Atopic Disease and Anemia in Korean Patients: Cross-Sectional Study with Propensity Score Analysis
Abstract
1. Introduction
2. Materials and Methods
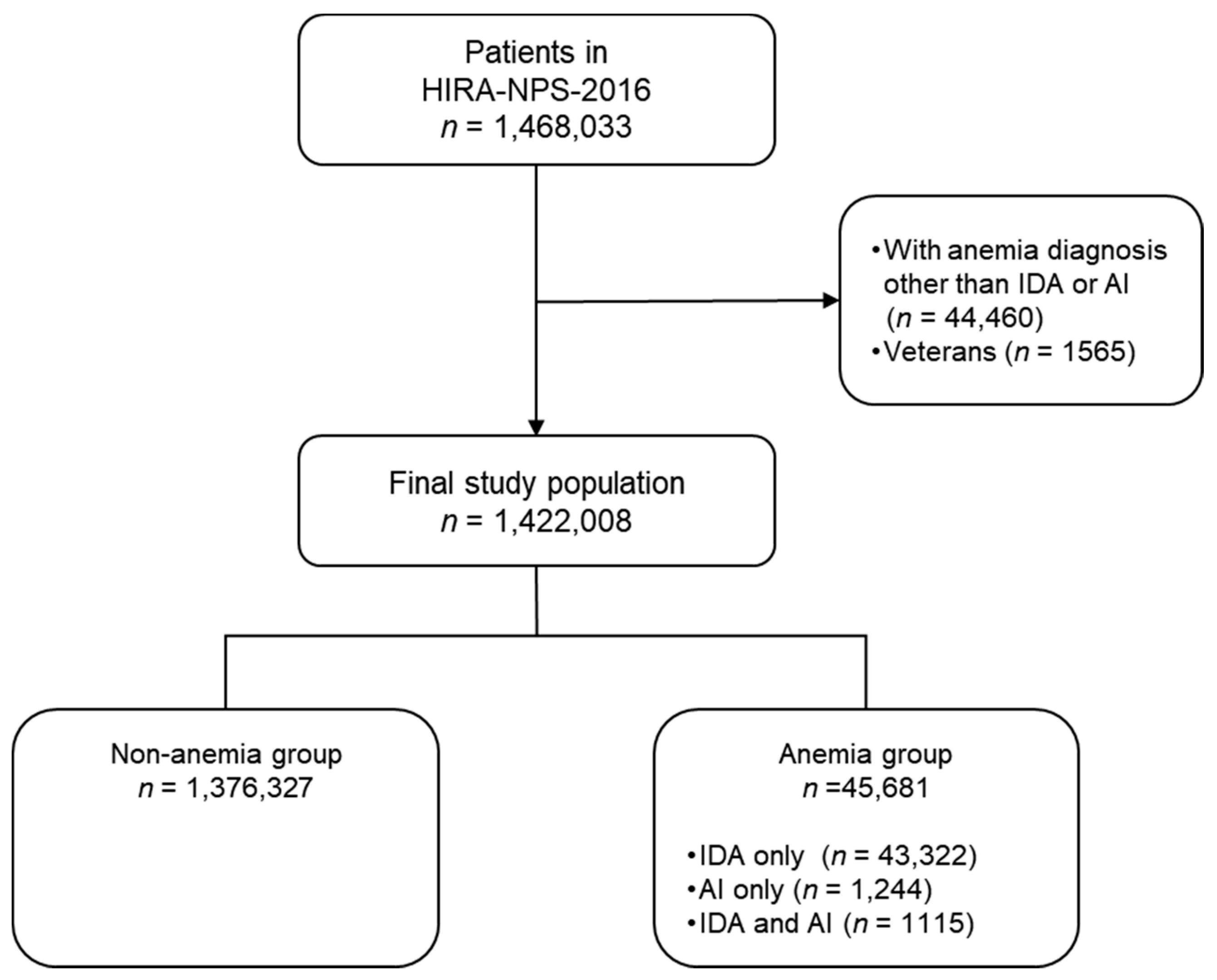
2.1. Study Subjects
2.2. Definition of Disease
2.3. Confounders
2.4. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Association between Atopic Disease and IDA
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AI | Anemia of inflammation |
| BJI | Bone and joint infection |
| CI | Confidence intervals |
| CKD | Chronic kidney disease |
| COPD | Chronic obstructive pulmonary disease |
| DM | Diabetes mellitus |
| GI | Gastrointestinal |
| HIRA | Health Insurance Review and Assessment Service |
| HF | Heart failure |
| IBD | Irritable bowel disease |
| ICD-10 | International Classification of Diseases 10th Revision |
| IDA | Iron deficiency anemia |
| IPTW | Inverse probability of treatment weighting |
| KCD-7 | The Korean Standard Classification of Disease and Cause of Death-7 |
| OR | Odds ratio |
| PUD | Peptic ulcer disease |
| RA | Rheumatoid arthritis |
| SLE | Systemic lupus erythematosus |
References
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Peña-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet Glob. Health 2013, 1, e16–e25. [Google Scholar] [CrossRef]
- Stauder, R.; Valent, P.; Theurl, I. Anemia at older age: Etiologies, clinical implications, and management. Blood 2018, 131, 505–514. [Google Scholar] [CrossRef]
- Allali, S.; Brousse, V.; Sacri, A.S.; Chalumeau, M.; de Montalembert, M. Anemia in children: Prevalence, causes, diagnostic work-up, and long-term consequences. Expert Rev. Hematol. 2017, 10, 1023–1028. [Google Scholar] [CrossRef]
- Milman, N. Iron prophylaxis in pregnancy—General or individual and in which dose? Ann. Hematol. 2006, 85, 821–828. [Google Scholar] [CrossRef]
- Sim, Y.E.; Wee, H.E.; Ang, A.L.; Ranjakunalan, N.; Ong, B.C.; Abdullah, H.R. Prevalence of preoperative anemia, abnormal mean corpuscular volume and red cell distribution width among surgical patients in Singapore, and their influence on one year mortality. PLoS ONE 2017, 12, e0182543. [Google Scholar] [CrossRef]
- Corona, L.P.; de Oliveira Duarte, Y.A.; Lebrão, M.L. Markers of nutritional status and mortality in older adults: The role of anemia and hypoalbuminemia. Geriatr Gerontol Int 2018, 18, 177–182. [Google Scholar] [CrossRef]
- Hansen, T.E.; Evjenth, B.; Holt, J. Increasing prevalence of asthma, allergic rhinoconjunctivitis and eczema among schoolchildren: Three surveys during the period 1985–2008. Acta Paediatr. 2013, 102, 47–52. [Google Scholar] [CrossRef]
