Parkinson’s Disease in Louisiana, 1999–2012: Based on Hospital Primary Discharge Diagnoses, Incidence, and Risk in Relation to Local Agricultural Crops, Pesticides, and Aquifer Recharge
Abstract
:1. Introduction
1.1. Epidemiology
1.2. Genetics
1.3. Pesticides
1.4. Pesticide Usage in Louisiana Agriculture
1.5. Other Exposures
2. Materials and Methods
| Fungicides: | hexachlorobenzene (HCB), hexachlorocyclopentadiene. |
| Herbicides: | 2,4-D, 2,4,5-TP, atrazine, dalapon, dinoseb, diquat, endothall, glyphosate, Lasso®, pentachlorophenol, picloram, simazine. |
| Insecticides: | BHC-Gamma, carbofuran, endrin, oxamyl, toxaphene. |
3. Results
3.1. Hospital Records
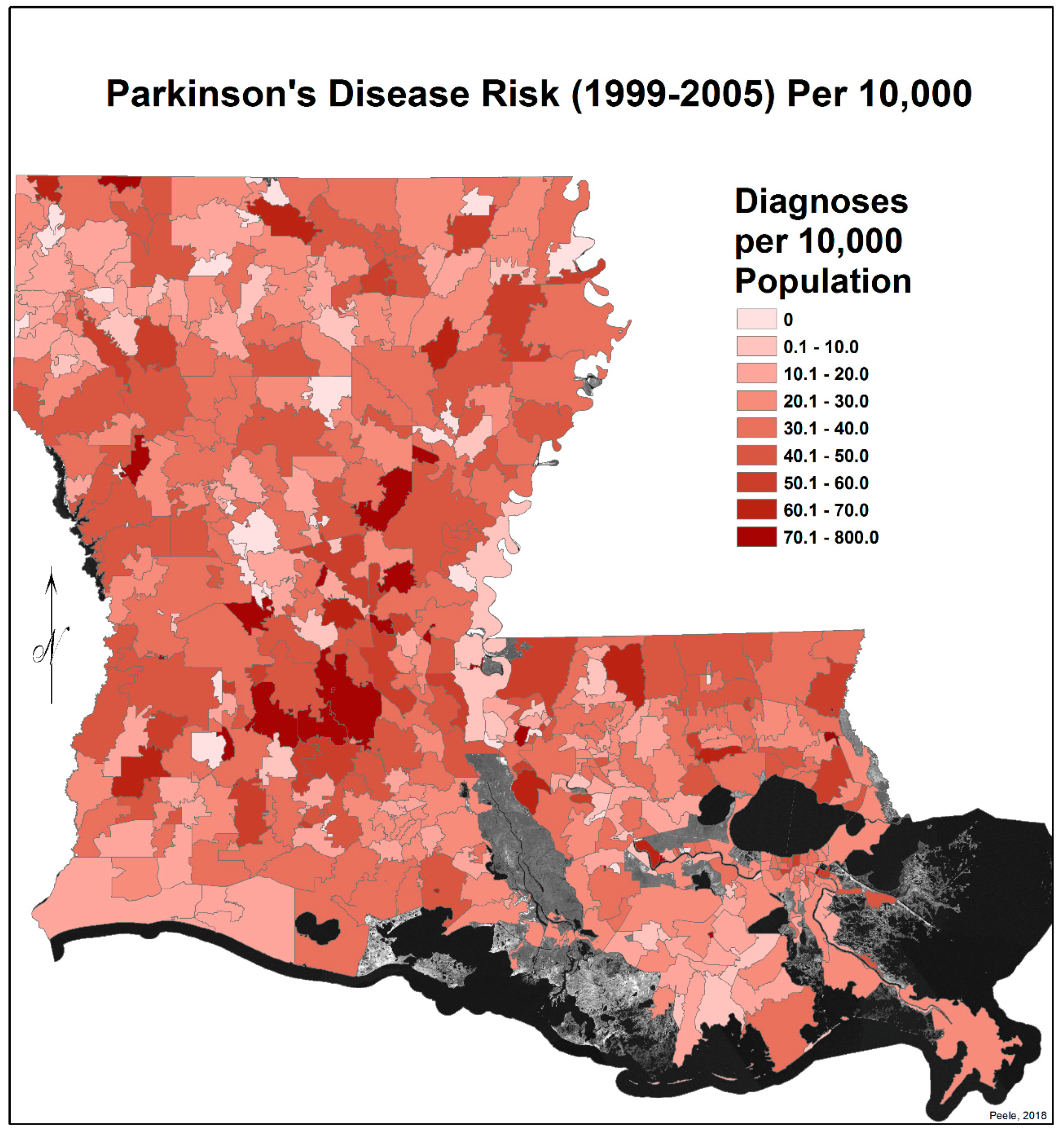
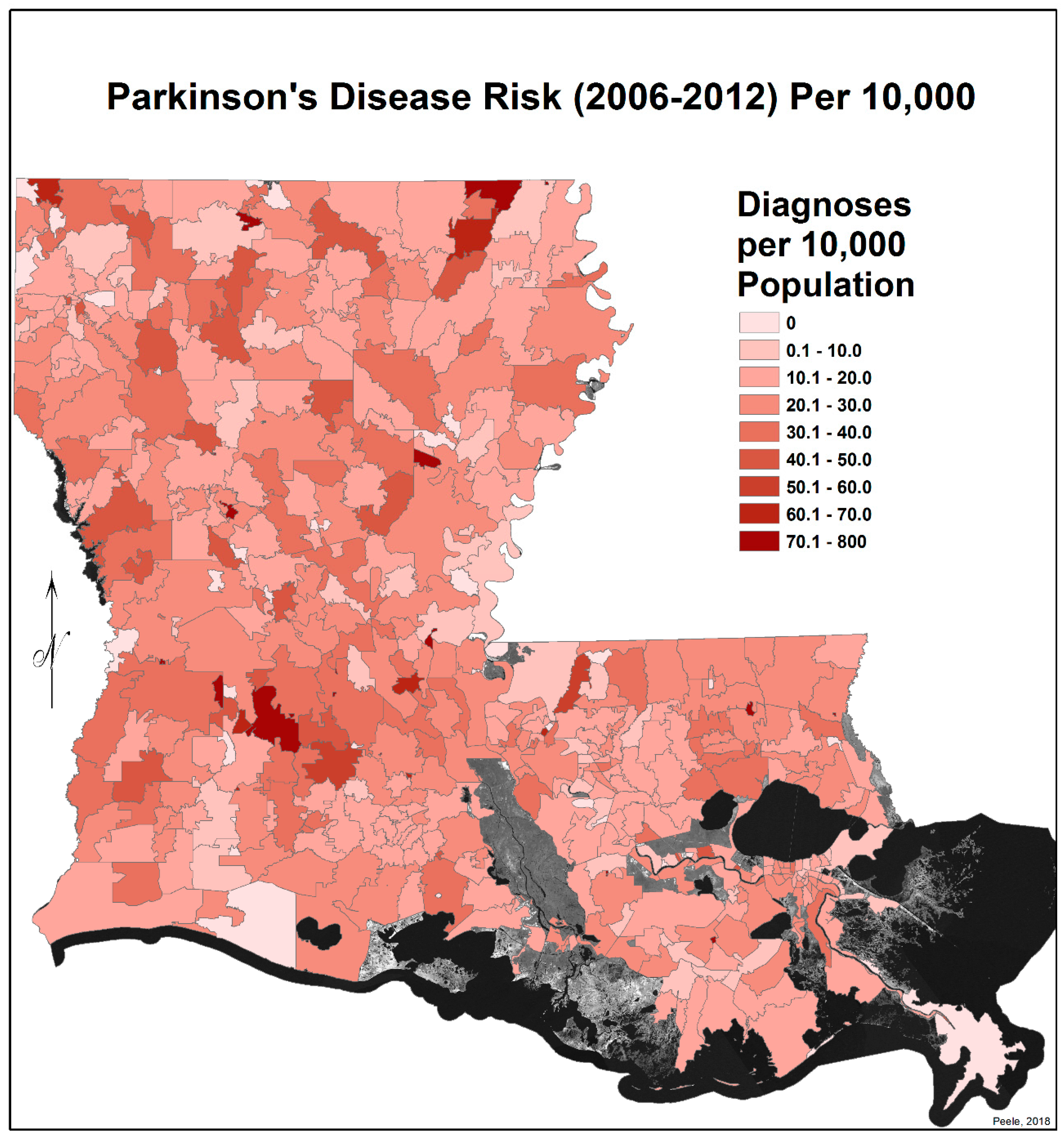
