Outcome Impacts Due to Pathogen-Specific Antimicrobial Resistance: A Narrative Review of Published Literature
Abstract
1. Introduction
2. Materials and Methods
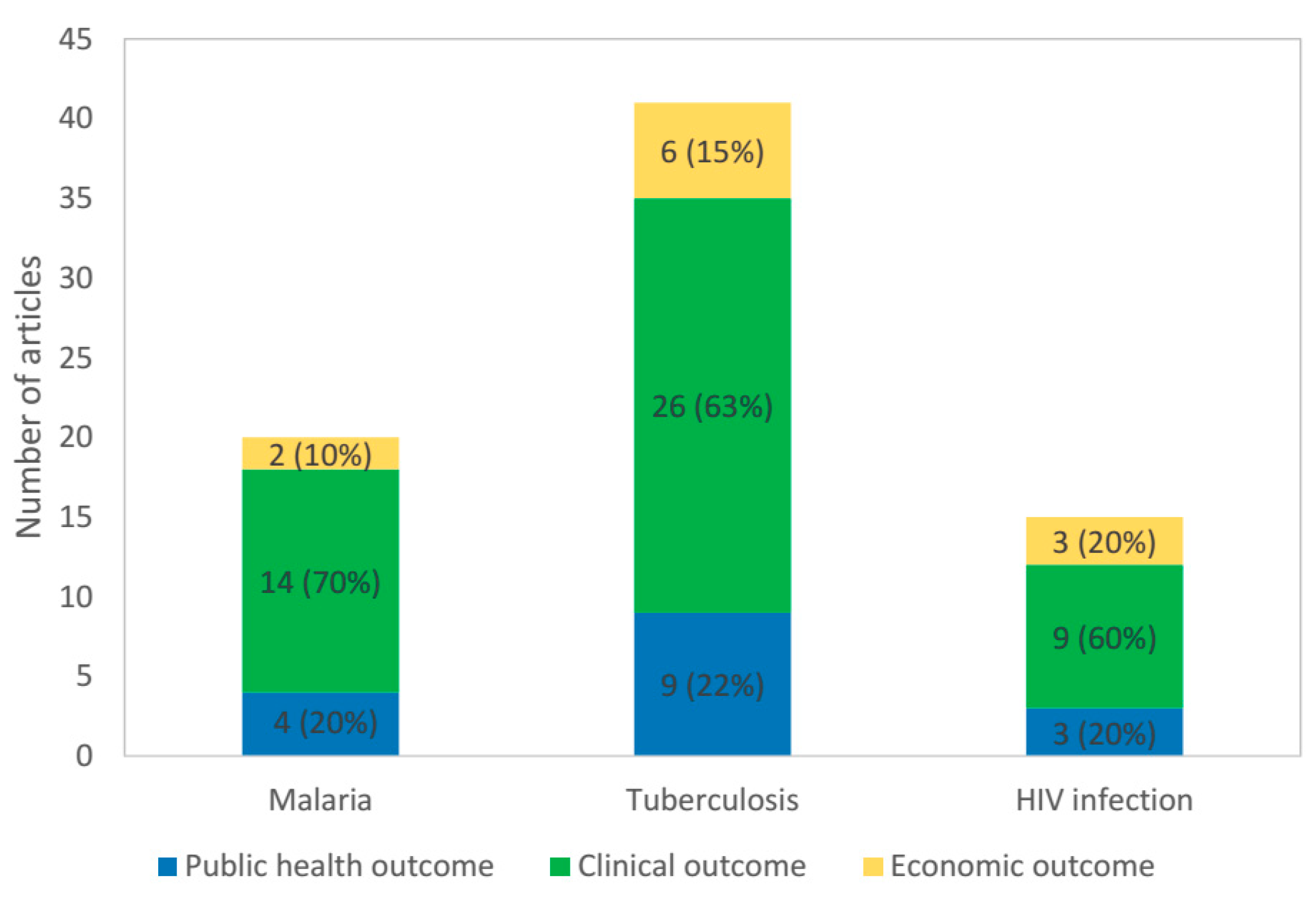
3. Results
3.1. Public Health Outcomes
3.1.1. Malaria
3.1.2. Tuberculosis
3.1.3. HIV
3.2. Clinical Outcomes
3.2.1. Malaria
3.2.2. Tuberculosis
3.2.3. HIV
3.3. Economic Outcomes
3.3.1. Malaria
3.3.2. Tuberculosis
3.3.3. HIV
3.4. Summary of Evidence
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- WHO. Antimicrobial Resistance Fact Sheet. Available online: http://www.who.int/mediacentre/factsheets/fs194/en/ (accessed on 6 May 2019).
- Mendelson, M.; Matsoso, M.P. The World Health Organization Global Action Plan for antimicrobial resistance. S. Afr. Med. J. 2015, 105, 325. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- WHO. Global Tuberculosis Report 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- WHO. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Olatunde, A. Chloroquine-resistant Plasmodium falciparum and malaria in Africa. Trans. R. Soc. Trop. Med. Hyg. 1977, 71, 80–81. [Google Scholar] [CrossRef]
- Bjorkman, A.; Phillips-Howard, P.A. The epidemiology of drug-resistant malaria. Trans. R. Soc. Trop. Med. Hyg. 1990, 84, 177–180. [Google Scholar] [CrossRef][Green Version]
- Amaratunga, C.; Lim, P.; Suon, S.; Sreng, S.; Mao, S.; Sopha, C.; Sam, B.; Dek, D.; Try, V.; Amato, R.; et al. Dihydroartemisinin-piperaquine resistance in Plasmodium falciparum malaria in Cambodia: A multisite prospective cohort study. Lancet Infect. Dis. 2016, 16, 357–365. [Google Scholar] [CrossRef]
- Youmans, G.P.; Williston, E.H. Increase in resistance of tubercle bacilli to streptomycin; a preliminary report. Proc. Staff Meet. Mayo Clin. 1946, 21, 126. [Google Scholar]
- Pablos-Mendez, A.; Raviglione, M.C.; Laszlo, A.; Binkin, N.; Rieder, H.L.; Bustreo, F.; Cohn, D.L.; Lambregts-van Weezenbeek, C.S.; Kim, S.J.; Chaulet, P.; et al. Global surveillance for antituberculosis-drug resistance, 1994-1997. World Health Organization-International Union against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. N. Engl. J. Med. 1998, 338, 1641–1649. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs—Worldwide, 2000–2004. MMWR Morb. Mortal. Wkly. Rep. 2006, 55, 301–305. [Google Scholar]
- Larder, B.A.; Darby, G.; Richman, D.D. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science 1989, 243, 1731–1734. [Google Scholar] [CrossRef]
- Thomas, B.H.; Ciliska, D.; Dobbins, M.; Micucci, S. A Process for systematically reviewing the literature: Providing the research evidence for public health nursing interventions. Worldviews Evid. Based Nurs. 2004, 1, 176–184. [Google Scholar] [CrossRef]
- Warsame, M.; Wernsdorfer, W.H.; Huldt, G.; Bjorkman, A. An epidemic of Plasmodium falciparum malaria in Balcad, Somalia, and its causation. Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 142–145. [Google Scholar] [CrossRef]
- Sharma, Y.D.; Biswas, S.; Pillai, C.R.; Ansari, M.A.; Adak, T.; Devi, C.U. High prevalence of chloroquine resistant Plasmodium falciparum infection in Rajasthan epidemic. Acta Trop. 1996, 62, 135–141. [Google Scholar] [CrossRef]
- Knight, S.E.; Anyachebelu, E.J.; Geddes, R.; Maharaj, R. Impact of delayed introduction of sulfadoxine-pyrimethamine and arthemeter-lumefantrine on malaria epidemiology in KwaZulu-Natal, South Africa. Trop. Med. Int. Health 2009, 14, 1086–1092. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Slater, H.C.; Griffin, J.T.; Ghani, A.C.; Okell, L.C. Assessing the potential impact of artemisinin and partner drug resistance in sub-Saharan Africa. Malar. J. 2016, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Reves, R.; Blakey, D.; Snider, D.E., Jr.; Farer, L.S. Transmission of multiple drug-resistant tuberculosis: Report of a school and community outbreak. Am. J. Epidemiol. 1981, 113, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Ussery, X.T.; Bierman, J.A.; Valway, S.E.; Seitz, T.A.; DiFerdinando, G.T., Jr.; Ostroff, S.M. Transmission of multidrug-resistant Mycobacterium tuberculosis among persons exposed in a medical examiner’s office, New York. Infect. Control Hosp. Epidemiol. 1995, 16, 160–165. [Google Scholar] [CrossRef]
- Coronado, V.G.; Beck-Sague, C.M.; Hutton, M.D.; Davis, B.J.; Nicholas, P.; Villareal, C.; Woodley, C.L.; Kilburn, J.O.; Crawford, J.T.; Frieden, T.R.; et al. Transmission of multidrug-resistant Mycobacterium tuberculosis among persons with human immunodeficiency virus infection in an urban hospital: Epidemiologic and restriction fragment length polymorphism analysis. J. Infect. Dis. 1993, 168, 1052–1055. [Google Scholar] [CrossRef]
- Friedman, C.R.; Stoeckle, M.Y.; Kreiswirth, B.N.; Johnson, W.D., Jr.; Manoach, S.M.; Berger, J.; Sathianathan, K.; Hafner, A.; Riley, L.W. Transmission of multidrug-resistant tuberculosis in a large urban setting. Am. J. Respir. Crit. Care Med. 1995, 152, 355–359. [Google Scholar] [CrossRef]
- Centers for Disease Control (CDC). Nosocomial transmission of multidrug-resistant tuberculosis among HIV-infected persons—Florida and New York, 1988–1991. MMWR Morb. Mortal. Wkly. Rep. 1991, 40, 585–591. [Google Scholar]
- Nivin, B.; Nicholas, P.; Gayer, M.; Frieden, T.R.; Fujiwara, P.I. A continuing outbreak of multidrug-resistant tuberculosis, with transmission in a hospital nursery. Clin. Infect. Dis. 1998, 26, 303–307. [Google Scholar] [CrossRef]
- Powell, K.M.; VanderEnde, D.S.; Holland, D.P.; Haddad, M.B.; Yarn, B.; Yamin, A.S.; Mohamed, O.; Sales, R.F.; DiMiceli, L.E.; Burns-Grant, G.; et al. Outbreak of Drug-Resistant Mycobacterium tuberculosis Among Homeless People in Atlanta, Georgia, 2008–2015. Public Health Rep. 2017, 132, 231–240. [Google Scholar] [CrossRef]
- Mehra, M.; Cossrow, N.; Kambili, C.; Underwood, R.; Makkar, R.; Potluri, R. Assessment of tuberculosis burden in China using a dynamic disease simulation model. Int. J. Tuberc. Lung Dis. 2013, 17, 1186–1194. [Google Scholar] [CrossRef]
- Sharma, A.; Hill, A.; Kurbatova, E.; van der Walt, M.; Kvasnovsky, C.; Tupasi, T.E.; Caoili, J.C.; Gler, M.T.; Volchenkov, G.V.; Kazennyy, B.Y.; et al. Estimating the future burden of multidrug-resistant and extensively drug-resistant tuberculosis in India, the Philippines, Russia, and South Africa: A mathematical modelling study. Lancet Infect. Dis. 2017, 17, 707–715. [Google Scholar] [CrossRef]