- Yoo, B.; Park, Y.; Park, K.; Kim, H. A 9-year Trend in the Prevalence of Allergic Disease Based on National Health Insurance Data. J. Prev. Med. Public Health 2015, 48, 301–309. [Google Scholar] [CrossRef]
- Schmitt, J.; Schwarz, K.; Baurecht, H.; Hotze, M.; Fölster-Holst, R.; Rodríguez, E.; Lee, Y.A.E.; Franke, A.; Degenhardt, F.; Lieb, W. Atopic dermatitis is associated with an increased risk for rheumatoid arthritis and inflammatory bowel disease, and a decreased risk for type 1 diabetes. J. Allergy Clin. Immunol. 2016, 137, 130–136. [Google Scholar] [CrossRef]
- Nicholas, M.N.; Gooderham, M.J. Atopic Dermatitis, Depression, and Suicidality. J. Cutan. Med. Surg. 2017, 21, 237–242. [Google Scholar] [CrossRef]
- Yaghmaie, P.; Koudelka, C.W.; Simpson, E.L. Mental health comorbidity in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2013, 131, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Nairz, M.; Theurl, I.; Wolf, D.; Weiss, G. Iron deficiency or anemia of inflammation? Differential diagnosis and mechanisms of anemia of inflammation. Wien. Med. Wochenschr 2016, 166, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Polin, V.; Coriat, R.; Perkins, G.; Dhooge, M.; Abitbol, V.; Leblanc, S.; Prat, F.; Chaussade, S. Iron deficiency: From diagnosis to treatment. Dig. Liver Dis. 2013, 45, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Drury, K.E.; Schaeffer, M.; Silverberg, J.I. Association Between Atopic Disease and Anemia in US Children. JAMA Pediatr. 2016, 170, 29–34. [Google Scholar] [CrossRef]
- Rhew, K.; Oh, J.M. Association between atopic disease and anemia in pediatrics: A cross-sectional study. BMC Pediatr. 2019, 19, 455. [Google Scholar] [CrossRef]
- Kim, L.; Kim, J.A.; Kim, S. A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol. Health 2014, 36, e2014008. [Google Scholar] [CrossRef]
- Balarajan, Y.; Ramakrishnan, U.; Ozaltin, E.; Shankar, A.H.; Subramanian, S.V. Anaemia in low-income and middle-income countries. Lancet 2011, 378, 2123–2135. [Google Scholar] [CrossRef]
- Guo, Y.F.; Gan, Y.Y.; Guo, C.N.; Sun, J.; Hao, L.P. Nutritional status of under-five children from urban low-income families in Xiangtan and Jilin in China. J. Huazhong Univ. Sci. Technolog. Med. Sci 2017, 37, 74–78. [Google Scholar] [CrossRef]
- Ning, S.Y.; Chang, N.B.; Han, X.Y.; Liu, X.; Duan, Y.W.; Liu, Y.H.; Liu, T.; Duan, X.L.; Li, N.H.; Guo, J. The prevalence and etiology of anemia in urban community dwelling elderly population in Beijing. Zhonghua Nei Ke Za Zhi. 2016, 55, 289–292. [Google Scholar]
- Le, C.H. The Prevalence of Anemia and Moderate-Severe Anemia in the US Population (NHANES 2003–2012). PLoS ONE 2016, 11, e0166635. [Google Scholar] [CrossRef]
