Serum Sclerostin But Not DKK-1 Correlated with Central Arterial Stiffness in End Stage Renal Disease Patients
Abstract
1. Introduction
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Patients
5.2. Anthropometric Analysis
5.3. Biochemical Investigations
5.4. Carotid-Femoral Pulse Wave Velocity Measurements
5.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stevens, P.E.; Levin, A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group, M. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N.; Collins, A.J. The USRDS: What you need to know about what it can and can’t tell us about ESRD. CJASN 2013, 8, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Lin, M.Y.; Chen, H.C.; Hwang, S.C.; Yang, W.C.; Hsu, C.C.; Chiu, H.C.; Mau, L.W. Increased risk of mortality in the elderly population with late-stage chronic kidney disease: A cohort study in Taiwan. Nephrol. Dial. Transplant. 2008, 23, 3192–3198. [Google Scholar] [CrossRef] [PubMed]
- Jono, S.; McKee, M.D.; Murry, C.E.; Shioi, A.; Nishizawa, Y.; Mori, K.; Morii, H.; Giachelli, C.M. Phosphate regulation of vascular smooth muscle cell calcification. Circ. Res. 2000, 87, E10–E17. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Blacher, J.; Guerin, A.P.; Pannier, B.; Marchais, S.J.; Safar, M.E.; London, G.M. Impact of aortic stiffness on survival in end-stage renal disease. Circulation 1999, 99, 2434–2439. [Google Scholar] [CrossRef]
- Moe, S.M.; Chen, N.X. Mechanisms of vascular calcification in chronic kidney disease. JASN 2008, 19, 213–216. [Google Scholar] [CrossRef]
- Morena, M.; Jaussent, I.; Dupuy, A.M.; Bargnoux, A.S.; Kuster, N.; Chenine, L.; Leray-Moragues, H.; Klouche, K.; Vernhet, H.; Canaud, B.; et al. Osteoprotegerin and sclerostin in chronic kidney disease prior to dialysis: Potential partners in vascular calcifications. Nephrol. Dial. Transplant. 2015, 30, 1345–1356. [Google Scholar] [CrossRef]
- Wang, J.H.; Lee, C.J.; Chen, M.L.; Yang, C.F.; Chen, Y.C.; Hsu, B.G. Association of serum osteoprotegerin levels with carotid-femoral pulse wave velocity in hypertensive patients. J. Clin. Hypertens. 2014, 16, 301–308. [Google Scholar] [CrossRef]
- Balemans, W.; Ebeling, M.; Patel, N.; Van Hul, E.; Olson, P.; Dioszegi, M.; Lacza, C.; Wuyts, W.; Van Den Ende, J.; Willems, P.; et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 2001, 10, 537–543. [Google Scholar] [CrossRef]
- Zhu, D.; Mackenzie, N.C.; Millan, J.L.; Farquharson, C.; MacRae, V.E. The appearance and modulation of osteocyte marker expression during calcification of vascular smooth muscle cells. PLoS ONE 2011, 6, e19595. [Google Scholar] [CrossRef] [PubMed]