- Blower, S.M.; Aschenbach, A.N.; Gershengorn, H.B.; Kahn, J.O. Predicting the unpredictable: Transmission of drug-resistant HIV. Nat. Med. 2001, 7, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J.; Okano, J.T.; Kahn, J.S.; Bodine, E.N.; Blower, S. Evolutionary dynamics of complex networks of HIV drug-resistant strains: The case of San Francisco. Science 2010, 327, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.N.; Stover, J.; Cambiano, V.; Nakagawa, F.; Jordan, M.R.; Pillay, D.; Doherty, M.; Revill, P.; Bertagnolio, S. Impact of HIV Drug Resistance on HIV/AIDS-Associated Mortality, New Infections, and Antiretroviral Therapy Program Costs in Sub-Saharan Africa. J. Infect. Dis. 2017, 215, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Nsanzabana, C.; Hastings, I.M.; Marfurt, J.; Müller, I.; Baea, K.; Rare, L.; Schapira, A.; Felger, I.; Betschart, B.; Smith, T.A.; et al. Quantifying the evolution and impact of antimalarial drug resistance: Drug use, spread of resistance, and drug failure over a 12-year period in Papua New Guinea. J. Infect. Dis. 2010, 201, 435–443. [Google Scholar] [CrossRef]
- Khoromana, C.O.; Campbell, C.C.; Wirima, J.J.; Heymann, D.L. In vivo efficacy of chloroquine treatment for Plasmodium falciparum in Malawian children under five years of age. Am. J. Trop. Med. Hyg. 1986, 35, 465–471. [Google Scholar] [CrossRef]
- Greenberg, A.E.; Ntumbanzondo, M.; Ntula, N.; Mawa, L.; Howell, J.; Davachi, F. Hospital-based surveillance of malaria-related paediatric morbidity and mortality in Kinshasa, Zaire. Bull. World Health Organ. 1989, 67, 189–196. [Google Scholar]
- Carme, B.; Yombi, B.; Bouquety, J.C.; Plassard, H.; Nzingoula, S.; Senga, J.; Akani, I. Child morbidity and mortality due to cerebral malaria in Brazzaville, Congo. A retrospective and prospective hospital-based study 1983–1989. Trop. Med. Parasitol. 1992, 43, 173–176. [Google Scholar]
- Asindi, A.A.; Ekanem, E.E.; Ibia, E.O.; Nwangwa, M.A. Upsurge of malaria-related convulsions in a paediatric emergency room in Nigeria. Consequence of emergence of chloroquine-resistant Plasmodium falciparum. Trop. Geogr. Med. 1993, 45, 110–113. [Google Scholar] [PubMed]
- Zucker, J.R.; Lackritz, E.M.; Ruebush, T.K., II; Hightower, A.W.; Adungosi, J.E.; Were, J.B.; Metchock, B.; Patrick, E.; Campbell, C.C. Childhood mortality during and after hospitalization in western Kenya: Effect of malaria treatment regimens. Am. J. Trop. Med. Hyg. 1996, 55, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Shanks, G.D.; Biomndo, K.; Hay, S.I.; Snow, R.W. Changing patterns of clinical malaria since 1965 among a tea estate population located in the Kenyan highlands. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 253–255. [Google Scholar] [CrossRef]
- Zucker, J.R.; Ruebush, T.K., 2nd; Obonyo, C.; Otieno, J.; Campbell, C.C. The mortality consequences of the continued use of chloroquine in Africa: Experience in Siaya, western Kenya. Am. J. Trop. Med. Hyg. 2003, 68, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Brewster, D.R.; Greenwood, B.M. Seasonal variation of paediatric diseases in The Gambia, west Africa. Ann. Trop. Paediatr. 1993, 13, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Poespoprodjo, J.R.; Fobia, W.; Kenangalem, E.; Lampah, D.A.; Sugiarto, P.; Tjitra, E.; Anstey, N.M.; Price, R.N. Treatment policy change to dihydroartemisinin-piperaquine contributes to the reduction of adverse maternal and pregnancy outcomes. Malar. J. 2015, 14, 272. [Google Scholar] [CrossRef]
- Leang, R.; Taylor, W.R.; Bouth, D.M.; Song, L.; Tarning, J. Evidence of Plasmodium falciparum Malaria Multidrug Resistance to Artemisinin and Piperaquine in Western Cambodia: Dihydroartemisinin-Piperaquine Open-Label Multicenter Clinical Assessment. Antimicrob. Agents Chemother. 2015, 59, 4719–4726. [Google Scholar] [CrossRef]
- Leang, R.; Barrette, A.; Bouth, D.M.; Menard, D.; Abdur, R.; Duong, S.; Ringwald, P. Efficacy of dihydroartemisinin-piperaquine for treatment of uncomplicated Plasmodium falciparum and Plasmodium vivax in Cambodia, 2008 to 2010. Antimicrob. Agents Chemother. 2013, 57, 818–826. [Google Scholar] [CrossRef]
- Winter, K.; Hastings, I.M. Development, evaluation, and application of an in silico model for antimalarial drug treatment and failure. Antimicrob. Agents Chemother. 2011, 55, 3380–3392. [Google Scholar] [CrossRef]
- Espinal, M.A.; Kim, S.J.; Suarez, P.G.; Kam, K.M.; Khomenko, A.G.; Migliori, G.B.; Baéz, J.; Kochi, A.; Dye, C.; Raviglione, M.C. Standard short-course chemotherapy for drug-resistant tuberculosis: Treatment outcomes in 6 countries. JAMA 2000, 283, 2537–2545. [Google Scholar] [CrossRef]
- Singla, R.; Al-Sharif, N.; Al-Sayegh, M.O.; Osman, M.M.; Shaikh, M.A. Influence of anti-tuberculosis drug resistance on the treatment outcome of pulmonary tuberculosis patients receiving DOTS in Riyadh, Saudi Arabia. Int. J. Tuberc. Lung Dis. 2002, 6, 585–591. [Google Scholar] [PubMed]
- Samman, Y.; Krayem, A.; Haidar, M.; Mimesh, S.; Osoba, A.; Al-Mowaallad, A.; Abdelaziz, M.; Wali, S. Treatment outcome of tuberculosis among Saudi nationals: Role of drug resistance and compliance. Clin. Microbiol. Infect. 2003, 9, 289–294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anuwatnonthakate, A.; Whitehead, S.J.; Varma, J.K.; Silachamroon, U.; Kasetjaroen, Y.; Moolphate, S.; Limsomboon, P.; Inyaphong, J.; Suriyon, N.; Kavinum, S.; et al. Effect of mycobacterial drug resistance patterns on patients’ survival: A cohort study in Thailand. Glob. J. Health Sci. 2013, 5, 60–72. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deepa, D.; Achanta, S.; Jaju, J.; Rao, K.; Samyukta, R.; Claassens, M.; Kumar, A.M.; Ph, V. The impact of isoniazid resistance on the treatment outcomes of smear positive re-treatment tuberculosis patients in the state of Andhra Pradesh, India. PLoS ONE 2013, 8, e76189. [Google Scholar] [CrossRef] [PubMed]
- Baez-Saldana, R.; Delgado-Sanchez, G.; Garcia-Garcia, L.; Cruz-Hervert, L.P.; Montesinos-Castillo, M.; Ferreyra-Reyes, L.; Bobadilla-Del-Valle, M.; Canizales-Quintero, S.; Ferreira-Guerrero, E.; Téllez-Vázquez, N.; et al. Isoniazid Mono-Resistant Tuberculosis: Impact on Treatment Outcome and Survival of Pulmonary Tuberculosis Patients in Southern Mexico 1995–2010. PLoS ONE 2016, 11, e0168955. [Google Scholar] [CrossRef]
- Nagu, T.J.; Aboud, S.; Matee, M.I.; Maeurer, M.J.; Fawzi, W.W.; Mugusi, F. Effects of isoniazid resistance on TB treatment outcomes under programmatic conditions in a high-TB and -HIV setting: A prospective multicentre study. J. Antimicrob. Chemother. 2017, 72, 876–881. [Google Scholar] [CrossRef][Green Version]
