Passive Smoking Exposure in Living Environments Reduces Cognitive Function: A Prospective Cohort Study in Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Assessment of Passive Smoking Exposure in Living Environments
2.3. Assessment of Cognitive Function
2.4. Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. A Report of the Surgeon General, How Tobacco Smoke Causes Disease: What it Means to You. 2010. Available online: https://www.cdc.gov/tobacco/data_statistics/sgr/2010/consumer_booklet/pdfs/consumer.pdf (accessed on 14 September 2019).
- Hecht, S.S. Research opportunities related to establishing standards for tobacco products under the Family Smoking Prevention and Tobacco Control Act. Nicotine Tob. Res. 2012, 14, 18–28. [Google Scholar] [CrossRef]
- Kim, A.S.; Ko, H.J.; Kwon, J.H.; Lee, J.M. Exposure to Secondhand Smoke and Risk of Cancer in Never Smokers: A Meta-Analysis of Epidemiologic Studies. Int. J. Environ. Res. Public Health 2018, 15, E1981. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Hwang, S.H.; Choi, H.; Kim, H. The association between smoking or passive smoking and cardiovascular diseases using a Bayesian hierarchical model: Based on the 2008–2013 Korea Community Health Survey. Epidemiol. Health 2017, 39, e2017026. [Google Scholar] [CrossRef] [PubMed]
- Shiue, I. Modeling the effects of indoor passive smoking at home, work, or other households on adult cardiovascular and mental health: The Scottish Health Survey, 2008–2011. Int. J. Environ. Res. Public Health 2014, 11, 3096–3107. [Google Scholar] [CrossRef] [PubMed]
- Stirland, L.E.; O’Shea, C.I.; Russ, T.C. Passive smoking as a risk factor for dementia and cognitive impairment: Systematic review of observational studies. Int. Psychogeriatr. 2018, 30, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Khorasanchi, Z.; Bahrami, A.; Avan, A.; Jaberi, N.; Rezaey, M.; Bahrami-Taghanaki, H.; Ferns, G.A.; Ghayour-Mobarhan, M. Passive smoking is associated with cognitive and emotional impairment in adolescent girls. J. Gen. Psychol 2019, 146, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Newhouse, P.; Kellar, K.; Aisen, P.; White, H.; Wesnes, K.; Coderre, E.; Pfaff, A.; Wilkins, H.; Howard, D.; Levin, E.D. Nicotine treatment of mild cognitive impairment: A 6-month double-blind pilot clinical trial. Neurology 2012, 78, 91–101. [Google Scholar] [CrossRef]
- White, H.K.; Levin, E.D. Four-week nicotine skin patch treatment effects on cognitive performance in Alzheimer’s disease. Psychopharmacology (Berl) 1999, 143, 158–165. [Google Scholar] [CrossRef]
- Sheng, Y.; He, F.; Lin, J.F.; Shen, W.; Qiu, Y.W. Tea and risk of age-related cataracts: A cross-sectional study in Zhejiang Province, China. J. Epidemiol. 2016, 26, 587–592. [Google Scholar] [CrossRef]
- Li, F.D.; He, F.; Chen, T.R.; Xiao, Y.Y.; Lin, S.T.; Shen, W.; Wang, X.Y.; Zhai, Y.J.; Shang, X.P.; Lin, J.F. Reproductive history and risk of cognitive impairment in elderly women: A cross-sectional study in Eastern China. J. Alzheimers Dis. 2016, 49, 139–147. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhang, M.Y.; Zhai, G.Y.; Chen, J.X.; Zhao, J. Application of the Chinese version of the Mini-Mental State Examination. Shanghai Arch. Psychiatr. 1989, 7, 108–111. [Google Scholar]
- Zhang, Z.J. Handbook of Behavioral Medical Scales; Chinese Medical Multimedia Press: Beijing, China, 2005. [Google Scholar]
- Chen, R.; Wilson, K.; Chen, Y.; Zhang, D.; Qin, X.; He, M.; Hu, Z.; Ma, Y.; Copeland, J.R. Association between environmental tobacco smoke exposure and dementia syndromes. Occup. Environ. Med. 2013, 70, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Orsitto, G.; Turi, V.; Venezia, A.; Fulvio, F.; Manca, C. Relation of secondhand smoking to mild cognitive impairment in older inpatients. Sci. World J. 2012, 2012, 726948. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.E.; Haight, T.J.; Mehta, K.M.; Carlson, M.C.; Kuller, L.H.; Tager, I.B. Secondhand smoke, vascular disease, and dementia incidence: Findings from the cardiovascular health cognition study. Am. J. Epidemiol. 2010, 171, 292–302. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. A Report of the Surgeon General, The Health Consequences of Involuntary Exposure to Tobacco Smoke. Available online: https://www.ncbi.nlm.nih.gov/books/NBK44324/pdf/Bookshelf_NBK44324.pdf (accessed on 15 September 2019).