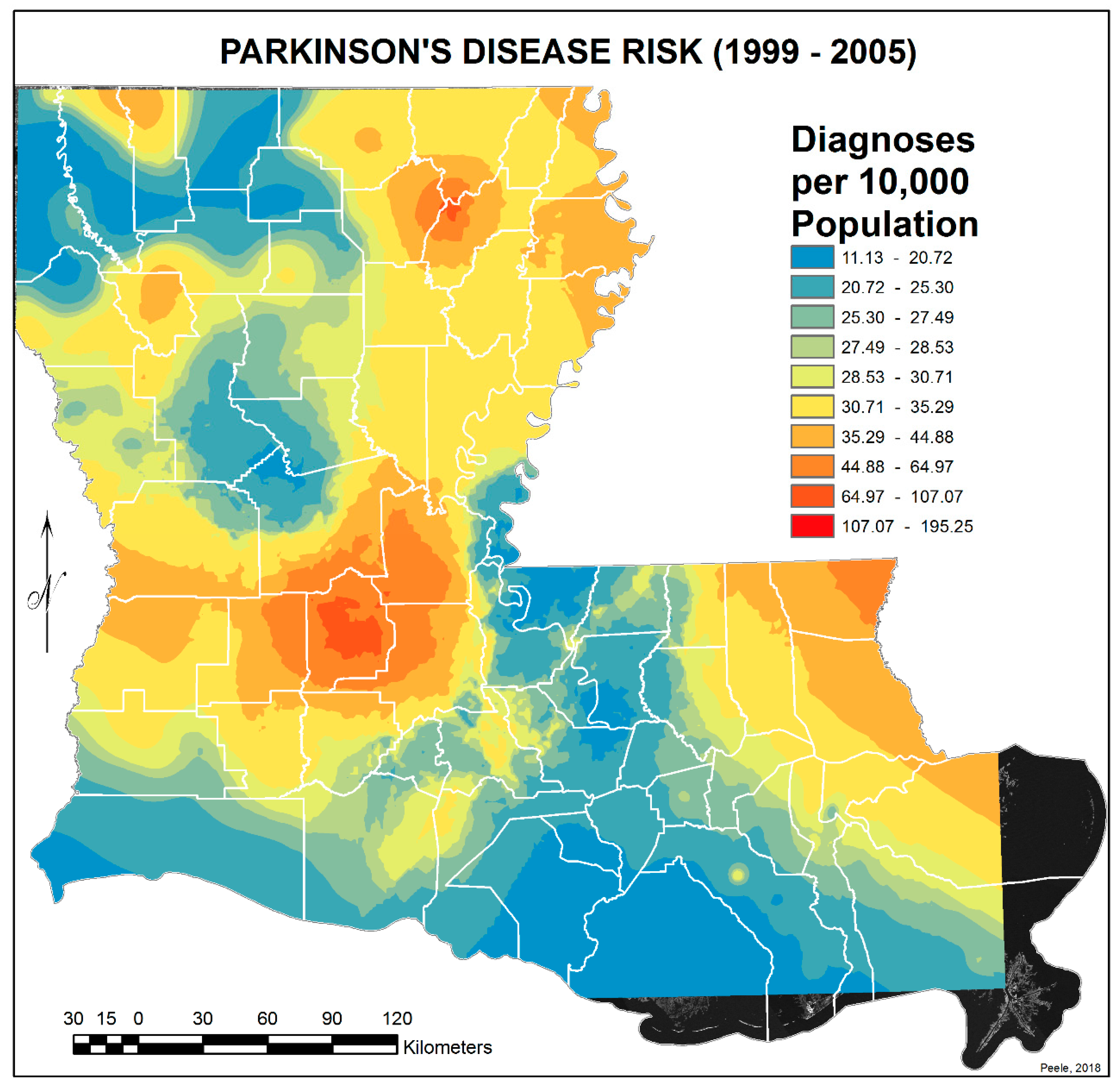
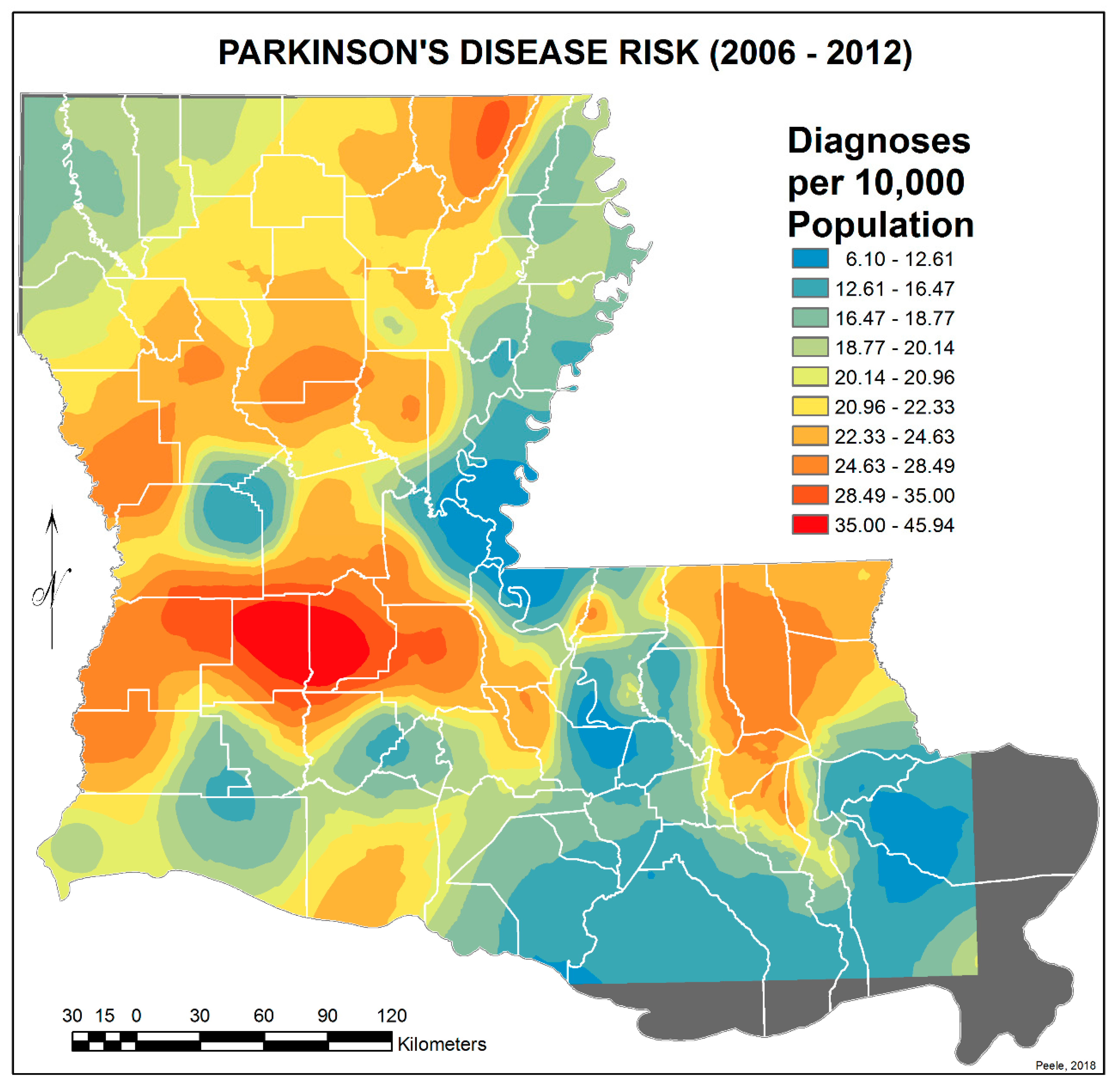
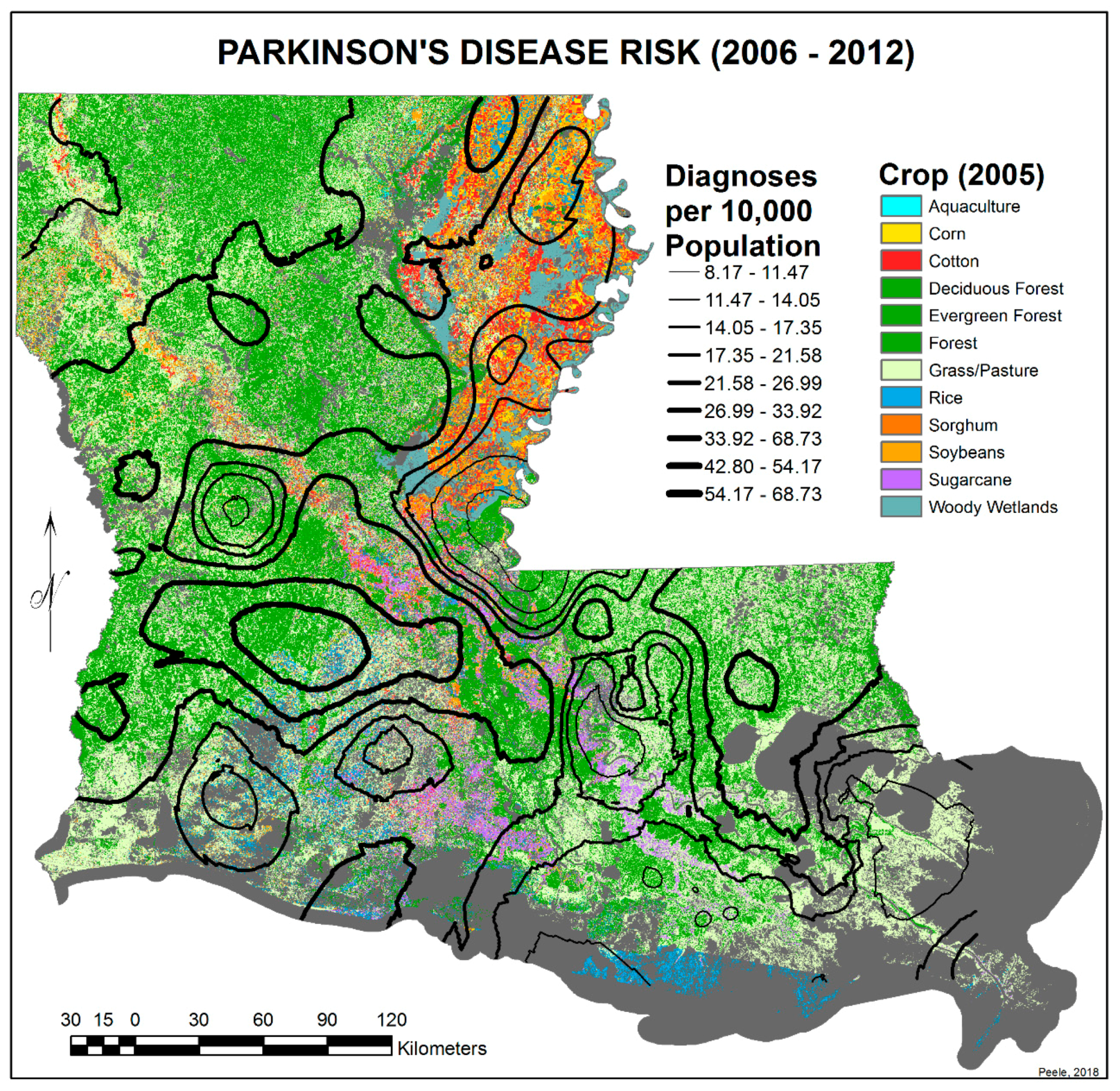
3.2. Spatial Distribution of Parkinson’s Disease
- The singular highest-incidence area in Figure 4 contains Mamou, Mittie, Oakdale, and Oberlin (Allen and Evangeline Parishes) and is part of a band from the east of the Atchafalaya River and in the west to DeRidder (Beauregard Parish), Starks (Calcasieu Parish), and the Sabine River. Within this high-risk area are numerous pastures and dense timber. In the east, it hooks south from Livonia to Rosedale, an area that also includes soybeans and sorghum.
- There is a second band of high risk from Sabine Parish, also on the Sabine River and the Texas border, which reaches northeast to West Carroll Parish and the Arkansas border and is similarly well populated with pastures and commercial woodlands. West Carroll has a higher PD risk level than Morehouse and East Carroll on either side. These latter parishes are characterized by agricultural crops, except in the far west of Morehouse with forestry, while West Carroll has forestry along its length but especially in the northern two-thirds, with cotton, soybeans, and sorghum in the bottom third; the water drains from north to south from similar woodlands in Chicot County, Arkansas. The 2006–2012 PD risk map also shows an area of increased PD risk south of Monroe in Caldwell Parish, at the time and place of intense cotton growing.