- Karo, B.; Kohlenberg, A.; Hollo, V.; Duarte, R.; Fiebig, L.; Jackson, S.; Kearns, C.; Ködmön, C.; Korzeniewska-Kosela, M.; Papaventsis, D.; et al. Isoniazid (INH) mono-resistance and tuberculosis (TB) treatment success: Analysis of European surveillance data, 2002 to 2014. Eur. Surveill. 2019, 24, 1800392. [Google Scholar] [CrossRef]
- Garcia-Garcia, M.L.; Ponce de Leon, A.; Jimenez-Corona, M.E.; Jiménez-Corona, A.; Palacios-Martínez, M.; Balandrano-Campos, S.; Ferreyra-Reyes, L.; Juárez-Sandino, L.; Sifuentes-Osornio, J.; Olivera-Díaz, H.; et al. Clinical consequences and transmissibility of drug-resistant tuberculosis in southern Mexico. Arch. Intern. Med. 2000, 160, 630–636. [Google Scholar] [CrossRef]
- Garcia-Garcia, M.L.; Jimenez-Corona, M.E.; Ponce-de-Leon, A.; Jiménez-Corona, A.; Palacios-Martínez, M.; Balandrano-Campos, S.; Ferreyra-Reyes, L.; Juárez-Sandino, L.; Sifuentes-Osornio, J.; Olivera-Díaz, H.; et al. Mycobacterium tuberculosis drug resistance in a suburban community in southern Mexico. Int. J. Tuberc. Lung Dis. 2000, 4, S168–S170. [Google Scholar]
- Garcia-Garcia, M.L.; Sifuentes-Osornio, J.; Jimenez-Corona, M.E.; Ponce-de-León, A.; Jiménez-Corona, A.; Bobadilla-del Valle, M.; Palacios-Martínez, M.; Canales, G.; Sanginés, A.; Jaramillo, Y.; et al. Drug resistance of Mycobacterium tuberculosis in Orizaba, Veracruz. Implications for the tuberculosis prevention and control program. Rev. Investig. Clin. 2001, 53, 315–323. [Google Scholar]
- Noeske, J.; Nguenko, P.N. Impact of resistance to anti-tuberculosis drugs on treatment outcome using World Health Organization standard regimens. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 429–433. [Google Scholar] [CrossRef]
- Toungoussova, O.S.; Nizovtseva, N.I.; Mariandyshev, A.O.; Caugant, D.A.; Sandven, P.; Bjune, G. Impact of drug-resistant Mycobacterium tuberculosis on treatment outcome of culture-positive cases of tuberculosis in the Archangel oblast, Russia, in 1999. Eur. J. Clin. Microbiol. Infect Dis. 2004, 23, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Ohkado, A.; Aguiman, L.; Adlawan, S.; Baring, E.; Quirante, O.; Suchi, M.; Endo, S.; Fujiki, A.; Mori, T. Tuberculosis drug resistance and treatment outcomes under DOTS settings in large cities in the Philippines. Int. J. Tuberc. Lung Dis. 2006, 10, 283–289. [Google Scholar] [PubMed]
- Cox, H.; Kebede, Y.; Allamuratova, S.; Ismailov, G.; Davletmuratova, Z.; Byrnes, G.; Stone, C.; Niemann, S.; Rüsch-Gerdes, S.; Blok, L.; et al. Tuberculosis recurrence and mortality after successful treatment: Impact of drug resistance. PLoS Med. 2006, 3, e384. [Google Scholar] [CrossRef]
- Matos, E.D.; Lemos, A.C.; Bittencourt, C.; Mesquita, C.L. Anti-tuberculosis drug resistance in strains of Mycobacterium tuberculosis isolated from patients in a tertiary hospital in Bahia. Br. J. Infect. Dis. 2007, 11, 331–338. [Google Scholar] [CrossRef][Green Version]
- Seddon, J.A.; Visser, D.H.; Bartens, M.; Jordaan, A.M.; Victor, T.C.; van Furth, A.M.; Schoeman, J.F.; Schaaf, H.S. Impact of drug resistance on clinical outcome in children with tuberculous meningitis. Pediatr. Infect. Dis. J. 2012, 31, 711–716. [Google Scholar] [CrossRef]
- Sun, Y.; Harley, D.; Vally, H.; Sleigh, A. Comparison of characteristics and mortality in multidrug resistant (MDR) and non-MDR tuberculosis patients in China. BMC Public Health 2015, 15, 1027. [Google Scholar] [CrossRef]
- Sun, Y.; Harley, D.; Vally, H.; Sleigh, A. Impact of Multidrug Resistance on Tuberculosis Recurrence and Long-Term Outcome in China. PLoS ONE 2017, 12, e0168865. [Google Scholar] [CrossRef]
- Lockman, S.; Kruuner, A.; Binkin, N.; Levina, K.; Wang, Y.; Danilovitsh, M.; Hoffner, S.; Tappero, J. Clinical outcomes of Estonian patients with primary multidrug-resistant versus drug-susceptible tuberculosis. Clin. Infect. Dis. 2001, 32, 373–380. [Google Scholar] [CrossRef][Green Version]
- Quy, H.T.; Cobelens, F.G.; Lan, N.T.; Buu, T.N.; Lambregts, C.S.; Borgdorff, M.W. Treatment outcomes by drug resistance and HIV status among tuberculosis patients in Ho Chi Minh City, Vietnam. Int. J. Tuberc. Lung Dis. 2006, 10, 45–51. [Google Scholar]
- Pradipta, I.S.; Van’t Boveneind-Vrubleuskaya, N.; Akkerman, O.W.; Alffenaar, J.C.; Hak, E. Treatment outcomes of drug-resistant tuberculosis in the Netherlands, 2005–2015. Antimicrob. Resist. Infect. Control 2019, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Eyob, G.; Guebrexabher, H.; Lemma, E.; Wolday, D.; Gebeyehu, M.; Abate, G.; Rigouts, L.; van Soolingen, D.; Fontanet, A.; Sanders, E.; et al. Drug susceptibility of Mycobacterium tuberculosis in HIV-infected and -uninfected Ethiopians and its impact on outcome after 24 months of follow-up. Int. J. Tuberc. Lung Dis. 2004, 8, 1388–1391. [Google Scholar] [PubMed]
- Sungkanuparph, S.; Eampokalap, B.; Chottanapund, S.; Thongyen, S.; Manosuthi, W. Impact of drug-resistant tuberculosis on the survival of HIV-infected patients. Int. J. Tuberc. Lung Dis. 2007, 11, 325–330. [Google Scholar]
- Mak, A.; Thomas, A.; Del Granado, M.; Zaleskis, R.; Mouzafarova, N.; Menzies, D. Influence of multidrug resistance on tuberculosis treatment outcomes with standardized regimens. Am. J. Respir. Crit. Care Med. 2008, 178, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Falzon, D.; Gandhi, N.; Migliori, G.B.; Sotgiu, G.; Cox, H.S.; Holtz, T.H.; Hollm-Delgado, M.G.; Keshavjee, S.; DeRiemer, K.; Centis, R.; et al. Resistance to fluoroquinolones and second-line injectable drugs: Impact on multidrug-resistant TB outcomes. Eur. Respir. J. 2013, 42, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Harrigan, P.R.; Hertogs, K.; Verbiest, W.; Pauwels, R.; Larder, B.; Kemp, S.; Bloor, S.; Yip, B.; Hogg, R.; Alexander, C.; et al. Baseline HIV drug resistance profile predicts response to ritonavir-saquinavir protease inhibitor therapy in a community setting. Aids 1999, 13, 1863–1871. [Google Scholar] [CrossRef][Green Version]
- Kuritzkes, D.R.; Lalama, C.M.; Ribaudo, H.J.; Marcial, M.; Meyer, M.A., 3rd; Shikuma, C.; Johnson, V.A.; Fiscus, S.A.; D’Aquila, R.T.; Schackman, B.R.; et al. Preexisting resistance to nonnucleoside reverse-transcriptase inhibitors predicts virologic failure of an efavirenz-based regimen in treatment-naive HIV-1-infected subjects. J. Infect. Dis. 2008, 197, 867–870. [Google Scholar] [CrossRef]
- Taniguchi, T.; Nurutdinova, D.; Grubb, J.R.; Önen, N.F.; Shacham, E.; Donovan, M.; Overton, E.T. Transmitted drug-resistant HIV type 1 remains prevalent and impacts virologic outcomes despite genotype-guided antiretroviral therapy. AIDS Res. Hum. Retroviruses 2012, 28, 259–264. [Google Scholar] [CrossRef]