- Zlokovic, B.V. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005, 28, 202–208. [Google Scholar] [CrossRef]
- Barnoya, J.; Glantz, S.A. Cardiovascular effects of secondhand smoke: Nearly as large as smoking. Circulation 2005, 111, 2684–2698. [Google Scholar] [CrossRef]
- Ho, Y.S.; Yang, X.; Yeung, S.C.; Chiu, K.; Lau, C.F.; Tsang, A.W.; Mak, J.C.; Chang, R.C. Cigarette smoking accelerated brain aging and induced pre-Alzheimer-like neuropathology in rats. PLoS ONE 2012, 7, e36752. [Google Scholar] [CrossRef]
- Ghosh, D.; Mishra, M.K.; Das, S.; Kaushik, D.K.; Basu, A. Tobacco carcinogen induces microglial activation and subsequent neuronal damage. J. Neurochem. 2009, 110, 1070–1081. [Google Scholar] [CrossRef]
- Llewellyn, D.J.; Lang, I.A.; Langa, K.M.; Naughton, F.; Matthews, F.E. Exposure to secondhand smoke and cognitive impairment in non-smokers: National cross-sectional study with cotinine measurement. BMJ 2009, 338, b462. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, D.; Chen, Y.; Hu, Z.; Wilson, K. Passive smoking and risk of cognitive impairment in women who never smoke. Arch. Intern. Med. 2012, 172, 271–273. [Google Scholar] [CrossRef]
- Chen, R.; Clifford, A.; Lang, L.; Anstey, K.J. Association of passive smoking with cognitive impairment in nonsmoking older adults: A systematic literature review and a new study of Chinese cohort. J. Geriatr. Psychiatry Neurol. 2013, 26, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Newhouse, P.A.; Potter, A.; Singh, A. Effects of nicotinic stimulation on cognitive performance. Curr. Opin. Pharmacol. 2004, 4, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Yolton, K.; Dietrich, K.; Auinger, P.; Lanphear, B.P.; Hornung, R. Exposure to environmental tobacco smoke and cognitive abilities among U.S. children and adolescents. Environ. Health Perspect. 2005, 113, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.N.; Abeles, N. Nicotine’s effect on neural and cognitive functioning in an aging population. Aging Ment. Health 2002, 6, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Teaktong, T.; Graham, A.J.; Johnson, M.; Court, J.A.; Perry, E.K. Selective changes in nicotinic acetylcholine receptor subtypes related to tobacco smoking: An immunohistochemical study. Neuropathol. Appl. Neurobiol. 2004, 30, 243–254. [Google Scholar] [CrossRef]
- Cervilla, J.A.; Prince, M.; Mann, A. Smoking, drinking, and incident cognitive impairment: A cohort community based study included in the Gospel Oak project. J. Neurol. Neurosurg. Psychiatry 2000, 68, 622–626. [Google Scholar] [CrossRef]