- Morales-Santana, S.; Garcia-Fontana, B.; Garcia-Martin, A.; Rozas-Moreno, P.; Garcia-Salcedo, J.A.; Reyes-Garcia, R.; Munoz-Torres, M. Atherosclerotic disease in type 2 diabetes is associated with an increase in sclerostin levels. Diabetes Care 2013, 36, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Guan, L.; Zhang, Y.; Yu, S.; Cao, B.; Ji, Y. Sclerostin as a new key factor in vascular calcification in chronic kidney disease stages 3 and 4. Int. Urol. Nephrol. 2016, 48, 2043–2050. [Google Scholar] [CrossRef]
- Qureshi, A.R.; Olauson, H.; Witasp, A.; Haarhaus, M.; Brandenburg, V.; Wernerson, A.; Lindholm, B.; Soderberg, M.; Wennberg, L.; Nordfors, L.; et al. Increased circulating sclerostin levels in end-stage renal disease predict biopsy-verified vascular medial calcification and coronary artery calcification. Kidney Int. 2015, 88, 1356–1364. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chang, Z.F.; Chau, Y.P.; Chen, A.; Yang, W.C.; Yang, A.H.; Lee, O.K. Circulating Wnt/beta-catenin signalling inhibitors and uraemic vascular calcifications. Nephrol. Dial. Transplant. 2015, 30, 1356–1363. [Google Scholar] [CrossRef]
- Jean, G.; Chazot, C.; Bresson, E.; Zaoui, E.; Cavalier, E. High Serum Sclerostin Levels Are Associated with a Better Outcome in Haemodialysis Patients. Nephron 2016, 132, 181–190. [Google Scholar] [CrossRef]
- Claes, K.J.; Viaene, L.; Heye, S.; Meijers, B.; d’Haese, P.; Evenepoel, P. Sclerostin: Another vascular calcification inhibitor? J. Clin. Endocrinol. Metab. 2013, 98, 3221–3228. [Google Scholar] [CrossRef]
- Szulc, P.; Schoppet, M.; Rachner, T.D.; Chapurlat, R.; Hofbauer, L.C. Severe abdominal aortic calcification in older men is negatively associated with DKK1 serum levels: The STRAMBO study. J. Clin. Endocrinol. Metab. 2014, 99, 617–624. [Google Scholar] [CrossRef]
- Krediet, R.T.; Balafa, O. Cardiovascular risk in the peritoneal dialysis patient. Nat. Rev. Nephrol. 2010, 6, 451–460. [Google Scholar] [CrossRef]
- Sipahioglu, M.H.; Kucuk, H.; Unal, A.; Kaya, M.G.; Oguz, F.; Tokgoz, B.; Oymak, O.; Utas, C. Impact of arterial stiffness on adverse cardiovascular outcomes and mortality in peritoneal dialysis patients. Perit. Dial. Int. 2012, 32, 73–80. [Google Scholar] [CrossRef]
- Szeto, C.C.; Kwan, B.C.; Chow, K.M.; Leung, C.B.; Law, M.C.; Li, P.K. Prognostic value of arterial pulse wave velocity in peritoneal dialysis patients. Am. J. Nephrol. 2012, 35, 127–133. [Google Scholar] [CrossRef] [PubMed]
- London, G.M.; Safar, M.E.; Pannier, B. Aortic Aging in ESRD: Structural, Hemodynamic, and Mortality Implications. JASN 2016, 27, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Chi, P.J.; Lin, Y.L.; Tasi, J.P.; Wang, C.H.; Hou, J.S.; Lee, C.J.; Hsu, B.G. Osteocalcin and carotid-femoral pulse wave velocity in patients on peritoneal dialysis. Tzu-Chi Med. J. 2019, 31, 23–28. [Google Scholar]