- Simen, B.B.; Simons, J.F.; Hullsiek, K.H.; Novak, R.M.; Macarthur, R.D.; Baxter, J.D.; Huang, C.; Lubeski, C.; Turenchalk, G.S.; Braverman, M.S.; et al. Low-abundance drug-resistant viral variants in chronically HIV-infected, antiretroviral treatment-naive patients significantly impact treatment outcomes. J. Infect. Dis. 2009, 199, 693–701. [Google Scholar] [CrossRef]
- Miller, V.; Phillips, A.; Rottmann, C.; Staszewski, S.; Pauwels, R.; Hertogs, K.; de Béthune, M.P.; Kemp, S.D.; Bloor, S.; Harrigan, P.R.; et al. Dual resistance to zidovudine and lamivudine in patients treated with zidovudine-lamivudine combination therapy: Association with therapy failure. J. Infect. Dis. 1998, 177, 1521–1532. [Google Scholar] [CrossRef][Green Version]
- Derdelinckx, I.; Van Laethem, K.; Maes, B.; Schrooten, Y.; De Wit, S.; Florence, E.; Fransen, K.; Ribas, S.G.; Marissens, D.; Moutschen, M.; et al. Current levels of drug resistance among therapy-naive HIV-infected patients have significant impact on treatment response. J. Acquir. Immune Defic. Syndr. 2004, 37, 1664–1666. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lai, C.C.; Hung, C.C.; Chen, M.Y.; Sun, H.Y.; Lu, C.L.; Tseng, Y.T.; Chang, S.F.; Su, Y.C.; Liu, W.C.; Hsieh, C.Y.; et al. Trends of transmitted drug resistance of HIV-1 and its impact on treatment response to first-line antiretroviral therapy in Taiwan. J. Antimicrob. Chemother. 2012, 67, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Zaccarelli, M.; Tozzi, V.; Lorenzini, P.; Trotta, M.P.; Forbici, F.; Visco-Comandini, U.; Gori, C.; Narciso, P.; Perno, C.F.; Antinori, A.; et al. Multiple drug class-wide resistance associated with poorer survival after treatment failure in a cohort of HIV-infected patients. Aids 2005, 19, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Cozzi-Lepri, A.; Phillips, A.N.; Clotet, B.; Mocroft, A.; Ruiz, L.; Kirk, O.; Lazzarin, A.; Wiercinska-Drapalo, A.; Karlsson, A.; Lundgren, J.D.; et al. Detection of HIV drug resistance during antiretroviral treatment and clinical progression in a large European cohort study. Aids 2008, 22, 2187–2198. [Google Scholar] [CrossRef]
- Phillips, M.; Phillips-Howard, P.A. Economic implications of resistance to antimalarial drugs. Pharmacoeconomics 1996, 10, 225–238. [Google Scholar] [CrossRef]
- Lubell, Y.; Dondorp, A.; Guerin, P.J.; Drake, T.; Meek, S.; Ashley, E.; Day, N.P.; White, N.J.; White, L.J. Artemisinin resistance—Modelling the potential human and economic costs. Malar. J. 2014, 13, 452. [Google Scholar] [CrossRef]
- Pooran, A.; Pieterson, E.; Davids, M.; Theron, G.; Dheda, K. What is the cost of diagnosis and management of drug resistant tuberculosis in South Africa? PLoS ONE 2013, 8, e54587. [Google Scholar] [CrossRef]
- Pichenda, K.; Nakamura, K.; Morita, A.; Kizuki, M.; Seino, K.; Takano, T. Non-hospital DOT and early diagnosis of tuberculosis reduce costs while achieving treatment success. Int. J. Tuberc. Lung Dis. 2012, 16, 828–834. [Google Scholar] [CrossRef]
- White, V.L.; Moore-Gillon, J. Resource implications of patients with multidrug resistant tuberculosis. Thora 2000, 55, 962–963. [Google Scholar] [CrossRef]
- Rouzier, V.A.; Oxlade, O.; Verduga, R.; Gresely, L.; Menzies, D. Patient and family costs associated with tuberculosis, including multidrug-resistant tuberculosis, in Ecuador. Int. J. Tuberc. Lung 2010, 14, 1316–1322. [Google Scholar]
- Marks, S.M.; Hirsch-Moverman, Y.; Salcedo, K.; Graviss, E.A.; Oh, P.; Seaworth, B.; Flood, J.; Armstrong, L.; Armitige, L.; Mase, S.; et al. Characteristics and costs of multidrug-resistant tuberculosis in-patient care in the United States, 2005–2007. Int. J. Tuberc. Lung 2016, 20, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Marks, S.M.; Flood, J.; Seaworth, B.; Hirsch-Moverman, Y.; Armstrong, L.; Mase, S.; Salcedo, K.; Oh, P.; Graviss, E.A.; Colson, P.W.; et al. Treatment practices, outcomes, and costs of multidrug-resistant and extensively drug-resistant tuberculosis, United States, 2005–2007. Emerg. Infect. Dis. 2014, 20, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Krentz, H.B.; Ko, K.; Beckthold, B.; Gill, M.J. The cost of antiretroviral drug resistance in HIV-positive patients. Antivir. Ther. 2014, 19, 341–348. [Google Scholar] [CrossRef]
- Martin, S. Incremental medical costs associated with increased changes in HAART regimens in a US patient sample. In Proceedings of the 4th IAS Conference on HIV Pathogenesis, Treatment and Prevention, Sydney, Australia, 22–25 July 2007. [Google Scholar]
- Meenan, R.T. Incidence-based Costs of Multiple HAART Switches Among HIV infected Patients in an HMO. Clin. Med. Res. 2010, 8, 52. [Google Scholar] [CrossRef][Green Version]
- Steketee, R.W.; Nahlen, B.L.; Parise, M.E.; Menendez, C. The burden of malaria in pregnancy in malaria-endemic areas. Am. J. Trop. Med. Hyg. 2001, 64, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.C.; Breman, J.G. Gaps in the childhood malaria burden in Africa: Cerebral malaria, neurological sequelae, anemia, respiratory distress, hypoglycemia, and complications of pregnancy. Am. J. Trop. Med. Hyg. 2001, 64, 57–67. [Google Scholar] [CrossRef]
- Ratcliff, A.; Siswantoro, H.; Kenangalem, E.; Maristela, R.; Wuwung, R.M.; Laihad, F.; Ebsworth, E.P.; Anstey, N.M.; Tjitra, E.; Price, R.N. Two fixed-dose artemisinin combinations for drug-resistant falciparum and vivax malaria in Papua, Indonesia: An open-label randomised comparison. Lancet 2007, 369, 757–765. [Google Scholar] [CrossRef]
- Snedecor, S.J.; Khachatryan, A.; Nedrow, K.; Chambers, R.; Li, C.; Haider, S.; Stephens, J. The prevalence of transmitted resistance to first-generation non-nucleoside reverse transcriptase inhibitors and its potential economic impact in HIV-infected patients. PLoS ONE 2013, 8, e72784. [Google Scholar] [CrossRef]
- Grad, Y.H.; Goldstein, E.; Lipsitch, M.; White, P.J. Improving Control of Antibiotic-Resistant Gonorrhea by Integrating Research Agendas Across Disciplines: Key Questions Arising from Mathematical Modeling. J. Infect. Dis. 2016, 213, 883–890. [Google Scholar] [CrossRef]
- Maragakis, L.L.; Perencevich, E.N.; Cosgrove, S.E. Clinical and economic burden of antimicrobial resistance. Expert Rev. Anti Infect. Ther. 2008, 6, 751–763. [Google Scholar] [CrossRef]
- Gandra, S.; Barter, D.M.; Laxminarayan, R. Economic burden of antibiotic resistance: How much do we really know? Clin. Microbiol. Infect. 2014, 20, 973–980. [Google Scholar] [CrossRef]
