| 2nd appointment | 27.83 | 28.50 | 1.89 | 3.58 | 23.00 | 30.80 |
| 3rd appointment | 28.45 | 27.80 | 2.47 | 6.12 | 23.60 | 32.70 |
| Humidity (%) | ||||||
| 1st appointment | 55.58 | 59.00 | 15.26 | 232.86 | 24.00 | 81.46 |
| 2nd appointment | 56.98 | 56.35 | 11.19 | 125.28 | 27.71 | 71.00 |
| 3rd appointment | 54.93 | 55.00 | 13.85 | 191.71 | 26.50 | 82.92 |
| Insolation (Gy) | ||||||
| 1st appointment | 5.87 | 5.70 | 1.61 | 2.58 | 1.90 | 8.60 |
| 2nd appointment | 8.75 | 6.80 | 13.75 | 189.806 | 3.70 | 82.5 |
| 3rd appointment | 6.32 | 6.90 | 1.96 | 3.84 | 2.50 | 9.60 |
| Disease Manifestation | CO (PPM) | PM2.5 (µm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Interstitial lung disease | 0.131 | 0.054 | 0.091 | 0.071 | 15.081 | 3.9411 | 11.177 | 6.805 * |
| Myocarditis | 0.163 | 0.079 | 0.085 | 0.061 * | 17.716 | 5.114 | 10.654 | 6.280 * |
| Myositis | 0.175 | 0.087 | 0.083 | 0.056 * | 18.498 | 7.455 | 10.514 | 5.685 * |
| Osteonecrosis | 0.206 | 0.049 | 0.087 | 0.062 * | 19.603 | 2.629 | 10.978 | 6.378 * |
| Pericarditis | 0.132 | 0.068 | 0.093 | 0.069 | 15.787 | 4.705 | 11.177 | 6.653 * |
| Pleuritis | 0.130 | 0.070 | 0.089 | 0.070 | 15.621 | 4.302 | 11.088 | 6.714 * |
| Dependent Variable: Variation in SLEDAI Score | ||||
|---|---|---|---|---|
| Parameters | β1 | Standard Deviation | Chi-square | Significance |
| Fires | 1.464 | 0.636 | 5.296 | 0.021 |
| CO | 8.451 | 3.410 | 6.141 | 0.013 |
| PM2.5 | 0.012 | 0.034 | 0.119 | 0.730 |
| Fires+CO | 10.800 | 3.198 | 11.401 | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaskievicz, P.H.; Silva, A.M.C.; Fernandes, V.; Junior, O.B.P.; Shimoya-Bittencourt, W.; Ferreira, S.M.B.; da Silva, C.A.L. Atmospheric Pollution Exposure Increases Disease Activity of Systemic Lupus Erythematosus. Int. J. Environ. Res. Public Health 2020, 17, 1984. https://doi.org/10.3390/ijerph17061984
Blaskievicz PH, Silva AMC, Fernandes V, Junior OBP, Shimoya-Bittencourt W, Ferreira SMB, da Silva CAL. Atmospheric Pollution Exposure Increases Disease Activity of Systemic Lupus Erythematosus. International Journal of Environmental Research and Public Health. 2020; 17(6):1984. https://doi.org/10.3390/ijerph17061984
Chicago/Turabian StyleBlaskievicz, Paula Henriques, Ageo Mario Candido Silva, Vander Fernandes, Osvaldo Borges Pinto Junior, Walkiria Shimoya-Bittencourt, Silvana Margarida Benevides Ferreira, and Cristhiane Almeida Leite da Silva. 2020. "Atmospheric Pollution Exposure Increases Disease Activity of Systemic Lupus Erythematosus" International Journal of Environmental Research and Public Health 17, no. 6: 1984. https://doi.org/10.3390/ijerph17061984
APA StyleBlaskievicz, P. H., Silva, A. M. C., Fernandes, V., Junior, O. B. P., Shimoya-Bittencourt, W., Ferreira, S. M. B., & da Silva, C. A. L. (2020). Atmospheric Pollution Exposure Increases Disease Activity of Systemic Lupus Erythematosus. International Journal of Environmental Research and Public Health, 17(6), 1984. https://doi.org/10.3390/ijerph17061984





