Severe Oral Mucositis in Pediatric Cancer Patients: Survival Analysis and Predictive Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
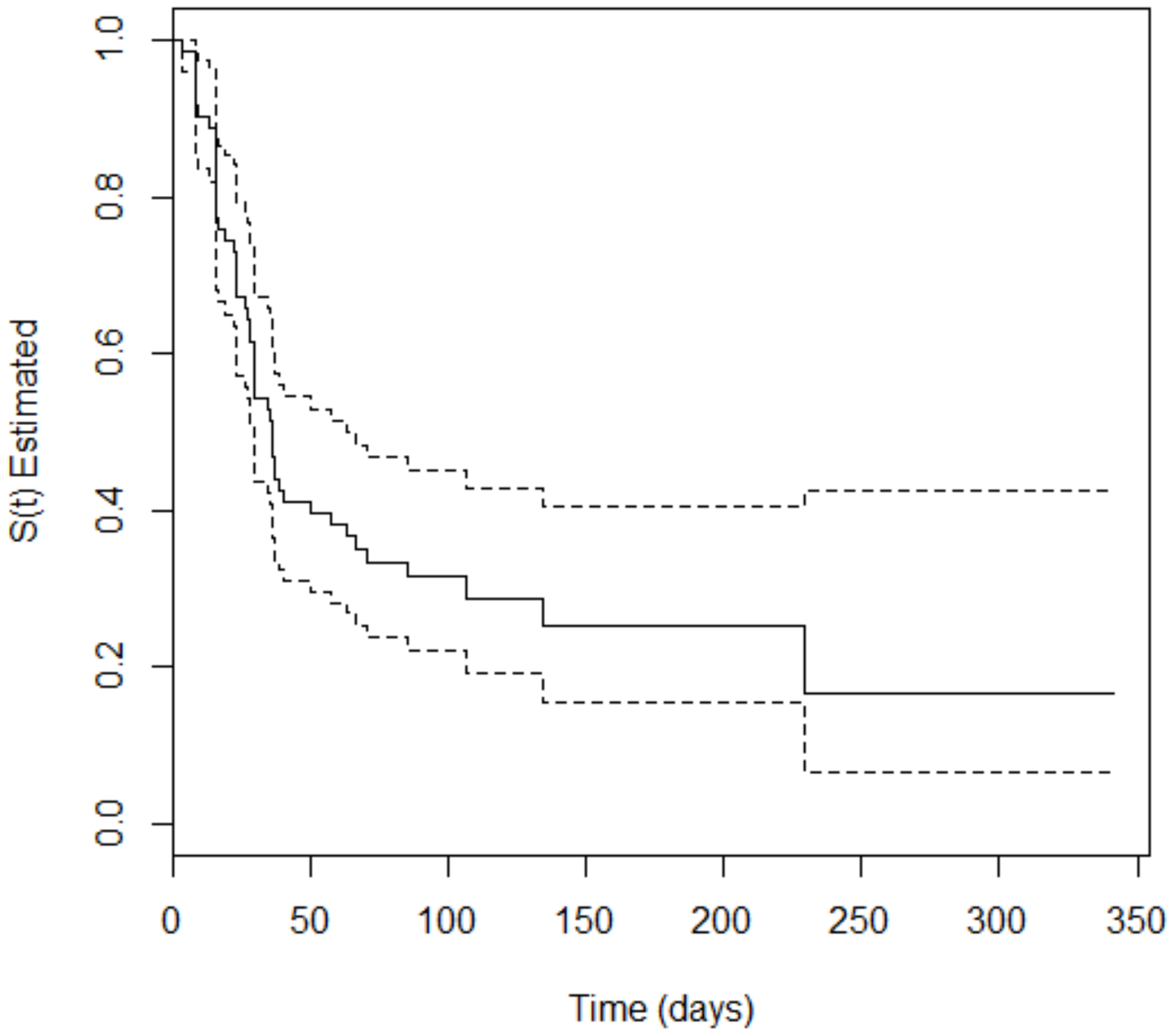
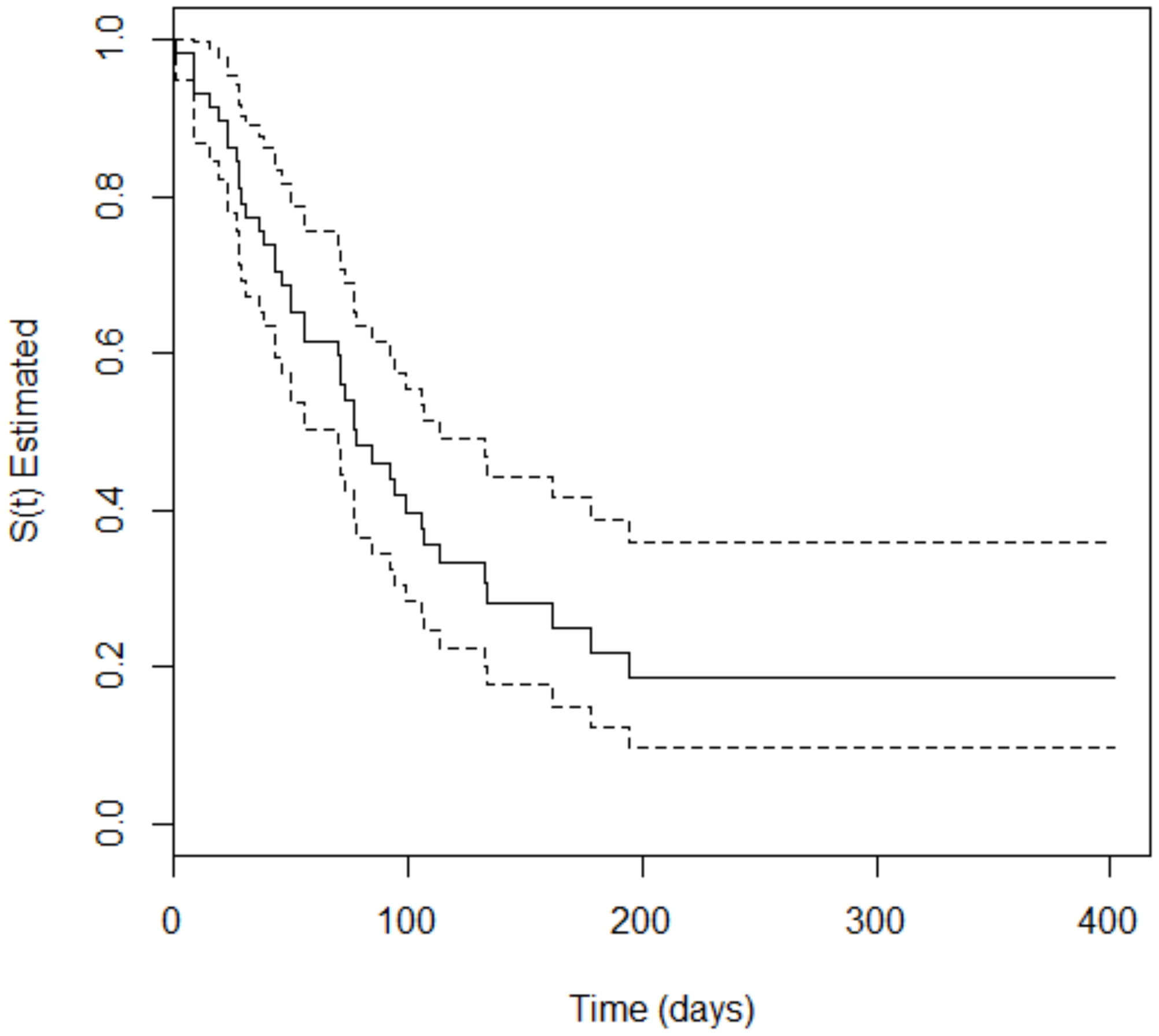
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ward, E.; DeSantis, C.; Robbins, A.; Kohler, B.; Jemal, A. Childhood and Adolescent Cancer Statistics. CA Cancer J. Clin. 2014, 64, 83–103. [Google Scholar] [CrossRef]
- Ye, Y.; Carlsson, G.; Agholme, M.B.; Wilson, J.A.L.; Roos, A.; Henriques-Normark, B.; Engstrand, L.; Modéer, T.; Pütsep, K. Oral Bacterial Community Dynamics in Paediatric Patients with Malignancies in Relation to Chemotherapy-Related Oral Mucositis: A Prospective Study. Clin. Microbiol. Infect. Off. Public Eur. Soc. Clin. Microbiol. Infect. Dis. 2013, 19, E559–E567. [Google Scholar] [CrossRef]
- Murshid, E.Z.; Azizalrahman, T.A.; AlJohar, A.J. Oral Mucositis in Leukemic Saudi Children Following Chemotherapy. Saudi J. Dent. Res. 2017, 8, 79–85. [Google Scholar] [CrossRef]
- Vitale, K.M.; Violago, L.; Cofnas, P.; Bishop, J.; Jin, Z.; Bhatia, M.; Kung, A.L.; George, D.; Garvin, J.; Satwani, P. Impact of Palifermin on Incidence of Oral Mucositis and Healthcare Utilization in Children Undergoing Autologous Hematopoietic Stem Cell Transplantation for Malignant Diseases. Pediatr. Transplant. 2014, 18, 211–216. [Google Scholar] [CrossRef]
- Yavuz, B.; Bal Yılmaz, H. Investigation of the Effects of Planned Mouth Care Education on the Degree of Oral Mucositis in Pediatric Oncology Patients. J. Pediatr. Oncol. Nurs. 2015, 32, 47–56. [Google Scholar] [CrossRef]
- Qutob, A.F.; Gue, S.; Revesz, T.; Logan, R.M.; Keefe, D. Prevention of Oral Mucositis in Children Receiving Cancer Therapy: A Systematic Review and Evidence-Based Analysis. Oral Oncol. 2013, 49, 102–107. [Google Scholar] [CrossRef]