- Hill, R.D.; Nilsson, L.G.; Nyberg, L.; Backman, L. Cigarette smoking and cognitive performance in healthy Swedish adults. Age Ageing 2003, 32, 548–550. [Google Scholar] [CrossRef]
- DeLorenze, G.N.; Kharrazi, M.; Kaufman, F.L.; Eskenazi, B.; Bernert, J.T. Exposure to environmental tobacco smoke in pregnant women: The association between self-report and serum cotinine. Environ. Res. 2002, 90, 21–32. [Google Scholar] [CrossRef]
- Ling, J.; Heffernan, T. The cognitive deficits associated with second-hand smoking. Front. Psychiatry 2016, 7, 46. [Google Scholar] [CrossRef]
- Benowitz, N.L. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol. Rev. 1996, 18, 188–204. [Google Scholar] [CrossRef]
- Bono, R.; Bellisario, V.; Tassinari, R.; Squillacioti, G.; Manetta, T.; Bugiani, M.; Migliore, E.; Piccioni, P. Bisphenol a, tobacco smoke, and age as predictors of oxidative stress in children and adolescents. Int J. Environ. Res. Public Health 2019, 16, E2025. [Google Scholar] [CrossRef] [PubMed]
- Oberg, M.; Jaakkola, M.S.; Woodward, A.; Peruga, A.; Pruss-Ustun, A. Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. Lancet 2011, 377, 139–146. [Google Scholar] [CrossRef]
| Variables | Passive Smoking Exposure | p-Value | |||
|---|---|---|---|---|---|
| No | Yes | ||||
| n | % | n | % | ||
| Age (years) | |||||
| Mean (SD) | 70.3 | (7.0) | 69.0 | (6.5) | <0.001 |
| Ethnicity | |||||
| Han | 5794 | 86.4 | 914 | 13.6 | <0.001 |
| Other | 172 | 68.5 | 79 | 31.5 | |
| Sex | |||||
| Female | 2932 | 82.1 | 641 | 17.9 | <0.001 |
| Male | 3035 | 89.6 | 352 | 10.4 | |
| BMI, kg/m2 | |||||
| 18.5–24.99 | 3740 | 86.4 | 589 | 13.6 | 0.434 |
| <18.5 | 299 | 87.7 | 42 | 12.3 | |
| >24.99 | 1663 | 85.5 | 283 | 14.5 | |
| Education | |||||
| Illiterate or semiliterate | 2902 | 85.8 | 482 | 14.2 | 0.448 |
| Primary school | 2616 | 85.3 | 450 | 14.7 | |
| Junior high school | 380 | 88.0 | 52 | 12.0 | |
| High school graduation or higher | 69 | 88.5 | 9 | 11.5 | |
| Marital status | |||||
| Unmarried | 80 | 83.3 | 16 | 16.7 | <0.001 |
| Married | 4637 | 84.6 | 847 | 15.4 | |
| Widowed | 1213 | 90.7 | 125 | 9.3 | |
| Divorced | 25 | 86.2 | 4 | 13.8 | |
| Job | |||||
| Never worked | 1356 | 80.8 | 322 | 19.2 | <0.001 |
| Farmers | 2697 | 88.2 | 361 | 11.8 | |
| Housework | 717 | 87.3 | 104 | 12.7 | |
| Workers | 616 | 84.5 | 113 | 15.5 | |