- Silverberg, D.S. The role of erythropoiesis stimulating agents and intravenous (IV) iron in the cardio renal anemia syndrome. Heart Fail. Rev. 2011, 16, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Boutou, A.K.; Pitsiou, G.G.; Stanopoulos, I.; Kontakiotis, T.; Kyriazis, G.; Argyropoulou, P. Levels of inflammatory mediators in chronic obstructive pulmonary disease patients with anemia of chronic disease: A case-control study. QJM 2012, 105, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Comin-Colet, J.; de Francisco, A.; Dignass, A.; Doehner, W.; Lam, C.S.; Macdougall, I.C.; Rogler, G.; Camaschella, C.; Kadir, R. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. Am. J. Hematol. 2017, 92, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Yu, J.; Oh, M.H.; Zhu, Z. The atopic march: Progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res. 2011, 3, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Spergel, J.M. From atopic dermatitis to asthma: The atopic march. Ann. Allergy Asthma Immunol. 2010, 105, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Ker, J.; Hartert, T.V. The atopic march: what’s the evidence? Ann. Allergy Asthma Immunol. 2009, 103, 282–289. [Google Scholar] [CrossRef]
- Roubenoff, R.; Harris, T.B.; Abad, L.W.; Wilson, P.W.; Dallal, G.E.; Dinarello, C.A. Monocyte cytokine production in an elderly population: Effect of age and inflammation. J. Gerontol. 1998, 53, M20–M26. [Google Scholar] [CrossRef]
- Milman, N.; Kirchhoff, M.; Jørgensen, T. Iron status markers, serum ferritin and hemoglobin in 1359 Danish women in relation to menstruation, hormonal contraception, parity, and postmenopausal hormone treatment. Ann. Hematol. 1992, 65, 96–102. [Google Scholar] [CrossRef]
- Andrade, A.T.; Souza, J.P.; Shaw, S.T., Jr.; Belsey, E.M.; Rowe, P.J. Menstrual blood loss and body iron stores in Brazilian women. Contraception 1991, 43, 241–249. [Google Scholar] [CrossRef]
- Youn, K.I. Comparisons of health care utilization patterns and outcome for national health insurance and medical aid program cancer patients. J. Health Info. Stat. 2014, 39, 42–59. [Google Scholar]
- Park, Y.H.; Lee, Y.J. Qualitative analysis of medical usage patterns of medical aid patients. J. KoCon. 2017, 17, 39–49. [Google Scholar]
- Lee, Y.J. Differences of Cancer Patient’s Health Care Utilizations between Medical Aid Program and National Health Insurance in the Elderly. J. KoCon. 2011, 11, 270–279. [Google Scholar]