- Unemo, M.; Del Rio, C.; Shafer, W.M. Antimicrobial Resistance Expressed by Neisseria gonorrhoeae: A Major Global Public Health Problem in the 21st Century. Microbiol. Spectr. 2016; 4. [Google Scholar]
- Chesson, H.W.; Kirkcaldy, R.D.; Gift, T.L.; Owusu-Edusei, K., Jr.; Weinstock, H.S. Ciprofloxacin resistance and gonorrhea incidence rates in 17 cities, United States, 1991–2006. Emerg. Infect. Dis. 2014, 20, 612–619. [Google Scholar] [CrossRef]
- Chesson, H.W.; Kirkcaldy, R.D.; Gift, T.L.; Owusu-Edusei, K., Jr.; Weinstock, H.S. An Illustration of the Potential Health and Economic Benefits of Combating Antibiotic-Resistant Gonorrhea. Sex Transm. Dis. 2018, 45, 250–253. [Google Scholar] [CrossRef]
- Head, M.G.; Fitchett, J.R.; Cooke, M.K.; Wurie, F.B.; Hayward, A.C.; Atun, R. UK investments in global infectious disease research 1997–2010: A case study. Lancet Infect. Dis. 2013, 13, 55–64. [Google Scholar] [CrossRef]
- Chen, X.S.; Yin, Y.P.; Li, X.Y. A Roadmap Plan to Address Research Needs for Gonococcal Antimicrobial Resistance in China. Clin. Infect. Dis. 2019, 68, 505–510. [Google Scholar] [CrossRef]

| Author by Disease | Study Year | Study Site | Sample Size | Study Design/Method | Outcome | Quality Assessment |
|---|---|---|---|---|---|---|
| Malaria | ||||||
| Warsame et al. [14] | 1982–1988 | Somalia | 109 | Retrospective study | The emergence of chloroquine resistance, accelerated by high drug pressure, low herd immunity and favourable meteorological conditions were identified as major causes of the epidemic of falciparum malaria in Balcad, Somalia. | STRONG |
| Sharma et al. [15] | 1994 | India | 32 | Cross-sectional study | In 1994, there was a falciparum malaria epidemic in Rajasthan, India, with many deaths, and most of the parasite isolates (95%) were resistant to chloroquine. | STRONG |
| Knight et al. [16] | 1982–1988, 1991–2001 | South Africa | 600000 | Ecological study | The relative risk for malaria infection after the level of drug resistance reached 10% was 4.5 (95% CI: 4.0–5.2) in the chloroquine period and 5.9 (95% CI: 5.7–6.1) in the sulfadoxine-pyrimethamine period. | MODERATE |
| Slater et al. [17] | 2016–2020 | Africa | - | An individual-based malaria transmission model | Artemisinin and partner drug resistance at levels similar to those observed in Oddar Meanchey province in Cambodia could result in an additional 78 million cases over a 5 year period, a 7% increase in cases compared to a scenario with no resistance. | WEAK |
| Tuberculosis | ||||||
| Reves et al. [18] | 1976 | USA | 15 | Retrospective investigation | An outbreak of tuberculosis in 1976 was caused by mycobacteria resistant to isoniazid (INH), streptomycin (SM), and para-aminosalicylic acid (PAS). | STRONG |
| Ussery et al. [19] | 1995 | USA | 18 | Cohort study | A trend between TST conversion and participation in autopsies on persons with MDR-TB was observed. | STRONG |
| Coronado et al. [20] | 1990–1991 | USA | 16 | Cross-sectional study | From January 1990 to December 1991, 16 patients with multidrug-resistant tuberculosis (MDR-TB) were caused by nosocomial transmission of MDR-TB resistant to isoniazid, rifampin, and streptomycin. | STRONG |
| Friedman et al. [21] | 1995 | USA | 167 | Cross-sectional study | Forty-three (34%) of 127 drug-susceptible isolates and 19 (79%) of 24 multidrug-resistant isolates had RFLP patterns representing possible recent exogenous infection. | STRONG |
| US CDC. [22] | 1990–1991 | USA | NR | Retrospective study | During 1990 and 1991, outbreaks of multidrug-resistant tuberculosis (MDR-TB) in four hospitals (one in Miami and three in New York City) were due to nosocomial transmission among HIV-infected persons. | STRONG |
| Nivin et al. [23] | 1998 | USA | 24 | Cross-sectional study | Nosocomial transmission appeared to account for an increase in cases of multidrug-resistant tuberculosis (MDRTB) at a large urban facility where a prior nosocomial outbreak of MDRTB had occurred. | STRONG |
| Powell et al. [24] | 2008–2015 | USA | 110 | Retrospective investigation | Of 110 outbreak cases in Georgia, 86 (78%) were culture confirmed and isoniazid resistant. | STRONG |
| Mehra et al. [25] | 2010–2050 | China | - | Dynamic state transition model | In China, by 2050, incidence, prevalence and mortality of MDR-TB will increase by respectively 60%, 48% and 35%, which attributed to inappropriate treatment, leading to high transmission of infection and increased LTBI prevalence. | WEAK |
| Sharma et al. [26] | 2000–2040 | India, Philippines, Russia and South Africa | - | A compartmental model | The model forecasted the percentage of MDR tuberculosis among incident cases of tuberculosis to increase, reaching 12.4% (95% prediction interval 9.4–16.2) in India, 8·9% (4.5–11.7) in the Philippines, 32.5% (27.0–35.8) in Russia, and 5·7% (3.0–7.6) in South Africa in 2040. It also predicted the percentage of XDR tuberculosis among incident MDR tuberculosis to increase, reaching 8·9% (95% prediction interval 5.1–12.9) in India, 9·0% (4.0–14.7) in the Philippines, 9·0% (4.8–14.2) in Russia, and 8·5% (2.5–14.7) in South Africa in 2040. | WEAK |
| HIV | ||||||
| Blower et al. [27] | 1996–2005 | USA | - | A mathematical model | The epidemic of resistance is being generated mainly by the conversion of drug-sensitive cases to drug-resistant cases, and not by the transmission of resistant strains. | WEAK |
| Smith et al. [28] | 2008–2013 | USA | - | A biologically complex multistrain network model | 60% of the currently circulating ARV-resistant strains in San Francisco are capable of causing self-sustaining epidemics, because each individual infected with one of these strains can cause, on average, more than one new resistant infection. | WEAK |
| Phillips et al. [29] | 2016–2030 | Sub-Saharan Africa | - | HIV Synthesis Model | In a situation in which current levels of pretreatment HIVDR are over 10% (mean, 15%), 16% of AIDS deaths (890 000 deaths), 9% of new infections (450 000), and 8% ($6.5 billion) of ART program costs in SSA in 2016–2030 will be attributable to HIVDR. | WEAK |
| Author by Disease | Study Year | Study Site | Sample Size | Study Design/Method | Outcome | Quality Assessment |
|---|---|---|---|---|---|---|
| Malaria | ||||||
| Nsanzabana et al. [30] | 1991–2002 | Papua New Guinea | 6678 | Retrospective study | Treatment failure rates multiplied by 3.5 between 1996 and 2000 but then decreased dramatically after treatment policy change. | STRONG |