- Schram, M.T.; Henry, R.M.; van Dijk, R.A.; Kostense, P.J.; Dekker, J.M.; Nijpels, G.; Heine, R.J.; Bouter, L.M.; Westerhof, N.; Stehouwer, C.D. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: The Hoorn Study. Hypertension 2004, 43, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Kuang, D.W.; Li, C.L.; Kuok, U.I.; Cheung, K.; Lio, W.I.; Xin, J. Risk factors associated with brachial-ankle pulse wave velocity among peritoneal dialysis patients in Macao. BMC Nephrol. 2012, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Moester, M.J.; Papapoulos, S.E.; Lowik, C.W.; van Bezooijen, R.L. Sclerostin: Current knowledge and future perspectives. Calcif. Tissue Int. 2010, 87, 99–107. [Google Scholar] [CrossRef]
- Baron, R.; Kneissel, M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef]
- Pelletier, S.; Dubourg, L.; Carlier, M.C.; Hadj-Aissa, A.; Fouque, D. The relation between renal function and serum sclerostin in adult patients with CKD. CJASN 2013, 8, 819–823. [Google Scholar] [CrossRef]
- Cejka, D.; Jager-Lansky, A.; Kieweg, H.; Weber, M.; Bieglmayer, C.; Haider, D.G.; Diarra, D.; Patsch, J.M.; Kainberger, F.; Bohle, B.; et al. Sclerostin serum levels correlate positively with bone mineral density and microarchitecture in haemodialysis patients. Nephrol. Dial. Transplant. 2012, 27, 226–230. [Google Scholar] [CrossRef]
- Bonani, M.; Rodriguez, D.; Fehr, T.; Mohebbi, N.; Brockmann, J.; Blum, M.; Graf, N.; Frey, D.; Wuthrich, R.P. Sclerostin blood levels before and after kidney transplantation. Kidney Blood Press. Res. 2014, 39, 230–239. [Google Scholar] [CrossRef]
- Thambiah, S.; Roplekar, R.; Manghat, P.; Fogelman, I.; Fraser, W.D.; Goldsmith, D.; Hampson, G. Circulating sclerostin and Dickkopf-1 (DKK1) in predialysis chronic kidney disease (CKD): Relationship with bone density and arterial stiffness. Calcif. Tissue Int. 2012, 90, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.H.; Lin, W.H.; Chao, J.Y.; Wu, A.B.; Tseng, C.C.; Chang, Y.T.; Liou, H.H.; Wang, M.C. Serum sclerostin levels are positively related to bone mineral density in peritoneal dialysis patients: A cross-sectional study. BMC Nephrol. 2019, 20, 266. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, D.; Inaba, M.; Imanishi, Y.; Hayashi, N.; Ohara, M.; Nagata, Y.; Kurajoh, M.; Yamada, S.; Mori, K.; Emoto, M. Denosumab Improves Glomerular Filtration Rate in Osteoporotic Patients With Normal Kidney Function by Lowering Serum Phosphorus. J. Bone Miner. Res. 2019, 34, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, L.; Liabeuf, S.; Oliveira, R.B.; Louvet, L.; Kamel, S.; Lemke, H.D.; Vanholder, R.; Choukroun, G.; Massy, Z.A.; European Uremic Toxin Work, G. Uremic toxicity and sclerostin in chronic kidney disease patients. Nephrol. Ther. 2014, 10, 463–470. [Google Scholar] [CrossRef]