- In Figure 4, 2006–2012, it can be seen in the Florida parishes that there is an eastern cluster of high risk stretching down through Washington, northeastern St. Tammany, and Tangipahoa parishes to St. John the Baptist, northern St. Charles Parish, and northwest Jefferson Parish, west of New Orleans. As the ZIP code areas reach out into Lakes Pontchartrain and Maurepas, the calculated risks follow but are, in fact, two clusters. In the eastern Florida parishes, central Tangipahoa Parish is traditionally a strawberry growing area, but otherwise, it is pastures with woodland along with St. Tammany and Washington Parishes. Then, there is the “Western New Orleans” cluster of St. John the Baptist, northern St. Charles, and Kenner, where the first two parishes contain petrochemical plants and storage, shipbuilding, and some agriculture and Kenner is a dormitory suburb providing workers to the former parishes.
- There is a noticeable low-incidence “sink” to the west of Alexandria in northeast Vernon Parish, south of the Kisatchie National Forest, corresponding to Fort Polk and its non-resident young soldiers under training, as well as along the parishes west of the Mississippi River from East Carroll Parish in the northeast down to Concordia and then on the east side of West Feliciana; after a slight increase on the West and East Feliciana border, Iberville and St. Martin Parishes are essentially free of cases, as well as the lower Atchafalaya basin down to Houma. The relative absence along the length of the Mississippi is clear but with some risk in Jefferson (Kenner), St. John the Baptist, and (northern) St. Charles Parishes, as noted above. In 1999–2005 (Figure 3), the northeast parishes along the Mississippi were once afflicted with Parkinson’s disease patients but are now relatively free. This is an area where cotton and soybeans were traditionally grown. PD is restricted to the northern third of the rice growing areas, suggesting that any PD risk may be from a north–south surface flow of dangerous pesticides into the rice growing area and not from pesticides used on rice itself. PD is essentially absent from the sugarcane growing areas of the lower Mississippi from East Baton Rouge south, along the Lafourche River, and the western bank of the Atchafalaya basin from Breaux Bridge south.
3.3. Pesticides and Drinking Water
3.4. Arbor–Pastoral Risk and Estimated Pesticide Use
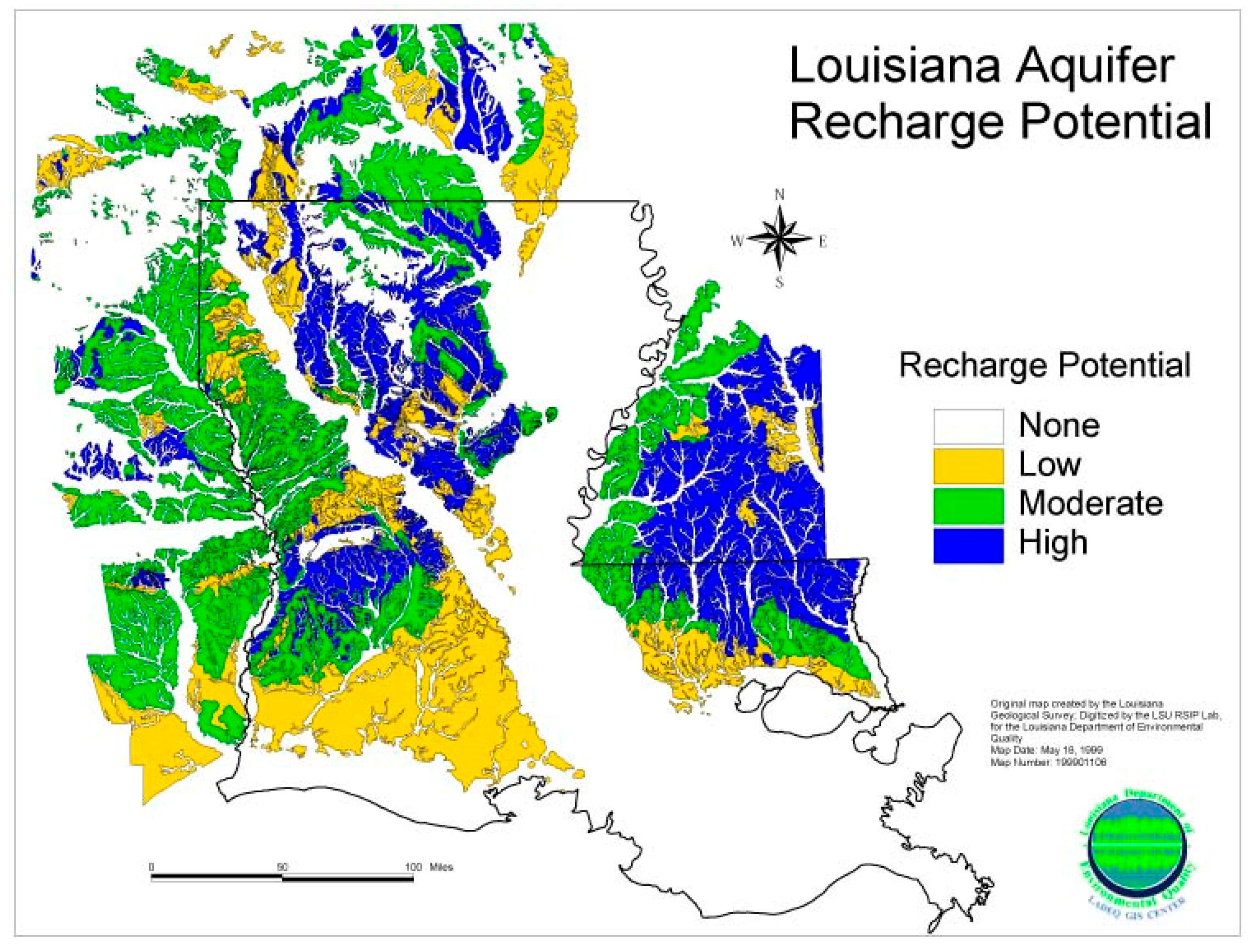
3.5. Potential Aquifer Recharge
4. Discussion
5. Conclusions
- The need to confirm the new apparent freedom from PD in the cotton, rice, sugar cane, and soybean areas in certain parishes. Why does there appear to be a persistent high risk in discrete other places in relation to cotton, rice, and soybeans and possibly other crops?
- The need to identify and quantify the risks associated with the high-incidence band east to west from the upper Atchafalaya to DeRidder and Starks on the Sabine River. The risks appear to be constant over time.
- The need to identify the herbicides most probably associated with the elevated PD risks in St. Tammany, Tangipahoa, and Washington Parishes, as well as upstream to the north in adjoining Mississippi counties and in the other arbor–pastoral parishes across Louisiana from Sabine to West Carroll.
- The need to identify and quantify the cause or causes of the relative freedom from PD in that discrete area in Vernon Parish to the north of Fort Polk and to the west of Alexandria and comparatively to the north of the latter city.
- The need to identify the local cause or causes of the higher risk of PD in Jefferson, St. John the Baptist, and St. Charles Parishes, which are possibly of an industrial nature.
- The need to share this information, where relevant, with the appropriate state departments in Arkansas, Mississippi, and Texas.