- Theurl, I.; Aigner, E.; Theurl, M.; Nairz, M.; Seifert, M.; Schroll, A.; Sonnweber, T.; Eberwein, L.; Witcher, D.R.; Murphy, A.T. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: Diagnostic and therapeutic implications. Blood 2009, 113, 5277–5286. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, G.; Di Sabatino, A.; Albertini, R.; Costanzo, F.; Guerci, M.; Masotti, M.; Pasini, A.; Massari, A.; Campostrini, N.; Corbella, M. Serum hepcidin in inflammatory bowel diseases: Biological and clinical significance. Inflamm. Bowel Dis. 2013, 19, 2166–2172. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Anemia of Inflammation. Hematol. Oncol. Clin. North. Am. 2014, 28, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Theurl, M.; Nairz, M.; Schroll, A.; Sonnweber, T.; Asshoff, M.; Haschka, D.; Seifert, M.; Willenbacher, W.; Wilflingseder, D.; Posch, W. Hepcidin as a predictive factor and therapeutic target in erythropoiesis-stimulating agent treatment for anemia of chronic disease in rats. Haematologica 2014, 99, 1516–1524. [Google Scholar] [CrossRef]
- Bregman, D.B.; Morris, D.; Koch, T.A.; He, A.; Goodnough, L.T. Hepcidin levels predict nonresponsiveness to oral iron therapy in patients with iron deficiency anemia. Am. J. Hematol. 2013, 88, 97–101. [Google Scholar] [CrossRef]
- Celakovska, J.; Bukac, J. Food hypersensitivity reactions and peripheral blood eosinophilia in patients suffering from atopic dermatitis. Food Agr. Immunol. 2017, 28, 35–43. [Google Scholar] [CrossRef]
- Celakovska, J.; Bukac, J. Eosinophils in patients suffering from atopic dermatitis and the relation to the occurrence of food allergy and other atopic diseases. Food Agr. Immunol. 2016, 5, 700–710. [Google Scholar] [CrossRef]
- Tran, T.N.; Zeiger, R.S.; Peters, S.P.; Colice, G.; Newbold, P.; Goldman, M.; Chipps, B.E. Overlap of Atopic, Eosinophilic, and TH2-high Asthma Phenotypes in a General Population with Current Asthma. Ann. Allergy Asthma Immunol. 2016, 116, 37–42. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food Allergy: Epidemiology, Pathogenesis, Diagnosis, and Treatment. J. Allergy Clin. Immunol. 2014, 133, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Fortes, C.; Mastroeni, S.; Mannooranparampil, T.J.; Di Lallo, D. Pre-natal folic acid and iron supplementation and atopic dermatitis in the first 6 years of life. Arch. Dermatol. Res. 2019, 311, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Bédard, A.; Lewis, S.J.; Burgess, S.; Henderson, A.J.; Shaheen, S.O. Maternal iron status during pregnancy and respiratory and atopic outcomes in the offspring: A Mendelian randomisation study. BMJ Open Respir Res. 2018, 5, e000275. [Google Scholar] [CrossRef] [PubMed]