- Mendonça, R.M.H.; Araújo, M.; Levy, C.E.; Morari, J.; Silva, R.A.; Yunes, J.A.; Brandalise, S.R. Oral Mucositis in Pediatric Acute Lymphoblastic Leukemia Patients: Evaluation of Microbiological and Hematological Factors. Pediatr. Hematol. Oncol. 2015, 32, 322–330. [Google Scholar]
- Miller, M.M.; Donald, D.V.; Hagemann, T.M. Prevention and Treatment of Oral Mucositis in Children with Cancer. J. Pediatr. Pharmacol. Ther. JPPT 2012, 17, 340–350. [Google Scholar] [CrossRef]
- Cheng, K.K.; Chang, A.M.; Yuen, M.P. Prevention of Oral Mucositis in Paediatric Patients Treated with Chemotherapy: A Randomised Crossover Trial Comparing Two Protocols of Oral Care. Eur. J. Cancer 2004, 40, 1208–1216. [Google Scholar]
- Lalla, R.V.; Saunders, D.P.; Peterson, D.E. Chemotherapy or Radiation-Induced Oral Mucositis. Dent. Clin. N. Am. 2014, 58, 341–349. [Google Scholar] [CrossRef]
- Ye, Y.; Carlsson, G.; Agholme, M.B.; Karlsson-Sjöberg, J.; Yucel-Lindberg, T.; Pütsep, K.; Modéer, T. Pretherapeutic Plasma Pro-and Anti-Inflammatory Mediators Are Related to High Risk of Oral Mucositis in Pediatric Patients with Acute Leukemia: A Prospective Cohort Study. PLoS ONE 2013, 8, e64918. [Google Scholar] [CrossRef]
- Berger Velten, D.; Zandonade, E.; Monteiro de Barros Miotto, M.H. Prevalence of Oral Manifestations in Children and Adolescents with Cancer Submitted to Chemotherapy. BMC Oral Health 2016, 16, 107. [Google Scholar] [CrossRef]
- Ribeiro, I.L.A. Differences between the oral changes presented by patients with solid and hematologic tumors during the chemotherapeutic treatment. J. Appl. Oral Sci. 2020, 28, e20190020. [Google Scholar] [CrossRef]
- Patini, R.; Coviello, V.; Riminucci, M.; Corsi, A.; Cicconetti, A. Early-stage diffuse large B-cell lymphoma of the submental region: A case report and review of the literature. Oral Surg. 2017, 10, 56–60. [Google Scholar] [CrossRef]