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
- Glyphosate is used presently with RoundupReady®-resistant varieties. It was approved for use with soybean crops in 1996, and by 1999, it represented >50% of the soybean crops sown; by 2006, it was essentially universal. When plants are over 8 inches tall, 2,4-D can be used as a directed spray as long as it goes no higher than 3 inches on the soybean plant in order to kill morning glory, cocklebur, pigweed, and prickly sida. Paraquat is used to control seedling grasses 2–4 inches tall, such as Johnson grass, crabgrasses, broadleaf signal grass, barnyard grass, and goose grass, but the soybean plants must be at least 8 inches tall with a directed spray at the weeds. Paraquat is also used as a preharvest “burner”. Trifluralin is suggested as a preplant treatment to be applied 2–3 inches deep for annual grasses, seedling Johnson grass, and some broadleaf weeds [34]. In 2000 [35] and 2007 [36], 2,4-D was recommended as a preplant and directed-spray herbicide, primarily for weeds not controlled by glyphosate, and trifluralin was recommended for preemergence application. Paraquat was detailed in the 2000 edition for preplanting and postemergence. Previously, in 1991 and 1994, trifluralin was recommended for soybeans for preemergence treatment, paraquat was recommended for preplant and postemergence treatments, and 2,4-D was only for postemergence directed-spray treatments [37,38].
References
- Parkinson’s Foundation, Understanding Parkinson’s, Statistics. Available online: https://www.parkinson.org/Understanding-Parkinsons/Statistics (accessed on 9 January 2019).
- Shapira, A.H.V. Parkinson’s disease. Br. Med. J. 1999, 318, 311–314. [Google Scholar]
- Hilker, R.; Schweitzer, K.; Coburger, S.; Ghaemi, M.; Weisenbach, S.; Jacobs, A.H.; Rudolf, J.; Herholz, K.; Heiss, W.D. Nonlinear progression of Parkinson disease as determined by serial positron emission tomographic imaging of striatal flurodopa F 18 activity. Arch. Neurol. 2005, 62, 378–382. [Google Scholar] [CrossRef] [Green Version]
- Cory-Slechta, D.A.; Thiruchelvam, M.; Barlow, B.K.; Richfield, E.K. Developmental pesticide models of the Parkinson disease phenotype. Environ. Health Perspect. 2005, 113, 1263–1270. [Google Scholar] [CrossRef] [Green Version]
- Logroscino, G. The role of early life environmental risk factors in Parkinson disease: what is the evidence? Environ. Health Perspect. 2005, 113, 1234–1238. [Google Scholar] [CrossRef] [Green Version]
- Schenck, C.H.; Boeve, B.F.; Mahowald, M.W. Delayed emergence of a Parkinson disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 2013, 14, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Iranzo, A.; Fernández-Arcos, A.; Tolosa, E.; Serradell, M.; Molinuevo, J.L.; Valldeoriola, F.; Gelpi, E.; Vilaseca, I.; Sánchez-Valle, R.; Lladó, A.; et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder study in 174 patients. PLoS ONE 2014, 9, e89741. [Google Scholar] [CrossRef] [PubMed]
- Boeve, B.F.; Silber, M.H.; Ferman, T.J.; Lin, S.C.; Benarroch, E.E.; Schmeichel, A.M.; Ahlskog, J.E.; Caselli, R.J.; Jacobson, S.; Sabbagh, M.; et al. Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep Med. 2013, 14, 754–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sixel-Döring, F.; Zimmermann, J.; Wegener, A.; Mollenhauer, B.; Trenkwalder, C. The evolution of REM sleep behavior disorder in early Parkinson disease. Sleep 2016, 39, 1737–1742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Gao, H.M.; Hong, J.S. Gene-environment interactions: Key to unravelling the mystery of Parkinson’s disease. Prog. Neurobiol. 2011, 94, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Litvan, I.; MacIntyre, A.; Goetz, C.G.; Wenning, G.K.; Jellinger, K.; Verny, M.; Bartko, J.J.; Jankovic, J.; McKee, A.; Brandel, J.P.; et al. Accuracy of the clinical diagnoses of Lewy body disease, Parkinson’s disease, and dementia with Lewy bodies: a clinicopathologic study. Arch Neurol. 1998, 55, 969–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbaz, A.; Tranchant, C. Epidemiologic studies of environmental exposures in Parkinson’s disease. J. Neurol. Sci. 2007, 262, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Wirdefeldt, K.; Adami, H.O.; Cole, P.; Trichopoulos, D.; Mandel, J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur. J. Epidemiol. 2011, 26, S1–S58. [Google Scholar] [CrossRef] [PubMed]
- Kwakye, G.F.; McMinimy, R.A.; Aschner, M. Disease-toxicant interactions in Parkinson’s disease neuropathology. Neurochem. Res. 2017, 42, 1772–1786. [Google Scholar] [CrossRef] [PubMed]
- Freire, C.; Koifman, S. Pesticide exposure and Parkinson’s disease: epidemiological association. Neurotoxicology. 2012, 33, 947–971. [Google Scholar] [CrossRef] [PubMed]
- Hristina, V.D.; Sipetic, S.B.; Maksimovic, J.M.; Marinkovic, J.M.; Dzoljic, E.D.; Ratkov, I.S.; Kostic, V.S. Environmental factors and Parkinson’s disease: A case-control study in Belgrade, Serbia. Int. J. Neurosci. 2010, 120, 361–367. [Google Scholar] [CrossRef]
- Gatto, N.M.; Cockburn, M.; Bronstein, J.; Manthripragada, A.D.; Ritz, B. Well-water consumption and Parkinson’s disease in rural California. Environ Health Perspect 2009, 117, 1912–1918. [Google Scholar] [CrossRef] [Green Version]
- Kab, S.; Spinosi, J.; Chaperon, L.; Dugravot, A.; Singh-Manoux, A.; Moisan, F.; Elbaz, A. Agricultural activities and the incidence of Parkinson’s disease in the general French population. Eur. J. Epidemiol. 2017, 32, 203–216. [Google Scholar] [CrossRef]