- Nwaru, B.I.; Hayes, H.; Gambling, L.; Craig, L.C.; Allan, K.; Prabhu, N.; Turner, S.W.; McNeill, G.; Erkkola, M.; Seaton, A. An exploratory study of the associations between maternal iron status in pregnancy and childhood wheeze and atopy. Br. J. Nutr. 2014, 112, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.O.; Macdonald-Wallis, C.; Lawlor, D.A.; Henderson, A.J. Haemoglobin concentrations in pregnancy and respiratory and allergic outcomes in childhood: Birth cohort study. Clin. Exp. Allergy. 2017, 47, 1615–1624. [Google Scholar] [CrossRef]
- Lai, F.P.; Yang, Y.J. The prevalence and characteristics of cow’s milk protein allergy in infants and young children with iron deficiency anemia. Pediatr. Neonatol. 2018, 59, 48–52. [Google Scholar] [CrossRef]
- Roth-Walter, F.; Pacios, L.F.; Bianchini, R.; Jensen-Jarolim, E. Linking iron-deficiency with allergy: Role of molecular allergens and the microbiome. Metallomics 2017, 9, 1676–1692. [Google Scholar] [CrossRef]
| Before Weighting, n (%) | After Weighting, n (%) | |||
|---|---|---|---|---|
| No Anemia (n = 1,376,327) | IDA/AI (n = 45,681) | No Anemia (n = 46,958) | IDA/AI (n = 45,681) | |
| Age group | ||||
| Children (<12) | 160,240 (11.64) | 4755 (10.41) | 4769 (10.16) | 4755 (10.41) |
| Adolescents (≥12, <18) | 91,508 (6.65) | 1332 (2.92) | 1325 (2.82) | 1332 (2.92) |
| Adults (≥18, <65) | 948,857 (68.94) | 27,093 (59.31) | 27,340 (58.22) | 27,093 (59.31) |
| Elderly (≥65) | 175,722 (12.77) | 12,501 (27.37) | 13,524 (28.80) | 12,501 (27.37) |
| Sex | ||||
| Male | 683,856 (49.69) | 14,568 (31.89) | 14,705 (31.31) | 14,568 (31.89) |
| Female | 692,471 (50.31) | 31,113 (68.11) | 32,253 (68.69) | 31,113 (68.11) |
| Insurance Type | ||||
| Health insurance | 1,338,718 (97.27) | 42,249 (92.49) | 43,105 (91.80) | 42,249 (92.49) |
| Medical aid | 37,609 (2.73) | 3432 (7.51) | 3853 (8.20) | 3432 (7.51) |
| Systemic infection | ||||
| Meningitis | 624 (0.05) | 76 (0.17) | 90 (0.19) | 76 (0.17) |
| BJI | 3727 (0.27) | 331 (0.72) | 408 (0.87) | 331 (0.72) |
| Sepsis | 2980 (0.22) | 777 (1.70) | 1071 (2.28) | 777 (1.70) |
| HEP | 23,828 (1.73) | 3403 (7.45) | 3950 (8.41) | 3403 (7.45) |
| CKD | 4767 (0.35) | 3284 (7.19) | 4116 (8.77) | 3284 (7.19) |
| HF | 18,356 (1.33) | 2842 (6.22) | 3296 (7.02) | 2842 (6.22) |
| DM | 137,858 (10.02) | 15,806 (34.60) | 17,120 (36.46) | 15,806 (34.60) |
| Mental disorder | ||||
| Depression | 57,754 (4.20) | 5020 (10.99) | 5630 (11.99) | 5020 (10.99) |
| Anxiety | 90,513 (6.58) | 7493 (16.40) | 8258 (17.59) | 7493 (16.40) |
| Chronic inflammation | ||||
| PUD | 123,328 (8.96) | 8839 (19.35) | 9,629 (20.51) | 8839 (19.35) |
| COPD | 45,100 (3.28) | 3586 (7.85) | 3991 (8.50) | 3586 (7.85) |
| SLE | 1633 (0.12) | 299 (0.65) | 371 (0.79) | 299 (0.65) |
| RA | 24,614 (1.79) | 2841 (6.22) | 3286 (7.00) | 2841 (6.22) |