| Others | 557 | 86.2 | 89 | 13.8 | |
| Family income (1000 ¥/year) | |||||
| <10 | 952 | 90.4 | 101 | 9.6 | <0.001 |
| 10–19 | 1350 | 88.6 | 174 | 11.4 | |
| 20–49 | 1636 | 85.6 | 276 | 14.4 | |
| 50–99 | 992 | 81.4 | 226 | 18.6 | |
| ≥100 | 1032 | 82.8 | 214 | 17.2 | |
| Participation in group activities | |||||
| Never | 3706 | 86.8 | 563 | 13.2 | 0.003 |
| Occasional | 1522 | 83.6 | 298 | 16.4 | |
| Frequent | 735 | 84.8 | 132 | 15.2 | |
| Smoking | |||||
| Non-smokers | 4211 | 85.4 | 718 | 14.6 | 0.284 |
| Current smokers | 1164 | 85.8 | 192 | 14.2 | |
| Ex-smokers | 592 | 87.7 | 83 | 12.3 | |
| Alcohol consumption | |||||
| Nondrinkers | 4012 | 85.9 | 656 | 14.1 | 0.076 |
| Current drinkers | 1569 | 86.1 | 253 | 13.9 | |
| Ex-drinkers | 378 | 82.2 | 82 | 17.8 | |
| Tea drinking | |||||
| Nondrinkers | 4389 | 87.2 | 646 | 12.8 | <0.001 |
| Current drinkers | 1453 | 82.0 | 319 | 18.0 | |
| Ex-drinkers | 110 | 84.6 | 20 | 15.4 | |
| Physical exercise | |||||
| No | 4824 | 86.1 | 776 | 13.9 | 0.064 |
| Yes | 1139 | 84.2 | 214 | 15.8 | |
| Physical work | |||||
| No | 3975 | 86.4 | 628 | 13.6 | 0.112 |
| Yes | 1981 | 84.9 | 351 | 15.1 | |
| Stroke | |||||
| No | 5807 | 85.7 | 971 | 14.3 | 0.314 |
| Yes | 159 | 88.3 | 21 | 11.7 | |
| High blood pressure | |||||
| No | 3140 | 85.2 | 546 | 14.8 | 0.169 |
| Yes | 2826 | 86.3 | 447 | 13.7 | |
| Hyperlipidemia | |||||
| No | 5710 | 85.8 | 946 | 14.2 | 0.437 |
| Yes | 250 | 84.2 | 47 | 15.8 | |
| Diabetes | |||||
| No | 5384 | 85.9 | 887 | 14.1 | 0.329 |
| Yes | 577 | 84.5 | 106 | 15.5 | |
| Coronary heart disease | |||||
| No | 5743 | 85.7 | 960 | 14.3 | 0.552 |
| Yes | 221 | 87.0 | 33 | 13.0 | |
| Chronic bronchitis | |||||
| No | 5851 | 85.9 | 959 | 14.1 | 0.003 |
| Yes | 112 | 77.2 | 33 | 22.8 | |
| Gallstones | |||||
| No | 5774 | 86.0 | 940 | 14.0 | 0.001 |
| Yes | 190 | 78.2 | 53 | 21.8 | |
| Tumor | |||||
| No | 5823 | 85.7 | 973 | 14.3 | 0.497 |
| Yes | 141 | 87.6 | 20 | 12.4 | |
| Arthritis | |||||
| No | 5768 | 85.9 | 946 | 14.1 | 0.017 |
| Yes | 188 | 80.3 | 46 | 19.7 | |
| Cataract | |||||
| No | 5687 | 85.9 | 930 | 14.1 | 0.020 |
| Yes | 276 | 81.4 | 63 | 18.6 | |
| Depressive symptoms | |||||
| Normal | 5157 | 85.6 | 869 | 14.4 | 0.524 |
| Mild depression | 607 | 86.3 | 96 | 13.7 | |
| Moderate depression | 164 | 86.8 | 25 | 13.2 | |
| Heavy depression | 39 | 92.9 | 3 | 7.1 | |
| Covariates | Cognitive Impairment | Multivariate Adjusted Regression Analyses | ||||