- Yitshak Sade, M.; Zlotnik, Y.; Kloog, I.; Novack, V.; Peretz, C.; Ifergane, G. Parkinson’s Disease Prevalence and Proximity to Agricultural Cultivated Fields. Parkinson’s Dis. 2015, 2015, 576564. [Google Scholar] [CrossRef]
- Bordelon, M.S. Environmental benefits realized from eradication of the non-indigenous insect Anthonomus grandis Boheman, the Cotton Boll Weevil. Master’s Thesis, Louisiana State University, Baton Rouge, LA, USA, 2005. [Google Scholar]
- Sanders, D.; Puls, E., Jr.; Koske, T.; Cannon, J.W.; Boudreaux, J.E. Cotton: Effectiveness of cotton preemergence herbicides. In Louisiana’s Suggested Chemical Weed Control Guide for 1991; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 1991; pp. 18–30. [Google Scholar]
- Sanders, D.; Puls, E., Jr.; Koske, T.; Cannon, J.M.; Boudreaux, J.E. Cotton: Effectiveness of cotton preemergence herbicides. In Louisiana’s Suggested Chemical Weed Control Guide for 1994; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 1994; pp. 15–26. [Google Scholar]
- Sanders, D.; Lencse, R.J.; Kelly, S.; Puls, E., Jr.; Koske, T.; Cannon, J.M.; Boudreaux, J.E.; Owings, A.D.; Strahan, R. Cotton: Effectiveness of cotton preemergence herbicides. In 2000 Louisiana Suggested Chemical Weed Control Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2000; pp. 20–27. [Google Scholar]
- Vidrine, P.R.; Sanders, D.E.; Strahan, R.E.; Koske, T.J.; Owings, A.D.; Boudreaux, J.E.; Saichuk, J.K.; Lancios, D.Y. Cotton: Effectiveness of cotton preemergence herbicides. In 2007 Louisiana Suggested Chemical Weed Control Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2007; pp. 29–37. [Google Scholar]
- Sanders, D.; Puls, E., Jr.; Koske, T.; Cannon, J.M.; Boudreaux, J.E. Permanent Pastures. In Louisiana’s Suggested Chemical Weed Control Guide for 1988; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 1988; pp. 30–33. [Google Scholar]
- Vidrine, P.R.; Sanders, D.E.; Strahan, R.E.; Koske, T.J.; Owings, A.D.; Boudreaux, J.E.; Saichuk, J.K.; Lancios, D.Y. Permanent Pastures. In 2007 Louisiana Suggested Chemical Weed Control Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2007; pp. 39–41. [Google Scholar]
- Fish, J.C.; Webster, E.P.; Blouin, D.C.; Bond, J.A. Imazamox plus propanil mixtures for grass weed management in imidazolinone-resistant rice. Weed Technol. 2016, 30, 29–35. [Google Scholar] [CrossRef]
- Webster, E.P.; Teló, G.M.; Blouin, D.C.; McKnight, B.M. Imazethapyr plus propanil mixtures in imidazolinone-resistant rice. Weed Technol. 2017, 32, 45–51. [Google Scholar] [CrossRef]
- Bagent, R.; Baldwin, J.L.; Boethel, D.; Johnson, S.J.; Leonard, R.; Meek, C.L. 2000 Louisiana soybean insect control recommendations. In 2000 Insect Pest Management Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2000; pp. 108–109. [Google Scholar]
- Brown, S.; Davis, J.; Foil, L.D.; Healy, K.; Huang, F.; Reagan, T.; Ring, D.; Schowalter, T.; Stout, M.; Smith, T.; et al. Crops—Commercial, Soybean. In 2017 Louisiana Insect Pest Management Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2017; pp. 40–46. [Google Scholar]
- Baldwin, J.L.; Foil, L.D.; Grodner, M.L.; Hammond, A.; Henderson, G.; Hummel, N.; Johnson, S.; Leonard, R.; Morgan, A.; Pollet, D.K.; et al. Louisiana recommendations for control of insects on soybeans. In 2009 Louisiana Insect Pest Management Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2009; pp. 92–94. [Google Scholar]
- Bagwell, R.; Baldwin, J.L.; Foil, L.D.; Fuxa, J.R.; Grodner, M.L.; Hall, M.; Hammond, A.; Henderson, G.; Johnson, S.; Leonard, R.; et al. Louisiana recommendations for control of insects on soybeans. In 2007 Louisiana Insect Pest Management Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2007; pp. 83–85. [Google Scholar]
- Stephenson, D.O.; Graham, C.J.; Miller, D.K.; Mudge, C.; Orgeron, A.; Price, R.; Strahan, R.E.; Webster, E.P. Soybean Weed Management. In 2017 Louisiana Suggested Chemical Weed Management Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2017; pp. 50–60. [Google Scholar]
- Sanders, D.; Lencse, R.J.; Kelly, S.; Puls, E., Jr.; Koske, T.; Cannon, J.M.; Boudreaux, J.E.; Owings, A.D.; Strahan, R. Soybeans. In 2000 Louisiana Suggested Chemical Weed Control Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2000; pp. 61–71. [Google Scholar]
- Vidrine, P.R.; Sanders, D.E.; Strahan, R.E.; Koske, T.J.; Owings, A.D.; Boudreaux, J.E.; Saichuk, J.K.; Lancios, D.Y. Soybeans. In 2007 Louisiana Suggested Chemical Weed Control Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2007; pp. 75–84. [Google Scholar]
- Sanders, D.; Puls, E., Jr.; Koske, T.; Cannon, J.W.; Boudreaux, J.E. Soybeans. In Louisiana’s Suggested Chemical Weed Control Guide for 1991; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 1991; pp. 57–70. [Google Scholar]
- Sanders, D.; Puls, E., Jr.; Koske, T.; Cannon, J.M.; Boudreaux, J.E. Soybeans. In Louisiana’s Suggested Chemical Weed Control Guide for 1994; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 1994; pp. 50–59. [Google Scholar]
- Bagent, J.L.; Baldwin, J.L.; Boethel, D.; Chapin, J.; Foil, L.D.; Fuxa, J.L.; Goyer, R.A. 1990 Small Fruit Spray Schedule, Strawberry. In 1990 Insect Control Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 1990; pp. 167–168. [Google Scholar]
- Brown, S.; Davis, J.; Foil, L.D.; Healy, K.; Huang, F.; Reagan, T.; Ring, D.; Schowalter, T.; Stout, M.; Smith, T.; et al. Fruit and Nuts—Commercial Strawberry Spray Guide. In 2017 Insect Pest Management Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2017; pp. 85–87. [Google Scholar]
- Stephenson, D.O.; Graham, C.J.; Miller, D.K.; Mudge, C.; Orgeron, A.; Price, R.; Strahan, R.E.; Webster, E.P. Fruit Crops Weed Management, Strawberry. In 2017 Louisiana Suggested Chemical Weed Management Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2017; pp. 109–110. [Google Scholar]