| IBD | 2025 (0.15) | 248 (0.54) | 322 (0.69) | 248 (0.54) |
| Cancer | 46,695 (3.39) | 5043 (11.04) | 5792 (12.33) | 5043 (11.04) |
| Atopic Diseases | Frequency (%) | Odds Ratio (95% CI) | p Value | ||
|---|---|---|---|---|---|
| No Anemia (n = 46,958) | IDA/AI (n = 45,681) | ||||
| Atopic dermatitis | No | 44,486 (94.73) | 42,382 (92.78) | 1 [Reference] | |
| Yes | 2472 (5.27) | 3299 (7.22) | 1.40 (1.33–1.48) | < 0.001 | |
| Allergic rhinitis | No | 34,777 (74.06) | 32,372 (70.87) | 1 [Reference] | |
| Yes | 12,181 (25.94) | 13,309 (29.13) | 1.17 (1.14–1.21) | < 0.001 | |
| Asthma | No | 38,907 (82.85) | 35,891 (78.57) | 1 [Reference] | |
| Yes | 8051 (17.15) | 9790 (21.43) | 1.32 (1.28–1.36) | < 0.001 | |
| Atopic diseases, n | 0 | 29,193 (62.17) | 26,022 (56.96) | 1 [Reference] | |
| 1 | 13,247 (28.21) | 13,744 (30.09) | 1.16 (1.13–1.20) | < 0.001 | |
| 2 | 4095 (8.72) | 5091 (11.14) | 1.40 (1.33–1.46) | < 0.001 | |
| 3 | 422 (0.90) | 824 (1.80) | 2.19 (1.94–2.46) | < 0.001 | |
| Patient Characteristics | Atopic Diseases | Frequency (%) | Odds Ratio (95% CI) | p value | ||
|---|---|---|---|---|---|---|
| No Anemia | IDA/AI | |||||
| Children (<12 years) | Atopic dermatitis | No | 3979 (83.42) | 3379 (71.06) | 1 [Reference] | |
| Yes | 790 (16.58) | 1376 (28.94) | 2.05 (1.86–2.26) | <0.001 | ||
| Allergic rhinitis | No | 2882 (60.44) | 2319 (48.77) | 1 [Reference] | ||
| Yes | 1887 (39.56) | 2436 (51.23) | 1.61 (1.48–1.74) | <0.001 | ||
| Asthma | No | 3010 (63.11) | 1737 (36.53) | 1 [Reference] | ||
| Yes | 1759 (36.89) | 3018 (63.47) | 2.97 (2.74–3.23) | <0.001 | ||
| Atopic diseases, n | 0 | 1806 (37.87) | 805 (16.93) | 1 [Reference] | ||
| 1 | 1712 (35.90) | 1662 (34.95) | 2.18 (1.96–2.42) | <0.001 | ||
| 2 | 1029 (21.58) | 1696 (35.67) | 3.70 (3.30-4.14) | <0.001 | ||
| 3 | 222 (4.66) | 592 (12.45) | 5.98 (5.02-7.13) | <0.001 | ||
| Adolescents (≥12, <18 years) | Atopic dermatitis | No | 1245 (93.95) | 1212 (90.99) | 1 [Reference] | |
| Yes | 80 (6.05) | 120 (9.01) | 1.54 (1.15–2.06) | 0.004 | ||
| Allergic rhinitis | No | 951 (71.78) | 869 (65.24) | 1 [Reference] | ||
| Yes | 374 (28.22) | 463 (34.76) | 1.36 (1.15–1.60) | <0.001 | ||
| Asthma | No | 1189 (89.74) | 1129 (84.76) | 1 [Reference] | ||
| Yes | 136 (10.26) | 203 (15.24) | 1.57 (1.25–1.98) | <0.001 | ||
| Atopic diseases, n | 0 | 832 (62.80) | 711 (53.38) | 1 [Reference] | ||
| 1 | 402 (30.37) | 470 (35.29) | 1.37 (1.16–1.62) | <0.001 | ||
| 2 | 84 (6.32) | 137 (10.29) | 1.92 (1.43–2.56) | <0.001 | ||
| 3 | 7 (0.51) | 14 (1.05) | 2.43 (0.97–6.13) | 0.059 | ||
| Adults (≥18, <65 years) | Atopic dermatitis | No | 26,341 (96.35) | 25,910 (95.63) | 1 [Reference] | |