- Gandhi, K.; Datta, G.; Ahuja, S.; Saxena, T.; Datta, A.G. Prevalence of Oral Complications Occurring in a Population of Pediatric Cancer Patients Receiving Chemotherapy. Int. J. Clin. Pediatr. Dent. 2017, 10, 166–171. [Google Scholar] [CrossRef]
- Ip, W.Y.; Epstein, J.B.; Lee, V.; Yuen, H.L.; Li, R.; Thompson, D.R.; Goggins, W.B.; Cheng, K.K.F. Oral Mucositis in Paediatric Patients after Chemotherapy for Cancer. Hong Kong Med. J. 2014, 20, 4–8. [Google Scholar]
- Cheng, K.K.F.; Lee, V.; Li, C.H.; Goggins, W.; Thompson, D.R.; Yuen, H.L.; Epstein, J.B. Incidence and Risk Factors of Oral Mucositis in Paediatric and Adolescent Patients Undergoing Chemotherapy. Oral Oncol. 2011, 47, 153–162. [Google Scholar] [CrossRef]
- Al-Ansari, S.; Zecha, J.A.E.M.; Barasch, A.; De Lange, J.; Rozema, F.R.; Raber-Durlacher, J.E. Oral Mucositis Induced by Anticancer Therapies. Curr. Oral Health Rep. 2015, 2, 202–211. [Google Scholar] [CrossRef]
- Cinausero, M.; Aprile, G.; Ermacora, P.; Basile, D.; Vitale, M.G.; Fanotto, V.; Parisi, G.; Calvetti, L.; Sonis, S.T. New Frontiers in the Pathobiology and Treatment of Cancer Regimen-Related Mucosal Injury. Front. Pharmacol. 2017, 8, 354. [Google Scholar] [CrossRef]
- Albano Lopes, I.; Nunes Nogueira, D. Manifestações Orais Decorrentes Da Quimioterapia Em Crianças de Um Centro de Tratamento Oncológico. Pesqui. Bras. Odontopediatr. Clín. Integrada 2012, 12, 113–119. [Google Scholar] [CrossRef]
- Ribeiro, I.L.A.; Valença, A.M.G.; Bonan, P.R.F. Treatment of Severe Oral Mucositis in a Pediatric Patient Undergoing Chemotherapy. RGO Rev. Gaúcha Odontol. 2015, 63, 467–471. [Google Scholar] [CrossRef][Green Version]
- Caze, M.O.; Bueno, D.; Dos Santos, M.E.F. Estudo referencial de um protocolo quimioterápico para leucemia linfocítica aguda infantil. Rev. HCPA Porto Alegre 2010, 30, 5–12. [Google Scholar]