|---|---|---|---|---|---|---|
| No | Yes | |||||
| n | % | n | % | Adjusted RR (95% CI) a | p-Value | |
| Age (years) | ||||||
| Mean (SD) | 69.4 | (6.5) | 73.3 | (7.8) | 1.04 (1.03–1.05) | <0.001 |
| Male sex | 2883 | 85.1 | 504 | 14.9 | 0.85 (0.73–0.969) | 0.040 |
| Han ethnicity | 5486 | 81.8 | 1222 | 18.2 | 1.30 (0.92–1.84) | 0.141 |
| BMI, kg/m2 | ||||||
| 18.5–24.99 | 3565 | 82.4 | 764 | 17.6 | 1.00 | |
| <18.50 | 241 | 70.7 | 100 | 29.3 | 1.21 (1.05–1.39) | 0.007 |
| >24.99 | 1635 | 84.0 | 311 | 16.0 | 0.94 (0.84–1.06) | 0.305 |
| Education | ||||||
| Illiterate or semiliterate | 2647 | 78.2 | 737 | 21.8 | 1.00 | |
| Primary school | 2612 | 85.2 | 454 | 14.8 | 0.99 (0.89–1.12) | 0.964 |
| Junior high school | 372 | 86.1 | 60 | 13.9 | 1.03 (0.80–1.33) | 0.825 |
| High school or higher | 69 | 88.5 | 9 | 11.5 | 0.86 (0.49–1.51) | 0.605 |
| Marital status | ||||||
| Married | 83 | 86.5 | 13 | 13.5 | 1.00 | |
| Unmarried | 4599 | 83.9 | 885 | 16.1 | 1.31 (0.77–2.25) | 0.319 |
| Widowed | 986 | 73.7 | 352 | 26.3 | 1.27 (0.74–2.19) | 0.390 |
| Divorced | 23 | 79.3 | 6 | 20.7 | 1.56 (0.57–4.24) | 0.388 |
| Job | ||||||
| Never worked | 1254 | 74.7 | 424 | 25.3 | 1.00 | |
| Farmers | 2545 | 83.2 | 513 | 16.8 | 0.88 (0.78–0.99) | 0.035 |
| Housework | 657 | 80.0 | 164 | 20.0 | 0.83 (0.71–0.98) | 0.023 |
| Workers | 642 | 88.1 | 87 | 11.9 | 0.78 (0.62–0.99) | 0.038 |
| Others | 577 | 89.3 | 69 | 10.7 | 0.77 (0.60–0.99) | 0.043 |
| Family income (1000 ¥/year) | ||||||
| <10 | 734 | 69.7 | 319 | 30.3 | 1.00 | |
| 10–19 | 1175 | 77.1 | 349 | 22.9 | 0.93 (0.82–1.06) | 0.273 |
| 20–49 | 1673 | 87.5 | 239 | 12.5 | 0.62 (0.52–0.73) | <0.001 |
| 50–99 | 1026 | 84.2 | 192 | 15.8 | 0.74 (0.62–0.87) | <0.001 |
| ≥100 | 1087 | 87.2 | 159 | 12.8 | 0.58 (0.48–0.69) | <0.001 |
| Participation in group activities | ||||||
| Never | 3359 | 78.7 | 910 | 21.3 | 1.00 | |
| Occasionally | 1559 | 85.7 | 261 | 14.3 | 0.75 (0.66–0.85) | <0.001 |
| Frequently | 778 | 89.7 | 89 | 10.3 | 0.56 (0.46–0.69) | <0.001 |
| Smoking | ||||||
| Non-smokers | 3951 | 80.2 | 978 | 19.8 | 1.00 | |
| Current smokers | 1163 | 85.8 | 193 | 14.2 | 1.01 (0.84–1.21) | 0.923 |
| Ex-smokers | 586 | 86.8 | 89 | 13.2 | 0.88 (0.69–1.12) | 0.295 |
| Alcohol consumption | ||||||
| Non-drinkers | 3751 | 80.4 | 917 | 19.6 | 1.00 | |
| Current drinkers | 1554 | 85.3 | 268 | 14.7 | 1.13 (0.97–1.30) | 0.109 |
| Ex-drinkers | 389 | 84.6 | 71 | 15.4 | 0.95 (0.74–1.20) | 0.648 |
| Tea consumption | ||||||