- Vidrine, P.R.; Sanders, D.E.; Strahan, R.E.; Koske, T.J.; Owings, A.D.; Boudreaux, J.E.; Saichuk, J.K.; Lancios, D.Y. Fruit Crops, Strawberry. In 2007 Louisiana Suggested Chemical Weed Control Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2007; p. 43. [Google Scholar]
- Sanders, D.; Lencse, R.J.; Kelly, S.; Puls, E., Jr.; Koske, T.; Cannon, J.M.; Boudreaux, J.E.; Owings, A.D.; Strahan, R. Fruit Crops, Strawberry. In 2000 Louisiana Suggested Chemical Weed Control Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2007; p. 31. [Google Scholar]
- Sanders, D.; Puls, E., Jr.; Koske, T.; Cannon, J.M.; Boudreaux, J.E. Fruit Crops, Strawberry. In Louisiana’s Suggested Chemical Weed Control Guide for 1994; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 1994; p. 70. [Google Scholar]
- Sanders, D.; Puls, E., Jr.; Koske, T.; Cannon, J.W.; Boudreaux, J.E. Fruit Crops, Strawberry. In Louisiana’s Suggested Chemical Weed Control Guide for 1991; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 1991; p. 85. [Google Scholar]
- Bagent, J.L.; Baldwin, J.L.; Boethel, D.; Chapin, J.; Foil, L.D.; Fuxa, J.L.; Goyer, R.A. 1990 Louisiana Recommendations for Sugarcane Insect Control. In 1990 Insect Control Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 1990; pp. 128–131. [Google Scholar]
- Brown, S.; Davis, J.; Foil, L.D.; Healy, K.; Huang, F.; Reagan, T.; Ring, D.; Schowalter, T.; Stout, M.; Smith, T.; et al. Louisiana Recommendations for Control of Sugarcane Insects. In 2017 Insect Pest Management Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2017; pp. 49–51. [Google Scholar]
- Stephenson, D.O.; Graham, C.J.; Miller, D.K.; Mudge, C.; Orgeron, A.; Price, R.; Strahan, R.E.; Webster, E.P. Sugarcane Weed Management. In 2017 Louisiana Suggested Chemical Weed Management Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2017; pp. 67–84. [Google Scholar]
- Vidrine, P.R.; Sanders, D.E.; Strahan, R.E.; Koske, T.J.; Owings, A.D.; Boudreaux, J.E.; Saichuk, J.K.; Lancios, D.Y. Sugarcane Weed Control. In 2007 Louisiana Suggested Chemical Weed Control Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2007; pp. 85–99. [Google Scholar]
- Sanders, D.; Lencse, R.J.; Kelly, S.; Puls, E., Jr.; Koske, T.; Cannon, J.M.; Boudreaux, J.E.; Owings, A.D.; Strahan, R. Sugarcane. In 2000 Louisiana Suggested Chemical Weed Control Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2007; pp. 72–77. [Google Scholar]
- Sanders, D.; Puls, E., Jr.; Koske, T.; Cannon, J.M.; Boudreaux, J.E. Sugarcane. In Louisiana’s Suggested Chemical Weed Control Guide for 1994; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 1994; pp. 60–66. [Google Scholar]
- Sanders, D.; Puls, E., Jr.; Koske, T.; Cannon, J.W.; Boudreaux, J.E. Sugarcane. In Louisiana’s Suggested Chemical Weed Control Guide for 1991; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 1991; pp. 71–81. [Google Scholar]
- Brown, S.; Davis, J.; Foil, L.D.; Healy, K.; Huang, F.; Reagan, T.; Ring, D.; Schowalter, T.; Stout, M.; Smith, T.; et al. Trees (Forest, Shade and Christmas). In 2017 Insect Pest Management Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2017; pp. 157–178. [Google Scholar]
- Bagent, J.L.; Baldwin, J.L.; Boethel, D.; Chapin, J.; Foil, L.D.; Fuxa, J.L.; Goyer, R.A. 1990 Louisiana Recommendations for Tree Insects—Forest, Shade, and Christmas. In 1990 Insect Control Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 1990; pp. 17–50. [Google Scholar]
- Stephenson, D.O.; Graham, C.J.; Miller, D.K.; Mudge, C.; Orgeron, A.; Price, R.; Strahan, R.E.; Webster, E.P. Woody Plants Weed Management. In 2017 Louisiana Suggested Chemical Weed Management Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2017; pp. 190–192. [Google Scholar]
- Sanders, D.; Lencse, R.J.; Kelly, S.; Puls, E., Jr.; Koske, T.; Cannon, J.M.; Boudreaux, J.E.; Owings, A.D.; Strahan, R. Woody Plants. In 2000 Louisiana Suggested Chemical Weed Control Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2007; pp. 99–100. [Google Scholar]
- Vidrine, P.R.; Sanders, D.E.; Strahan, R.E.; Koske, T.J.; Owings, A.D.; Boudreaux, J.E.; Saichuk, J.K.; Lancios, D.Y. Woody Plants. In 2007 Louisiana Suggested Chemical Weed Control Guide; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2007; pp. 127–128. [Google Scholar]
- Sanders, D.; Puls, E., Jr.; Koske, T.; Cannon, J.W.; Boudreaux, J.E. Woody Plants. In Louisiana’s Suggested Chemical Weed Control Guide for 1991; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 1991; pp. 177–180. [Google Scholar]
- Sanders, D.; Puls, E., Jr.; Koske, T.; Cannon, J.M.; Boudreaux, J.E. Woody Plants. In Louisiana’s Suggested Chemical Weed Control Guide for 1994; Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 1994; pp. 141–143. [Google Scholar]
- Shaner, D.L. Imazapyr. In Herbicide Handbook, 10th ed.; Weed Science Society of America: Champaign, IL, USA, 2014; pp. 258–259. [Google Scholar]
| Years | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | |
| Primary PD Discharges | ||||||||||||||
| All | 2427 | 2087 | 1282 | 2.480 | 2106 | 1940 | 1582 | 1469 | 1270 | 1306 | 1349 | 1289 | 1332 | 1305 |
| Male | 1179 | 952 | 668 | 1154 | 1010 | 937 | 804 | 747 | 630 | 641 | 674 | 679 | 690 | 691 |
| Female | 1248 | 1135 | 614 | 1326 | 1096 | 1003 | 778 | 722 | 640 | 665 | 675 | 610 | 642 | 614 |
| Estimated Population in Millions | ||||||||||||||
| Total | 4.461 | 4.472 | 4.478 | 4.497 | 4.521 | 4.552 | 4.577 | 4.303 | 4.376 | 4.436 | 4.492 | 4.546 | 4.576 | 4.605 |