- Chaveli-López, B.; Bagán-Sebastián, J.V. Treatment of Oral Mucositis Due to Chemotherapy. J. Clin. Exp. Dent. 2016, 8, e201–e209. [Google Scholar] [CrossRef]
- Viana, M.B.; Cunha, K.C.; Ramos, G.; Murao, M. Leucemia Mielóide Aguda Na Criança: Experiência de 15 Anos Em Uma Única Instituição. J. Pediatr. 2003, 79, 489–496. [Google Scholar] [CrossRef]
- Pereira, J.; Neto, A.H.; Pracchia, L.F.; Alcântara, A.; Maurino, B.B.; Dorliac-Llacer, P.E.; Chamone, D.A. Quimioterapia Associada à Terapia Anti-Retroviral de Alta Eficácia No Tratamento Dos Linfomas Não-Hodgkin Agressivos Relacionados à Síndrome da Imunodeficiência Adquirida. Rev. Bras. Hematol. E Hemoter. 2004, 26, 177–182. [Google Scholar] [CrossRef]
- Allen, G.; Logan, R.; Gue, S. Oral Manifestations of Cancer Treatment in Children: A Review of the Literature. Clin. J. Oncol. Nurs. 2010, 14, 481–490. [Google Scholar] [CrossRef]
- Kwitkowski, V.E.; Prowell, T.M.; Ibrahim, A.; Farrell, A.T.; Justice, R.; Mitchell, S.S.; Sridhara, R.; Pazdur, R. FDA Approval Summary: Temsirolimus as Treatment for Advanced Renal Cell Carcinoma. Oncologist 2010, 15, 428–435. [Google Scholar] [CrossRef]
- Vokurka, S.; Bystrická, E.; Koza, V.; Scudlová, J.; Pavlicová, V.; Valentová, D.; Visokaiová, M.; Misaniová, L. Higher Incidence of Chemotherapy Induced Oral Mucositis in Females: A Supplement of Multivariate Analysis to a Randomized Multicentre Study. Support. Care Cancer 2006, 14, 974–976. [Google Scholar]
- Brasil. Lei No 8.080, de 19 de Setembro de 1990. Lei Orgânica Da Saúde: Dispõe Sobre as Condições Para a Promoção, Proteção e Recuperação Da Saúde, a Organização Eo Funcionamento Dos Serviços Correspondentes e Dá Outras Providências. Diário Of. Repúb. Federativa Brasil 1990, 128, 182.