| Khoromana et al. [31] | 1978–1983 | Malawi | 224 | Retrospective study | Parasitological failure ranged from 41–65% following administration of chloroquine 25 mg (base)/kg. | MODERATE |
| Greenberg et al. [32] | 1982–1986 | Zaire | 6208 | Retrospective study | The proportional malaria admission rate increased from 29.5% in 1983 to 56.4% in 1986, and the proportional malaria mortality rate, from 4.8% in 1982 to 15.3% in 1986, which were temporally related to the emergence of chloroquine-resistant Plasmodium falciparum malaria in Kinshasa. | STRONG |
| Carme et al. [33] | 1983–1989 | Congo | NR | A retrospective and prospective hospital-based study | The results show a marked increase in hospitalizations for malaria, noticeable since 1985, and which now account for about 50% of the overall non-surgical hospitalizations. | STRONG |
| Asindi et al. [34] | 1986–1988 | Nigeria | 134 | Retrospective study | Malaria was the dominant cause (73%) of febrile convulsion (FC); 81% of these cases did not respond to chloroquine. | STRONG |
| Zucker et al. [35] | 1991 | Kenya | 1223 | Prospective study | Treatment for malaria with chloroquine was associated with a 33% case fatality rate compared with 11% for children treated with more effective regimens (pyrimethamine/sulfa, quinine, or trimethoprim/sulfamethoxazole for five days). | STRONG |
| Shanks et al. [36] | 1980–1997 | Kenya | 10169 | Retrospective study | The dramatic increases in the numbers of malaria admissions (6.5 to 32.5% of all admissions), case fatality (1.3 to 6%) and patients originating from low-risk, highland areas (34 to 59%) were probably due to chloroquine resistance during the late 1980s in the subregion. | STRONG |
| Zucker et al. [37] | 1991–1994 | western Kenya | 1223 | Prospective study | The trend in case-fatality rates for malaria decreased as an increasing proportion of children received an effective treatment regimen; adjusted malaria case-fatality rates were 5.1%, 3.6%, and 3.3% in 1992, 1993, and 1994, respectively, when 85% of children in 1992 and 97% of children in 1993–1994 received effective therapy. | STRONG |
| Brewster et al. [38] | 1988–1990 | Gambia | 9584 | Prospective study | With the emergence of chloroquine-resistant malaria over the 3 years, there was a 27% annual increase in severe anaemia owing to malaria. | STRONG |
| Poespoprodjo et al. [39] | 2004–2010 | Indonesia | 7744 | Prospective cohort study | In those with history of malaria during pregnancy, the increasing use of DHP was associated with a 54% fall in the proportion of maternal malaria at delivery and a 98% decrease in congenital malaria (from 7.1% prior to 0.1% after policy change). | STRONG |
| Amaratunga et al. [8] | 2012–2013 | Cambodia | 241 | Prospective cohort study | In Pursat, where artemisinin resistance is entrenched, 37 (46%) of 81 patients had parasite recrudescence. In Preah Vihear, where artemisinin resistance is emerging, ten (16%) of 63 patients had recrudescence and in Ratanakiri, where artemisinin resistance is rare, one (2%) of 60 patients did. | STRONG |
| Leang et al. [40] | 2011–2013 | Cambodia | 425 | A prospective multicenter open-label study | The most significant risk factor associated with DHA-PP treatment failure was infection by parasites carrying the K13 mutant allele (odds ratio [OR], 17.5; 95% confidence interval [CI], 1 to 308; p = 0.04). | STRONG |
| Leang et al. [41] | 2008–2011 | Cambodia | 438 | Prospective cohort study | In 2010, the PCR-corrected treatment failure rates for DP on day 42 were 25% (95% confidence interval [CI] = 10 to 51%) in Pailin and 10.7% (95% CI = 4 to 23%) in Pursat, while the therapeutic efficacy of DP remained high (100%) in Ratanakiri and Preah Vihear provinces, located in northern and eastern Cambodia. | STRONG |
| Winter et al. [42] | NR | NR | - | In Silico Model for Antimalarial Drug Treatment and Failure | The development of artemisinin tolerance and resistance will, unless checked, have an immediate, large impact on the protection afforded to its partner drug and on the likely clinical efficacy of artemisinin combination therapies. | WEAK |
| Tuberculosis | ||||||
| Espinal et al. [43] | 1994–1996 | Dominican Republic, Hong Kong Special Administrative Region, Italy, Ivanovo Oblast, the Republic of Korea, and Peru | 6402 | Retrospective cohort study | Treatment failure (relative risk [RR], 15.4; 95% confidence interval [CI], 10.6–22.4; p < 0.001) and mortality (RR, 3.73; 95% CI, 2.13–6.53; p < 0.001) were higher among new multidrug-resistant TB cases than among new susceptible cases. | STRONG |
| Singla et al. [44] | 1998–1999 | Saudi Arabia | 515 | Retrospective cohort study | Sputum smear conversion rates at the end of 3 months of treatment in patients with any rifampicin resistance or with multidrug resistance were inferior to those of patients with sensitive strains (89.8% vs. 96.3%, p = 0.016 and 80% vs. 96.3%, p = 0.008, respectively). | MODERATE |
| Samman et al. [45] | 1993–1999 | Saudi Arabia | 147 | Retrospective cohort study | The prevalence of poor compliance and multiply drug-resistant Mycobacterium tuberculosis were found to be significantly higher among those with treatment failure than among those in whom treatment was successful. | STRONG |
| Anuwatnonthakate et al. [46] | 2004–2008 | Thailand | 9736 | Retrospective cohort study | Cox regression analysis showed a significantly higher risk of death among patients with rifampicin resistance (adjusted hazard ratio (aHR) 1.9, 95% confident interval (CI), 1.5–2.5) and isoniazid monoresistance (aHR 1.4, 95% CI 1.1–1.7) than those with pan-susceptible group. | STRONG |
| Deepa et al. [47] | 2011 | India | 1947 | Retrospective record review | Of 144 INH resistant cases, 64 (44%) had poor treatment outcomes (25 (17%) default, 22 (15%) death, 12 (8%) failure and 5 (3%) transfer out) as compared to 287 (31%) among INH sensitive cases [aRR 1.46; 95% CI (1.19–1.78)]. | STRONG |
| Báez-Saldaña et al. [48] | 1995–2010 | Southern Mexico | 1243 | Prospective cohort study | IMR patients had a higher probability of failure (adjusted hazard ratio (HR) 12.35, 95% CI 3.38–45.15) and death due to TB among HIV negative patients (aHR 3.30. 95% CI 1.00–10.84). | STRONG |
| Nagu et al. [49] | 2010–2011 | Tanzania | 1365 | A multicentre, prospective observational study | Isoniazid resistance [relative risk (RR) = 6.0; 95% CI = 1.9–18.7; p < 0.01] was an independent predictor of poor treatment outcomes. | STRONG |