| Yes | 999 (3.65) | 1183 (4.37) | 1.20 (1.11–1.31) | <0.001 | ||
| Allergic rhinitis | No | 20,673 (75.62) | 19,710 (72.75) | 1 [Reference] | ||
| Yes | 6667 (24.38) | 7383 (27.25) | 1.16 (1.12–1.21) | <0.001 | ||
| Asthma | No | 24,244 (88.67) | 23,368 (86.25) | 1 [Reference] | ||
| Yes | 3096 (11.33) | 3725 (13.75) | 1.25 (1.19–1.31) | <0.001 | ||
| Atopic diseases, n | 0 | 18,455 (67.50) | 17,124 (63.20) | 1 [Reference] | ||
| 1 | 7108 (26.00) | 7782 (28.72) | 1.18 (1.136–1.23) | <0.001 | ||
| 2 | 1677 (6.13) | 2052 (7.57) | 1.32 (1.233–1.41) | <0.001 | ||
| 3 | 100 (0.37) | 135 (0.50) | 1.45 (1.122–1.88) | 0.005 | ||
| Elderly (≥65 years) | Atopic dermatitis | No | 12,921 (95.54) | 11,881 (95.04) | 1 [Reference] | |
| Yes | 603 (4.46) | 620 (4.96) | 1.12 (1.00–1.25) | 0.057 | ||
| Allergic rhinitis | No | 10,270 (75.94) | 9474 (75.79) | 1 [Reference] | ||
| Yes | 3254 (24.06) | 3027 (24.21) | 1.01 (0.95–1.07) | 0.771 | ||
| Asthma | No | 10,464 (77.38) | 9657 (77.25) | 1 [Reference] | ||
| Yes | 3060 (22.62 | 2844 (22.75) | 1.01 (0.95–1.07) | 0.808 | ||
| Atopic diseases, n | 0 | 8100 (59.89) | 7382 (59.05) | 1 [Reference] | ||
| 1 | 4025 (29.76) | 3830 (30.64) | 1.04 (0.99–1.10) | 0.119 | ||
| 2 | 1306 (9.66) | 1206 (9.65) | 1.01 (0.93–1.10) | 0.758 | ||
| 3 | 93 (0.69) | 83 (0.66) | 0.98 (0.72–1.31) | 0.867 | ||
| Male | Atopic dermatitis | No | 13,967 (94.98) | 13,265 (91.06) | 1 [Reference] | |
| Yes | 738 (5.02) | 1303 (8.94) | 1.89 (1.69–2.04) | <0.001 | ||
| Allergic rhinitis | No | 11,259 (76.57) | 10,411 (71.46) | 1 [Reference] | ||
| Yes | 3446 (23.43) | 4157 (28.54) | 1.31 (1.24–1.38) | <0.001 | ||
| Asthma | No | 12,391 (84.26) | 10,904 (74.85) | 1 [Reference] | ||
| Yes | 2314 (15.74) | 3664 (25.15) | 1.80 (1.70–1.91) | <0.001 | ||
| Atopic diseases, n | 0 | 9618 (65.41) | 8025 (55.09) | 1 [Reference] | ||
| 1 | 3808 (25.89) | 4345 (29.83) | 1.39 (1.30–1.44) | <0.001 | ||
| 2 | 1146 (7.79) | 1815 (12.46) | 1.90 (1.75–2.06) | <0.001 | ||
| 3 | 133 (0.90) | 383 (2.63) | 3.46 (2.83–4.22) | <0.001 | ||
| Female | Atopic dermatitis | No | 30,519 (94.62) | 29,117 (93.58) | 1 [Reference] | |
| Yes | 1734 (5.38) | 1996 (6.42) | 1.21 (1.13–1.29) | <0.001 | ||
| Allergic rhinitis | No | 23,518 (72.92) | 21,961 (70.58) | 1 [Reference] | ||
| Yes | 8735 (27.08) | 9152 (29.42) | 1.12 (1.08–1.16) | <0.001 | ||
| Asthma | No | 26,516 (82.21) | 24,987 (80.31) | 1 [Reference] | ||
| Yes | 5737 (17.79) | 6126 (19.69) | 1.13 (1.09–1.18) | <0.001 | ||
| Atopic diseases, n | 0 | 19,575 (60.69) | 17,997 (57.84) | 1 [Reference] | ||
| 1 | 9440 (29.27) | 9399 (30.21) | 1.08 (1.05–1.12) | <0.001 | ||