- Zouain-Figueiredo, G.P.; Zandonade, E.; Amorim, M.H.C.; Figueiredo, L.Z.; Binda, L.A. Perfil Epidemiológico Dos Casos Novos de Câncer Infanto-Juvenil Em Hospital de Referência No Espírito Santo, Brasil, de 1986 a 2010. Rev. Brasileira Pesqui. Saúde 2016, 17, 109–120. [Google Scholar]
- Smith, L.; Norman, P.; Kapetanstrataki, M.; Fleming, S.; Fraser, L.K.; Parslow, R.C.; Feltbower, R.G. Comparison of Ethnic Group Classification Using Naming Analysis and Routinely Collected Data: Application to Cancer Incidence Trends in Children and Young People. BMJ Open 2017, 7, e016332. [Google Scholar] [CrossRef]
- Felgenhauer, J.; Hawkins, D.; Pendergrass, T.; Lindsley, K.; Conrad, E.U.; Miser, J.S. Very Intensive, Short-Term Chemotherapy for Children and Adolescents with Metastatic Sarcomas. Pediatr. Blood Cancer 2000, 34, 29–38. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Cartenì, G.; Autorino, R.; Gonnella, A.; Perdonà, S.; Ferro, M.; Longo, N.; Rescigno, P.; Doria, F.; Faiella, A.; et al. Activity and Toxicity of Paclitaxel in Pretreated Metastatic Penile Cancer Patients. Anticancer Drugs 2009, 20, 277–280. [Google Scholar] [CrossRef]
- Dalton, R.N. Serum Creatinine and Glomerular Filtration Rate: Perception and Reality. J. Bras. Patol. E Med. Lab. 2011, 47, 8–11. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, L.; Chen, X.; Pan, B.; Mao, J.; Song, H.; Li, J.; Tang, Y. Serum Creatinine and Creatinine Clearance for Predicting Plasma Methotrexate Concentrations after High-Dose Methotrexate Chemotherapy for the Treatment for Childhood Lymphoblastic Malignancies. Cancer Chemother. Pharmacol. 2014, 73, 79–86. [Google Scholar] [CrossRef]
- Yang, S.-L.; Zhao, F.-Y.; Song, H.; Shen, D.-Y.; Xu, X.-J. Methotrexate Associated Renal Impairment Is Related to Delayed Elimination of High-Dose Methotrexate. Sci. World J. 2015, 2015. [Google Scholar] [CrossRef]
- Abidi, M.H.; Agarwal, R.; Ayash, L.; Deol, A.; Al-Kadhimi, Z.; Abrams, J.; Cronin, S.; Ventimiglia, M.; Lum, L.; Zonder, J.; et al. Melphalan 180 Mg/M2 Can Be Safely Administered as Conditioning Regimen before an Autologous Stem Cell Transplantation (ASCT) in Multiple Myeloma Patients with Creatinine Clearance 60 ML/Min/1.73 M2 or Lower with Use of Palifermin for Cytoprotection: Results of a Phase I Trial. Biol. Blood Marrow Transplant. 2012, 18, 1455–1461. [Google Scholar] [CrossRef]
- Da Silva, S.R.; de Ávila, F.F.; Soares, M.B.O. Perfil Hematológico e Bioquímico Sérico de Pacientes Submetidas à Quimioterapia Antineoplásica. Rev. Enferm. E Atenção À Saúde 2013, 2, 32–45. [Google Scholar]
- Pontes, L.B.; Antunes, Y.P.P.V.; Bugano, D.D.G.; Karnakis, T.; Giglio del, A.; Kaliks, R.A.; Pontes, L.B.; Antunes, Y.P.P.V.; Bugano, D.D.G.; Karnakis, T.; et al. Prevalence of Renal Insufficiency in Elderly Cancer Patients in a Tertiary Cancer Center. Einstein São Paulo 2014, 12, 300–303. [Google Scholar] [CrossRef]