| Karo B et al. [50] | 2002–2014 | European Union/European Economic Area | 194948 | Retrospective cohort study | Treatment success was lower among INH mono-resistant cases (Odds ratio (OR): 0.7; 95% confidence interval (CI): 0.6–0.9; adjusted absolute difference in treatment success: 5.3%). | STRONG |
| García-García et al. [51] | 1995–1998 | Southern Mexico | 2525 | Prospective cohort study | Patients with multi–drug-resistant TB had a significantly poorer prognosis than patients with fully susceptible strains or with other resistant strains (p = 0.03). | STRONG |
| García-García et al. [52] | 1995–1999 | Mexico | 387 | Prospective cohort study | Cox-adjusted relative risks showed that MDR (RR 2.5, 95%CI 1.02–6.16, p = 0.04)was associated with mortality, controlling for age. | STRONG |
| García-García et al. [53] | 1995–1999 | Southern Mexico | 371 | Prospective cohort study | Patients with drug resistance had a higher probability of treatment failure (OR = 16.9, CI 95% 4.5–63.0) and patients with MDR strains had a higher probability of need of re-treatment (RR = 24.4, CI 95% 8.8–67.6), and of death (RR = 4.0, CI 95% 1.5–10.7). | STRONG |
| Noeske et al. [54] | 1997–1998 | Cameroon | 560 | Retrospective cohort study | 332 of the 410 patients (81%) with DS-TB were cured, compared to 109/150 (72.7%) patients with DR-TB (odds ratio [OR] = 0.62, 95% confidence interval [CI] 0.40–0.99). Seven patients (1.7%) failed treatment in the DS-TB group vs. 9 (6.0%) in the DR-TB group (OR = 3.67, 95% CI 1.23–11.18). No significant difference was found in rates of death, default or transfer. | STRONG |
| Toungoussova et al. [55] | 1999 | Russia | 235 | Retrospective cohort study | The high rates of death (16.7%) and failure (66.7%) among patients infected with multidrug-resistant strains illustrate the negative impact of multidrug resistance on the outcome of tuberculosis treatment. Pan-resistance was significantly associated with treatment failure (p < 0.001). | STRONG |
| Ohkado et al. [56] | 2000 | Philippines | 457 | Cross-sectional survey & cohort analysis of treatment | Over 90% of the new cases, either pan-susceptible or mono-resistant, were successfully treated with the standard regimen, but four of nine MDR new cases could not be cured. | STRONG |
| Cox et al. [57] | 2001–2002 | Uzbekistan | 213 | Retrospective observational study | Mortality was high, with an average of 15% (95% confidence interval, 11% to 19%) dying per year after diagnosis (6% of 73 pansusceptible cases and 43% of 55 MDR TB cases also died per year). | STRONG |
| Matos et al. [58] | 2001–2003 | Brazil | 396 | Prospective cohort study | An association was found between resistance and mortality from tuberculosis (adjusted OR: 7.13; 95%CI: 2.25–22.57; p < 0.001). | STRONG |
| Seddon et al. [59] | 2003–2009 | South Africa | 142 | Prospective cohort study | Multidrug-resistant tuberculosis (adjusted odds ratio: 12.4 [95% confidence interval: 1.17–132.3]; p = 0.037) was a risk factor for unfavorable outcome, and multidrug-resistant tuberculosis remained a risk for death (adjusted odds ratio: 63.9 [95% confidence interval: 4.84–843.2]; p = 0.002). | STRONG |
| Sun et al. [60] | 2010 | China | 234 | Cohort study | Nine years after the diagnosis of TB, 69 or 29.5% of the 234 patients had died (32 or 21.6% of non-MDR-TB versus 37 or 43.0% of MDR-TB) and the overall mortality rate was 39/1000 per year (PY) (27/1000 PY among non-MDR versus 63/1000 PY among MDR-TB). | STRONG |
| Sun et al. [61] | 2010 | China | 250 | Cohort study | The mean time for recurrence among MDR-TB patients was 5.7 years, compared to 7.2 years among non-MDR-TB patients. | STRONG |
| Lockman et al. [62] | 1998 | Estonia | 103 | Retrospective observational study | MDR tuberculosis (hazard ratio [HR], 7.8; 95% CI, 1.6–37.4) was associated with death due to tuberculosis in multivariable analysis. | STRONG |
| Quy et al. [63] | 1998–2000 | Vietnam | 2293 | Retrospective cohort study | Failure was associated with multidrug resistance (adjusted odds ratios [aOR] 49.6 and 16.6, respectively) and combined resistance to isoniazid (INH) and streptomycin (SM) (aOR 13.4 and 4.8) | STRONG |
| Pradipta et al. [64] | 2005–2015 | Netherlands | 10303 | Retrospective cohort study | Among all DR-TB cases, patients with Multi Drug-Resistant Tuberculosis (MDR-TB) (OR 4.43; 95% CI 1.70–11.60) were more likely to have unsuccessful treatment. | STRONG |
| Eyob et al. [65] | 1999–2001 | Ethiopia | 490 | Prospective cohort study | Among HIV-infected TB patients who died during follow-up, survival time in those with a resistant Mycobacterium tuberculosis strain was significantly shorter compared to those with a sensitive strain (6 vs. 13 months). | STRONG |
| Sungkanuparph et al. [66] | 1999–2004 | Thailand | 225 | Retrospective cohort study | INH resistance, RMP resistance and MDR-TB were associated with shorter survival (log-rank test, p < 0.005). MDR-TB (hazard ratio [HR] 11.7; 95% CI 2.1–64.9) was significant risk factors for death. | STRONG |
| Mak et al. [67] | 2003 & 2004 | 155 countries | 121 countries | Ecologic study | Among countries using one of two standardized initial regimens, failure rates averaged 5.0%, and relapse rates averaged 12.8% in the 20 countries where prevalence of initial multidrug resistance exceeded 3%, compared with an average of 1.6% (p < 0.0001) and 8.1% (p = 0.0002), respectively, in 83 countries where initial multidrug resistance prevalence was less than 3%. | WEAK |
| Falzon et al. [68] | 1980–2009 | 31 centres | - | Meta-analysis | Compared with treatment failure, relapse and death, treatment success was higher in MDR-TB patients infected with strains without additional resistance (n = 4763; 64%, 95% CI 57–72%) or with resistance to second-line injectable drugs only (n = 1130; 56%, 95% CI 45–66%), than in those having resistance to fluoroquinolones alone (n = 426; 48%, 95% CI 36–60%) or to fluoroquinolones plus second-line injectable drugs (extensively drug resistant (XDR)-TB) (n = 405; 40%, 95% CI 27–53%). | MODERATE |
| HIV | ||||||
| Harrigan et al. [69] | 1996–1997 | Canada | 297 | prospective cohort study | Patients classified as resistant to either drug using either method had median decreases in plasma viral load of 0.05 log10 HIV RNA copies/mL or less, compared to >0.8 log10 for those with sensitive virus. | STRONG |
| Kuritzkes et al. [70] | 2008 | USA | 220 | A case-cohort study | The risk of virologic failure for subjects with baseline NNRTI resistance was higher than that for subjects without such resistance (hazard ratio 2.27 [95% confidence interval], 1.15–4.49; p = 0.018). | STRONG |