| 2 | 2949 (9.14) | 3276 (10.53) | 1.21 (1.15–1.28) | <0.001 | ||
| 3 | 289 (0.90) | 441 (1.42) | 1.66 (1.43–1.92) | <0.001 | ||
| Health insurance | Atopic dermatitis | No | 40,857 (94.78) | 39,190 (92.76) | 1 [Reference] | |
| Yes | 2248 (5.22) | 3059 (7.24) | 1.42 (1.34–1.50) | <0.001 | ||
| Allergic rhinitis | No | 31,960 (74.14) | 29,901 (70.77) | 1 [Reference] | ||
| Yes | 11,145 (25.86) | 12,348 (29.23) | 1.18 (1.15–1.22) | <0.001 | ||
| Asthma | No | 36,055 (83.64) | 33,293 (78.80) | 1 [Reference] | ||
| Yes | 7050 (16.36) | 8956 (21.20) | 1.37 (1.33–1.42) | <0.001 | ||
| Atopic diseases, n | 0 | 27,053 (62.76) | 24,130 (57.11) | 1 [Reference] | ||
| 1 | 12,048 (27.95) | 12,657 (29.96) | 1.18 (1.14–1.21) | <0.001 | ||
| 2 | 3617 (8.39) | 4680 (11.08) | 1.45 (1.38–1.52) | <0.001 | ||
| 3 | 387 (0.90) | 782 (1.85) | 2.27 (2.00–2.56) | <0.001 | ||
| Medical aid | Atopic dermatitis | No | 3629 (94.19) | 3192 (93.01) | 1 [Reference] | |
| Yes | 224 (5.81) | 240 (6.99) | 1.22 (1.01–1.47) | 0.039 | ||
| Allergic rhinitis | No | 2818 (73.13) | 2471 (72.00) | 1 [Reference] | ||
| Yes | 1035 (26.87) | 961 (28.00) | 1.06 (0.96–1.17) | 0.280 | ||
| Asthma | No | 2852 (74.01) | 2598 (75.70) | 1 [Reference] | ||
| Yes | 1001 (25.99) | 834 (24.30) | 0.91 (0.82–1.02) | 0.099 | ||
| Atopic diseases, n | 0 | 2141 (55.56) | 1892 (55.13) | 1 [Reference] | ||
| 1 | 1199 (31.13) | 1087 (31.67) | 1.02 (0.93–1.14) | 0.632 | ||
| 2 | 478 (12.40) | 411 (11.98) | 0.97 (0.84–1.13) | 0.718 | ||
| 3 | 35 (0.92) | 42 (1.22) | 1.35 (0.86–2.12) | 0.195 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhew, K.; Brown, J.D.; Oh, J.M. Atopic Disease and Anemia in Korean Patients: Cross-Sectional Study with Propensity Score Analysis. Int. J. Environ. Res. Public Health 2020, 17, 1978. https://doi.org/10.3390/ijerph17061978
Rhew K, Brown JD, Oh JM. Atopic Disease and Anemia in Korean Patients: Cross-Sectional Study with Propensity Score Analysis. International Journal of Environmental Research and Public Health. 2020; 17(6):1978. https://doi.org/10.3390/ijerph17061978
Chicago/Turabian StyleRhew, Kiyon, Joshua D Brown, and Jung Mi Oh. 2020. "Atopic Disease and Anemia in Korean Patients: Cross-Sectional Study with Propensity Score Analysis" International Journal of Environmental Research and Public Health 17, no. 6: 1978. https://doi.org/10.3390/ijerph17061978
APA StyleRhew, K., Brown, J. D., & Oh, J. M. (2020). Atopic Disease and Anemia in Korean Patients: Cross-Sectional Study with Propensity Score Analysis. International Journal of Environmental Research and Public Health, 17(6), 1978. https://doi.org/10.3390/ijerph17061978