- Elad, S.; Zadik, Y.; Yarom, N. Oral Complications of Nonsurgical Cancer Therapies. Atlas Oral Maxillofac. Surg. Clin. N. Am. 2017, 25, 133–147. [Google Scholar] [CrossRef]
- Rose-Ped, A.M.; Bellm, L.A.; Epstein, J.B.; Trotti, A.; Gwede, C.; Fuchs, H.J. Complications of Radiation Therapy for Head and Neck Cancers: The Patient’s Perspective. Cancer Nurs. 2002, 25, 461–467. [Google Scholar]
- Volpato, L.E.R.; Silva, T.C.; Oliveira, T.M.; Sakai, V.T.; Machado, M.A.A.M. Radiation Therapy and Chemotherapy-Induced Oral Mucositis. Rev. Bras. Otorrinolaringol. 2007, 73, 562–568. [Google Scholar] [CrossRef]
- Peterson, D.E.; Ohrn, K.; Bowen, J.; Fliedner, M.; Lees, J.; Loprinzi, C.; Mori, T.; Osaguona, A.; Weikel, D.S.; Elad, S.; et al. Systematic Review of Oral Cryotherapy for Management of Oral Mucositis Caused by Cancer Therapy. Support. Care Cancer 2013, 21, 327–332. [Google Scholar] [CrossRef]
- Raber-Durlacher, J.E.; von Bültzingslöwen, I.; Logan, R.M.; Bowen, J.; Al-Azri, A.R.; Everaus, H.; Gerber, E.; Gomez, J.G.; Pettersson, B.G.; Soga, Y.; et al. Systematic Review of Cytokines and Growth Factors for the Management of Oral Mucositis in Cancer Patients. Support. Care Cancer 2013, 21, 343–355. [Google Scholar] [CrossRef]
- Sayles, C.; Hickerson, S.C.; Bhat, R.R.; Hall, J.; Garey, K.W.; Trivedi, M.V. Oral Glutamine in Preventing Treatment-Related Mucositis in Adult Patients with Cancer: A Systematic Review. Nutr. Clin. Pract. 2016, 31, 171–179. [Google Scholar] [CrossRef]
| Variable | Relative Frequency % | Absolute Frequency n |
|---|---|---|
| Sex | ||
| Male | 51.4 | 73 |
| Female | 48.6 | 69 |
| Ethnicity | ||
| Mixed | 51.4 | 73 |
| White | 33.1 | 47 |
| Black | 14.8 | 21 |
| Indigenous | 0.7 | 1 |
| Cancer type | ||
| Hematologic malignant | 56.3 | 80 |
| Solid tumor | 43.7 | 62 |
| Disease | ||
| Acute lymphoblastic leukemia | 38.8 | 55 |
| Wilms’ tumor | 14.1 | 20 |
| Osteosarcoma | 13.4 | 19 |
| Non-Hodgkin lymphoma | 9.2 | 13 |
| Acute myeloid leukemia | 6.3 | 09 |
| Embryonal rhabdomyosarcoma | 4.2 | 06 |
| Adenocarcinoma | 2.8 | 04 |
| Hodgkin lymphoma | 2.1 | 03 |
| Neuroblastoma | 2.1 | 03 |
| Brain stem tumor | 2.1 | 03 |
| Bladder tumor | 0.7 | 01 |
| Spinal tumor | 0.7 | 01 |
| Germ cell tumor | 0.7 | 01 |
| Melanoma | 0.7 | 01 |
| Lymphoepithelioma | 0.7 | 01 |
| Alveolar soft part sarcoma | 0.7 | 01 |
| Synovial sarcoma | 0.7 | 01 |
| Metastasis | ||
| No | 90.1 | 128 |
| Yes | 9.9 | 14 |
| Death | ||
| No | 86.6 | 123 |
| Yes | 13.4 | 19 |
| Treatment | ||
| Chemotherapy | 69.0 | 98 |
| Chemotherapy + surgery | 24.0 | 34 |
| Chemotherapy + radiotherapy | 3.5 | 5 |
| Chemotherapy + radiotherapy + surgery | 3.5 | 5 |
| Class 1—Alkylating agents | ||
| No | 83.1 | 118 |
| Yes | 16.9 | 24 |
| Class 2—Antimetabolites | ||
| No | 55.6 | 79 |
| Yes | 44.4 | 63 |
| Class 3—Natural agents | ||
| Yes | 58.5 | 83 |
| No | 41.5 | 59 |
| Class 4—Miscellaneous | ||
| No | 83.1 | 118 |
| Yes | 16.9 | 24 |
| Bone marrow transplantation | ||
| No | 97.2 | 138 |