| Taniguchi et al. [71] | 2001–2009 | USA | 801 | Retrospective study | In multivariate analysis, nonnucleoside reverse transcriptase inhibitor (NNRTI) resistance was associated with a 1.5-fold increased risk of virologic failure. | STRONG |
| Simen et al. [72] | 2005 | USA | 491 | Cohort study | The risk of VF was higher for those who had an NNRTI-resistance mutation detected by both methods (hazard ratio [HR], 12.40 [95% confidence interval {CI}, 3.41–45.10]) and those who had mutation(s) detected only with ultra-deep sequencing (HR, 2.50 [95% CI, 1.17–5.36]). | STRONG |
| Miller et al. [73] | 1998 | Germany | 43 | Cross-sectional study | After adjustment for all variables, phenotypic resistance to zidovudine remained the only significantly associated factor of therapy response. | STRONG |
| Derdelinckx et al. [74] | 2000 | Belgium | 93 | Retrospective study | In a multivariate logistic model, controlled for log VL and CD4 count at treatment start, the association of transmitted resistance with treatment failure remained significant (OR: 148, 95% CI: 3.34- > 999.9, p = 0.027). | STRONG |
| Lai et al. [75] | 2000–2010 | Taiwan | 1349 | Matched case-control study | Compared with regimens with GSS >2.5, initiation of regimens with GSS ≤2.5 was associated with a higher treatment failure rate (39.3% versus 15.7%, p = 0.02) and shorter time to treatment failure (log-rank p < 0.001). | STRONG |
| Zaccarelli et al. [76] | 1999–2003 | Italy | 623 | Observational, longitudinal cohort study | Kaplan-Meier analyses for end-points at 48 months in patients with no CWR, one CWR, two CWR or three CWR were 8.9, 11.7, 13.4 and 27.1%, respectively, for death; 6.1, 9.9, 13.4 and 21.5%, respectively, for AIDS-related death; and 16.0, 17.7, 19.3 and 35.9%, respectively, for new AIDS event/death. | STRONG |
| Cozzi-Lepri et al. [77] | 2003–2007 | UK | 8229 | Cohort study | By 96 months from baseline, the proportion of patients with a new AIDS diagnosis or death was 20.3% (95% CI:17.7–22.9) in patients with no evidence of virological failure and 53% (39.3–66.7) in those with virological failure and mutations to three drug classes (p = 0.0001) | STRONG |
| Author by Disease | Study Year | Study Site | Sample Size | Study Design/Method | Outcome | Quality Assessment |
|---|---|---|---|---|---|---|
| Malaria | ||||||
| Phillips et al. [78] | 1996 | NR | NR | Simplifying assumptions and illustrative calculations | The use of quinine versus chloroquine as first-line therapy in 150 million patients with malaria would increase spending by as much as $100 million. | WEAK |
| Lubell et al. [79] | 2014 | All malaria-endemic areas | - | Decision tree model | The predicted medical costs for retreatment of clinical failures and for management of severe malaria exceed US$32 million per year. Productivity losses resulting from excess morbidity and mortality were estimated at US$385 million for each year during which failing ACT remained in use as first-line treatment. | WEAK |
| Tuberculosis | ||||||
| Pooran et al. [80] | 2011 | South Africa | - | Cost analysis | Assuming adherence to national DR-TB management guidelines, the per patient cost of XDR-TB was $26,392, four times greater than MDR-TB ($6772), and 103 times greater than drug-sensitive TB ($257). | WEAK |
| Pichenda et al. [81] | 2011 | Cambodia | 277 | Cross-sectional survey | The mean total household cost for TB patients was $477, compared to $1525 in MDR-TB cases. | STRONG |
| White et al. [82] | 1996–1999 | UK | 9 | Retrospective study | The mean cost of managing a case of pulmonary MDR TB was in excess of 60,000 pounds sterling and for sensitive disease it was 6040 pounds sterling. | STRONG |
| Rouzier et al. [83] | 2007 | Ecuador | 118 | Cross-sectional survey | Among 104 non-MDR-TB patients, total TB-related patient costs averaged US$960 per patient, compared to an average total cost of US$6880 for 14 participating MDR-TB patients. | STRONG |
| Marks et al. [84] | 2005–2007 | USA | 98 | Population-based study | Average hospitalization cost per XDR-TB patient (US$285,000) was 3.5 times that per MDR-TB patient (US$81,000), in 2010 dollars | STRONG |
| Marks et al. [85] | 2005–2007 | USA | 135 | Retrospective study | Direct costs, mostly covered by the public sector, averaged $134,000 per MDR TB and $430,000 per XDR TB patient; in comparison, estimated cost per non-MDR TB patient is $17,000. | STRONG |
| HIV | ||||||
| Krentz et al. [86] | 2007–2011 | Canada | NR | An observational cohort study | Patients with no resistance had mean PPPM costs of CDN 1058, by contrast with the CDN 1291 costs of patients with secondary/acquired resistance. Mean costs for one, two or three ARV class resistance was CDN 1278, 1337 and 1801, respectively. | STRONG |
| Martin S. [87] | 2007 | NR | NR | NR | Total annual costs ranged from $31 700 for a patient on a 1st-line highly active antiretroviral therapy (HAART) regimen to $42,600 on 6th-line. | WEAK |
| Meenan RT. [88] | 2010 | NR | NR | NR | Total mean healthcare costs were $35,000 higher for patients on a 3rd- or greater line treatment regimen compared to patients on a 1st- or 2nd-line treatment regimen. | WEAK |
| Malaria | Tuberculosis | HIV Infection | |
|---|---|---|---|
| Public health outcome | +++ | +++ | + |
| Clinical outcome | +++ | +++ | +++ |
| Economic outcome | + | +++ | ++ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, T.; Chen, X.-S. Outcome Impacts Due to Pathogen-Specific Antimicrobial Resistance: A Narrative Review of Published Literature. Int. J. Environ. Res. Public Health 2020, 17, 1395. https://doi.org/10.3390/ijerph17041395
Jiang T, Chen X-S. Outcome Impacts Due to Pathogen-Specific Antimicrobial Resistance: A Narrative Review of Published Literature. International Journal of Environmental Research and Public Health. 2020; 17(4):1395. https://doi.org/10.3390/ijerph17041395
Chicago/Turabian StyleJiang, Tingting, and Xiang-Sheng Chen. 2020. "Outcome Impacts Due to Pathogen-Specific Antimicrobial Resistance: A Narrative Review of Published Literature" International Journal of Environmental Research and Public Health 17, no. 4: 1395. https://doi.org/10.3390/ijerph17041395
APA StyleJiang, T., & Chen, X.-S. (2020). Outcome Impacts Due to Pathogen-Specific Antimicrobial Resistance: A Narrative Review of Published Literature. International Journal of Environmental Research and Public Health, 17(4), 1395. https://doi.org/10.3390/ijerph17041395