| Male | 2.147 | 2.165 | 2.170 | 2.182 | 2.194 | 2.213 | 2.227 | 2.099 | 2.136 | 2.166 | 2.196 | 2.226 | 2.238 | 2.252 |
| Female | 2.314 | 2.310 | 2.308 | 2.316 | 2.327 | 2.339 | 2.349 | 2.204 | 2.239 | 2.270 | 2.295 | 2.320 | 2.338 | 2.353 |
| Results/10,000 | ||||||||||||||
| All | 5.44 | 4.67 | 2.86 | 5.51 | 4.66 | 4.26 | 3.46 | 3.41 | 2.90 | 2.94 | 3.00 | 2.84 | 2.91 | 2.83 |
| Male | 5.49 | 4.40 | 3.08 | 5.29 | 4.60 | 4.29 | 3.61 | 3.56 | 2.95 | 2.96 | 3.07 | 3.05 | 3.08 | 3.07 |
| Female | 5.39 | 4.92 | 2.66 | 5.73 | 4.71 | 4.23 | 3.31 | 3.28 | 2.86 | 2.93 | 2.94 | 2.63 | 2.75 | 2.61 |
| Risk per 10,000 | ZIPs 1 (1999–2005) | ZIPs 2 (2006–2012) |
|---|---|---|
| 0–9 | 39 | 43 |
| 10–19 | 32 | 46 |
| 20–29 | 86 | 167 |
| 30–39 | 111 | 133 |
| 40–49 | 102 | 63 |
| 50–59 | 65 | 26 |
| 60–60 | 32 | 2 |
| 70–70 | 12 | 7 |
| 80–89 | 2 | 7 |
| 90–99 | 9 | 3 |
| 100–149 | 3 | 2 |
| 150–199 | 10 | 4 |
| 200–249 | 2 | 1 |
| 250–299 | 0 | 1 |
| ≥300 | 1 | 2 |
| 1999–2005 | 2006–2012 | |||||
|---|---|---|---|---|---|---|
| Age in Years | Male | Female | Total | Male | Female | Total |
| <20 | 3 | 1 | 4 | 2 | 0 | 2 |
| 20–29 | 11 | 6 | 17 | 11 | 10 | 21 |
| 30–39 | 30 | 19 | 49 | 17 | 16 | 33 |
| 40–49 | 74 | 64 | 138 | 67 | 66 | 133 |
| 50–59 | 318 | 219 | 537 | 342 | 257 | 599 |
| 60–60 | 945 | 832 | 1777 | 860 | 685 | 1545 |
| 70–79 | 2751 | 2617 | 5368 | 1717 | 1511 | 3228 |
| 80–89 | 2248 | 2828 | 5076 | 1532 | 1696 | 3228 |
| 90–99 | 321 | 600 | 921 | 203 | 322 | 525 |
| ≥100 | 3 | 14 | 17 | 1 | 5 | 6 |
| Totals | 6704 | 7200 | 13,904 | 4752 | 4568 | 9320 |
| (a) 2,4-D | ||||||||
|---|---|---|---|---|---|---|---|---|
| Louisiana Parish | 2,4-D Kg, Epest-low | 2,4-D Kg Epest-high | ||||||
| 1992 | 1996 | 2000 | 2004 | 1992 | 1996 | 2000 | 2004 | |
| Allen | 6535.5 | 4426.4 | 4050.4 | 7175 | 5781.5 | 4209.9 | ||
| Beauregard | 966.8 | 1876 | 4260.2 | 2441.5 | 2454.3 | 4281.3 | 4390.6 | 2683.3 |
| Bienville | 369.1 | 3217.2 | 6535.5 | 622.5 | 465.1 | 3234.3 | 377.5 | |
| Claiborne | 12.5 | 4679 | 0.6 | 316 | 596.1 | 4679.2 | 552.3 | |
| East Feliciana | 792.8 | 809.2 | 360.9 | 700.1 | 1503.4 | 1006.3 | 938.4 | 734.4 |
| Grant | 221.8 | 374.2 | 160.4 | 768.1 | 383.9 | 942 | 200.4 | 922.2 |
| La Salle | 745.3 | 213.8 | 32.8 | 158.5 | 785.1 | 301.5 | 32.8 | 158.5 |
| Sabine | 165.7 | 26.1 | 1329.1 | 3602 | 464.2 | 779.8 | 1329.1 | 3602 |
| St. Helena | 2355.6 | 896.8 | 18 | 239.8 | 2553.2 | 1046.1 | 235.5 | 274.7 |
| Tangipahoa | 3926.9 | 1622.2 | 481.7 | 677 | 4507.3 | 1938.9 | 1035 | 734 |
| Washington | 2728.3 | 1407.7 | 353.6 | 433.1 | 3606.7 | 1718.6 | 688.8 | 508.6 |
| Winn | 3.8 | 1919.9 | 104.1 | 199.7 | 1919.9 | 221.9 | ||
| (b) Chlorpyrifos | ||||||||
| Louisiana Parish | Chlorpyrifos Kg, Epest-low | Chlorpyrifos Kg Epest-high | ||||||
| 1992 | 1996 | 2000 | 2004 | 1992 | 1996 | 2000 | 2004 | |
| Allen | 24.9 | 10.3 | 35.2 | 2.5 | 67.6 | 151.3 | 58.7 | 13.8 |
| Beauregard | 70.7 | 46.1 | 313.5 | 44.7 | 187.1 | 637 | 443.7 | 89 |
| Bienville | 1.6 | 104.4 | 7.7 | 160.3 | 106.9 | 8 | 10.2 | |
| Claiborne | 2.1 | 0.9 | 16.1 | 2.1 | 1.9 | 0.9 | ||
| East Feliciana | 13.9 | 486.6 | 182.8 | 502 | 141.4 | 112.2 | ||
| Grant | 479.3 | 504.3 | 416 | 22.8 | 491.5 | 1040.1 | 569.6 | 160.6 |
| La Salle | 7.1 | 3.7 | 3.2 | 4.4 | 15.6 | 4.4 | 5 | 7.1 |
| Sabine | 0.8 | 15 | 2.8 | 3.5 | 17.5 | 1.1 | 2.8 | |
| St. Helena | 7.3 | 987.3 | 0.8 | 96.2 | 993.7 | 73.6 | 98.9 | |
| Tangipahoa | 44.5 | 1441.8 | 0.6 | 283.1 | 1461.3 | 223.7 | 174.2 | |
| Washington | 74.1 | 2238.8 | 3.4 | 449.9 | 2254.5 | 194.6 | 214.3 | |
| Winn | 0.6 | 37.1 | 1.6 | 4.8 | 37.1 | 16 | ||
| (c) Paraquat | ||||||||
| Louisiana Parish | Paraquat Kg, Epest-low | Paraquat Kg Epest-high | ||||||
| 1992 | 1996 | 2000 | 2004 | 1992 | 1996 | 2000 | 2004 | |
| Allen | 6.4 | 59.1 | 223.3 | 146.4 | 54.5 | |||
| Beauregard | 2.5 | 4.8 | 15.5 | 5.6 | 89.2 | 272.7 | 318.5 | 135.1 |
| Bienville | 4.2 | 1.8 | 69.6 | 449.8 | 27.1 | 3.3 | 69.6 | |
| Claiborne | 711.8 | 14 | 0.6 | |||||
| East Feliciana | 259.1 | 45.1 | 20.1 | 5.4 | 277.9 | 95.9 | 165.3 | 112.8 |
| Grant | 50 | 86.3 | 699.7 | 45.9 | 160.1 | 117.7 | 793.6 | 174.6 |
| La Salle | 4.9 | 12.1 | 34.9 | 10.8 | 11.8 | 12.3 | 34.9 | 23.9 |
| Sabine | 0.5 | 1.9 | 1.7 | 17.1 | 6.2 | |||
| St. Helena | 5.4 | 0.2 | 1.1 | 1.1 | 21 | 72.7 | 153.9 | 110.7 |
| Tangipahoa | 407.3 | 576.2 | 15.2 | 1.2 | 435.3 | 692.5 | 352 | 179.7 |
| Washington | 191.5 | 83.2 | 22.9 | 7.3 | 224.1 | 251.8 | 421.7 | 244.2 |
| Winn | 208.7 | 4.1 | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hugh-Jones, M.E.; Peele, R.H.; Wilson, V.L. Parkinson’s Disease in Louisiana, 1999–2012: Based on Hospital Primary Discharge Diagnoses, Incidence, and Risk in Relation to Local Agricultural Crops, Pesticides, and Aquifer Recharge. Int. J. Environ. Res. Public Health 2020, 17, 1584. https://doi.org/10.3390/ijerph17051584
Hugh-Jones ME, Peele RH, Wilson VL. Parkinson’s Disease in Louisiana, 1999–2012: Based on Hospital Primary Discharge Diagnoses, Incidence, and Risk in Relation to Local Agricultural Crops, Pesticides, and Aquifer Recharge. International Journal of Environmental Research and Public Health. 2020; 17(5):1584. https://doi.org/10.3390/ijerph17051584
Chicago/Turabian StyleHugh-Jones, Martin E., R. Hampton Peele, and Vincent L. Wilson. 2020. "Parkinson’s Disease in Louisiana, 1999–2012: Based on Hospital Primary Discharge Diagnoses, Incidence, and Risk in Relation to Local Agricultural Crops, Pesticides, and Aquifer Recharge" International Journal of Environmental Research and Public Health 17, no. 5: 1584. https://doi.org/10.3390/ijerph17051584