| Yes | 2.8 | 4 |
| Limb amputation | ||
| No | 73.2 | 104 |
| Yes | 26.8 | 38 |
| Leukocytes | ||
| 3400 to 9500/mm3 | 48.6 | 69 |
| Less than 3400/mm3 | 33.8 | 48 |
| Greater than 9500/mm3 | 17.6 | 25 |
| Platelets | ||
| 150,000 to 450,000/mm3 | 52.8 | 75 |
| Less than 150,000/mm3 | 26.1 | 37 |
| Greater than 450,000/mm3 | 21.1 | 30 |
| Creatinine | ||
| 0.4 to 1.3 mg/dL | 81.7 | 116 |
| Less than 0.4 mg/dL | 17.6 | 25 |
| Greater than 1.3 mg/dL | 0.7 | 1 |
| Hematologic Malignancies | ||||||
|---|---|---|---|---|---|---|
| Covariables | Risk Category | Estimate | Standard Error | p-Value | RR | 95% CI (RR) |
| Leukocytes | Greater than 9500/mm3 | −1.169 | 0.513 | 0.023 | 0.311 | 0.114–0.849 |
| Platelets | Greater than 450,000/mm3 | 0.772 | 0.357 | 0.031 | 2.164 | 1.075–4.357 |
| Class 3—Natural agents | Use of natural chemotherapeutic agents | 0.673 | 0.299 | 0.025 | 1.961 | 1.089–3.528 |
| Solid Tumors | ||||||
|---|---|---|---|---|---|---|
| Covariable | Risk Category | Estimate | Standard Error | p-Value | RR | 95% CI (RR) |
| Sex | Female | 1.033 | 0.392 | 0.008 | 2.809 | 1.304–6.052 |
| Ethnicity | Nonmixed | −0.768 | 0.391 | 0.049 | 0.464 | 0.216–0.997 |
| Metastasis | With metastasis | 1.129 | 0.458 | 0.014 | 3.092 | 1.259–7.592 |
| Creatinine | 0.4 to 1.3 mg/dL | 1.359 | 0.619 | 0.009 | 3.892 | 1.157–13.095 |
| Treatment | Chemotherapy + surgery + radiotherapy | 1.829 | 0.582 | 0.002 | 6.226 | 1.989–19.483 |
| Chemotherapeutic agent class | Miscellaneous | 1.063 | 0.407 | 0.028 | 2.890 | 1.303–6.432 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damascena, L.C.L.; de Lucena, N.N.N.; Ribeiro, I.L.A.; Pereira, T.L.; Lima-Filho, L.M.A.; Valença, A.M.G. Severe Oral Mucositis in Pediatric Cancer Patients: Survival Analysis and Predictive Factors. Int. J. Environ. Res. Public Health 2020, 17, 1235. https://doi.org/10.3390/ijerph17041235
Damascena LCL, de Lucena NNN, Ribeiro ILA, Pereira TL, Lima-Filho LMA, Valença AMG. Severe Oral Mucositis in Pediatric Cancer Patients: Survival Analysis and Predictive Factors. International Journal of Environmental Research and Public Health. 2020; 17(4):1235. https://doi.org/10.3390/ijerph17041235
Chicago/Turabian StyleDamascena, Lecidamia Cristina Leite, Nyellisonn Nando Nóbrega de Lucena, Isabella Lima Arrais Ribeiro, Tarciana Liberal Pereira, Luiz Medeiros Araújo Lima-Filho, and Ana Maria Gondim Valença. 2020. "Severe Oral Mucositis in Pediatric Cancer Patients: Survival Analysis and Predictive Factors" International Journal of Environmental Research and Public Health 17, no. 4: 1235. https://doi.org/10.3390/ijerph17041235
APA StyleDamascena, L. C. L., de Lucena, N. N. N., Ribeiro, I. L. A., Pereira, T. L., Lima-Filho, L. M. A., & Valença, A. M. G. (2020). Severe Oral Mucositis in Pediatric Cancer Patients: Survival Analysis and Predictive Factors. International Journal of Environmental Research and Public Health, 17(4), 1235. https://doi.org/10.3390/ijerph17041235