| Non-drinkers | 4032 | 80.1 | 1003 | 19.9 | 1.00 | |
| Current drinkers | 1534 | 86.6 | 238 | 13.4 | 0.81 (0.70–0.94) | 0.004 |
| Ex-drinkers | 117 | 90.0 | 13 | 10.0 | 0.49 (0.27–0.88) | 0.016 |
| Physical exercise | 1198 | 88.5 | 155 | 11.5 | 0.68 (0.58–0.81) | <0.001 |
| Physical work | 2083 | 89.3 | 249 | 10.7 | 0.70 (0.61–0.81) | <0.001 |
| Passive smoking exposure | 819 | 82.5 | 174 | 17.5 | 1.16 (1.01–1.35) | 0.047 |
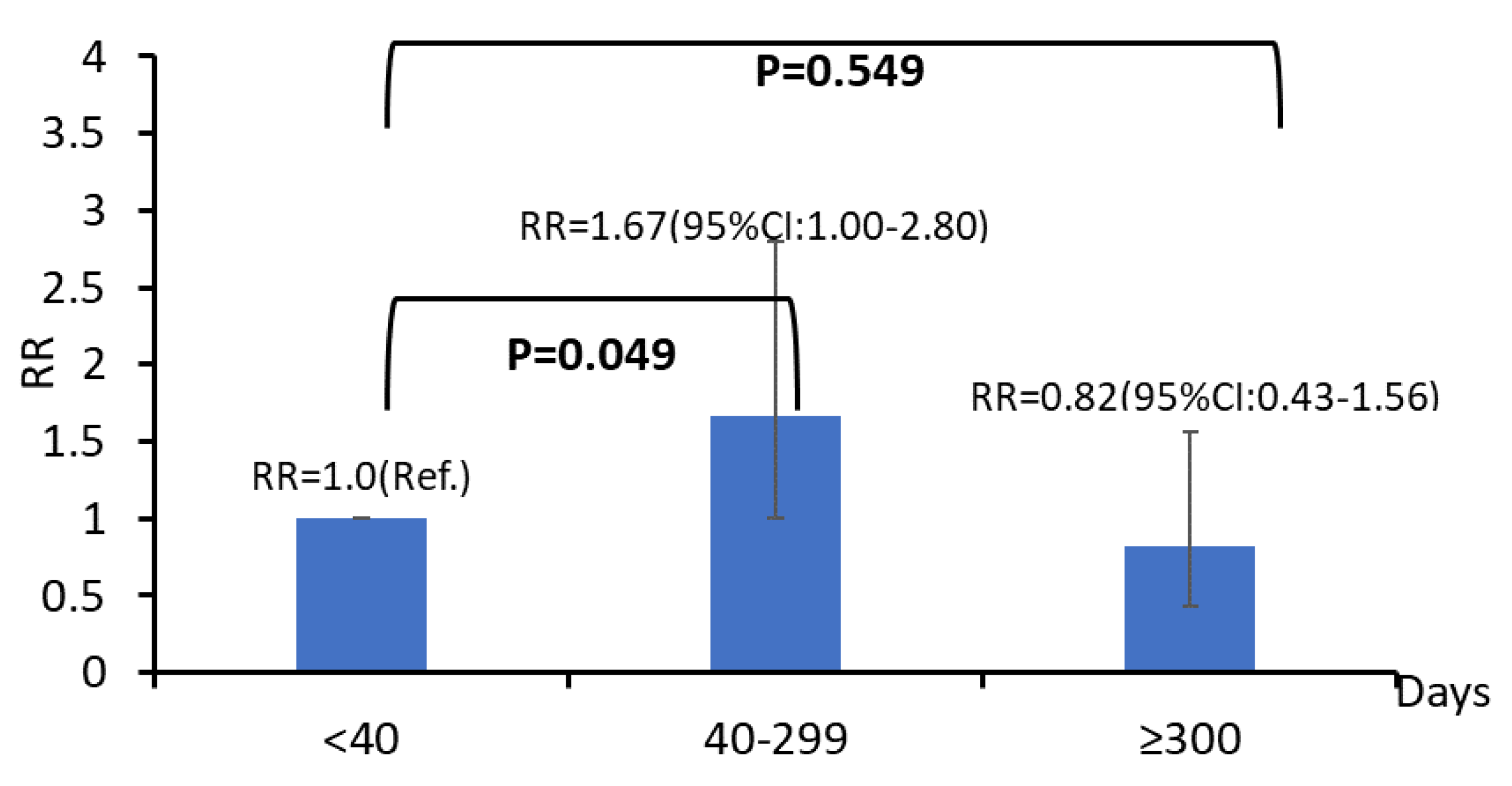
| Cumulative dose of passive smoking exposure (days) | ||||||
| <40 | 174 | 81.3 | 40 | 18.7 | 1.00 | |
| 40–299 | 336 | 80.8 | 80 | 19.2 | 1.17 (0.62–1.46) | 0.277 |
| ≥300 | 199 | 87.3 | 29 | 12.7 | 0.72 (0.41–1.32) | 0.421 |
| Active Smoking | Passive Smoking Exposure | Cognitive Impairment | Multivariate Adjusted Regression Analyses | ||||
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| n | % | n | % | Adjusted RR a (95% CI) | p-Value | ||
| No | No | 3884 | 80.9 | 919 | 19.1 | 1.00 | |
| Yes | 653 | 81.5 | 148 | 18.5 | 1.24 (1.06–1.46) | 0.008 | |
| Yes | No | 997 | 85.7 | 167 | 14.3 | 1.00 | |
| Yes | 166 | 86.5 | 26 | 13.5 | 1.11 (0.71–1.92) | 0.610 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, F.; Li, T.; Lin, J.; Li, F.; Zhai, Y.; Zhang, T.; Gu, X.; Zhao, G. Passive Smoking Exposure in Living Environments Reduces Cognitive Function: A Prospective Cohort Study in Older Adults. Int. J. Environ. Res. Public Health 2020, 17, 1402. https://doi.org/10.3390/ijerph17041402
He F, Li T, Lin J, Li F, Zhai Y, Zhang T, Gu X, Zhao G. Passive Smoking Exposure in Living Environments Reduces Cognitive Function: A Prospective Cohort Study in Older Adults. International Journal of Environmental Research and Public Health. 2020; 17(4):1402. https://doi.org/10.3390/ijerph17041402
Chicago/Turabian StyleHe, Fan, Tian Li, Junfen Lin, Fudong Li, Yujia Zhai, Tao Zhang, Xue Gu, and Genming Zhao. 2020. "Passive Smoking Exposure in Living Environments Reduces Cognitive Function: A Prospective Cohort Study in Older Adults" International Journal of Environmental Research and Public Health 17, no. 4: 1402. https://doi.org/10.3390/ijerph17041402
APA StyleHe, F., Li, T., Lin, J., Li, F., Zhai, Y., Zhang, T., Gu, X., & Zhao, G. (2020). Passive Smoking Exposure in Living Environments Reduces Cognitive Function: A Prospective Cohort Study in Older Adults. International Journal of Environmental Research and Public Health, 17(4), 1402. https://doi.org/10.3390/ijerph17041402