- Hsu, B.G.; Liou, H.H.; Lee, C.J.; Chen, Y.C.; Ho, G.J.; Lee, M.C. Serum Sclerostin as an Independent Marker of Peripheral Arterial Stiffness in Renal Transplantation Recipients: A Cross-Sectional Study. Medicine 2016, 95, e3300. [Google Scholar] [CrossRef]
- Martinez-Moreno, J.M.; Munoz-Castaneda, J.R.; Herencia, C.; Oca, A.M.; Estepa, J.C.; Canalejo, R.; Rodriguez-Ortiz, M.E.; Perez-Martinez, P.; Aguilera-Tejero, E.; Canalejo, A.; et al. In vascular smooth muscle cells paricalcitol prevents phosphate-induced Wnt/beta-catenin activation. Am. J. Physiol. Ren. Physiol. 2012, 303, F1136–F1144. [Google Scholar] [CrossRef]
- Lee, C.J.; Wang, J.H.; Chen, M.L.; Yang, C.F.; Chen, Y.C.; Hsu, B.G. Serum osteoprotegerin is associated with arterial stiffness assessed according to the cardio-ankle vascular index in hypertensive patients. J. Atheroscler. Thromb. 2015, 22, 304–312. [Google Scholar] [CrossRef][Green Version]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
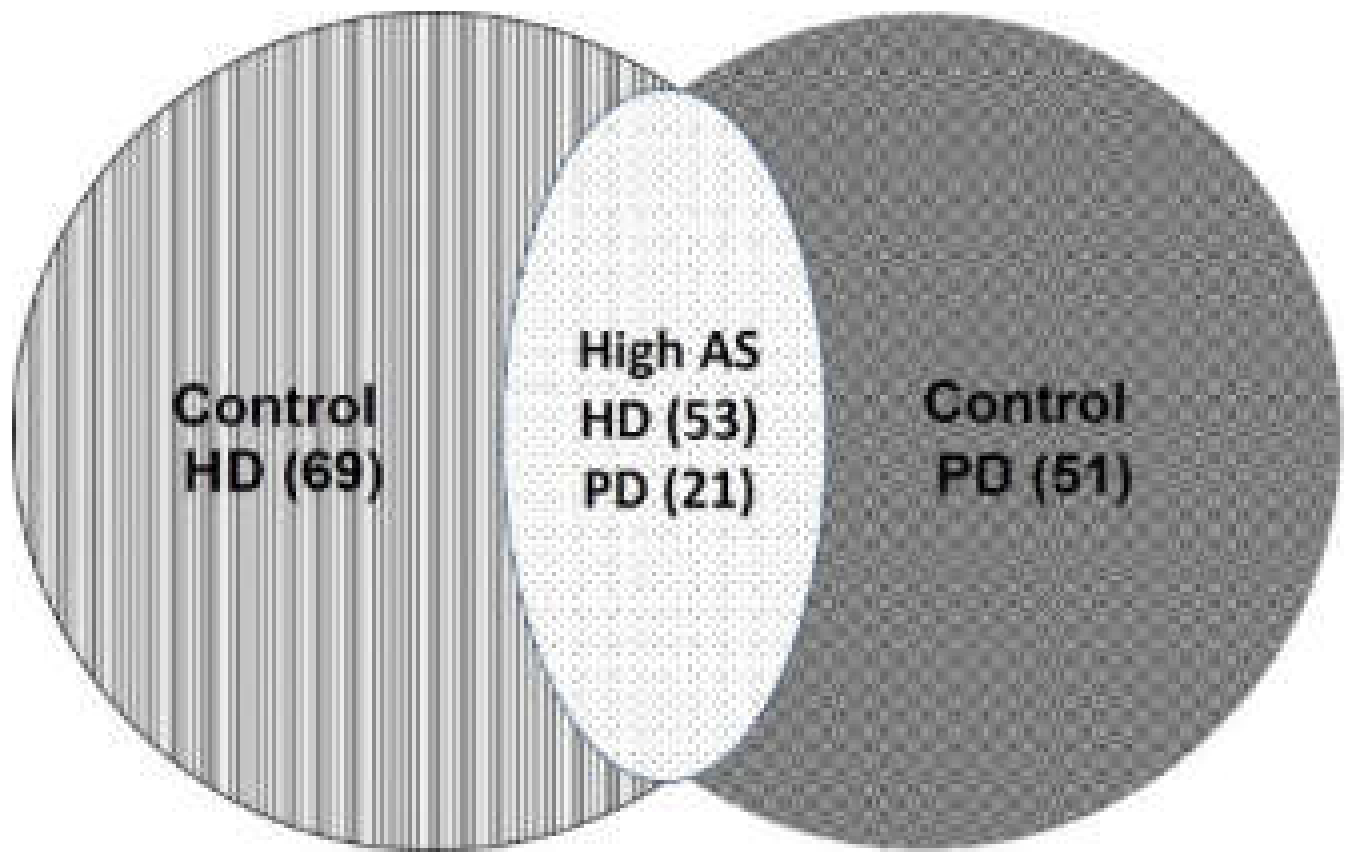
| Characteristics | All (n = 194) | Control (n = 120) | High AS (n = 74) | p |
|---|---|---|---|---|
| Age (years) | 61 (52–71) | 58 (47.5–67.5) | 65 (59 –73) | 0.0001 |
| Female, n (%) | 101 (52.1) | 69 (57.5) | 32 (43.2) | 0.075 |
| Dialysis duration (mo) | 48.5 (22–96) | 42.5 (18.5–96.5) | 57 (28–90) | 0.077 |
| BMI (Kg/m2) | 24.91 ± 4.82 | 24.58 ± 4.83 | 25.45 ± 4.80 | 0.226 |
| SBP (mmHg) | 142.29 ± 24.94 | 138.09 ± 25.54 | 149.11 ± 22.46 | 0.003 * |
| DBP (mmHg) | 79.66 ± 15.74 | 78.81 ± 14.84 | 81.05 ± 17.12 | 0.336 |
| cfPWV (m/s) | 9.0 (7.5–11.7) | 7.7 (7.0–8.9) | 12.3 (11.4–14.3) | <0.001 * |
| BUN (mg/dL) | 60 (50–70) | 60.0 (49.5–67.5) | 61.0 (50.0–71.0) | 0.564 |
| Creatinine (mg/dL) | 9.91 ± 2.67 | 9.90 ± 2.83 | 9.95 ± 2.43 | 0.901 |
| Calcium (mg/dL) | 9.02 ± 0.76 | 8.96 ± 0.71 | 9.11± 0.84 | 0.197 |
| IP (mg/dL) | 4.92 ± 1.34 | 4.94 ± 1.37 | 4.90 ± 1.29 | 0.842 |
| Albumin (mg/dL) | 4.06 (3.7–4.2) | 4.01 (3.70–4.30) | 4.10 (3.70–4.20) | 0.644 |
| TCH (mg/dL) | 154.73 ± 39.13 | 157.55 ± 40.58 | 150.15 ± 36.47 | 0.202 |
| TG (mg/dL) | 125 (87–197) | 114.0 (87.5–199.0) | 133.0 (87.0–189.0) | 0.473 |
| Glucose (mg/dL) | 120 (100–149) | 114.5 (97.0–142.0) | 131.0 (110.0–182.0) | 0.001 |
| CRP (mg/dL) | 0.32 (0.09–0.90) | 0.195 (0.06–0.780) | 0.465 (0.25–1.05) | 0.0001 * |
| iPTH (pg/mL) | 230.15 (89.91–486.30) | 252.15 (123.07–503.86) | 167.25 (73.01–434.10) | 0.111 |
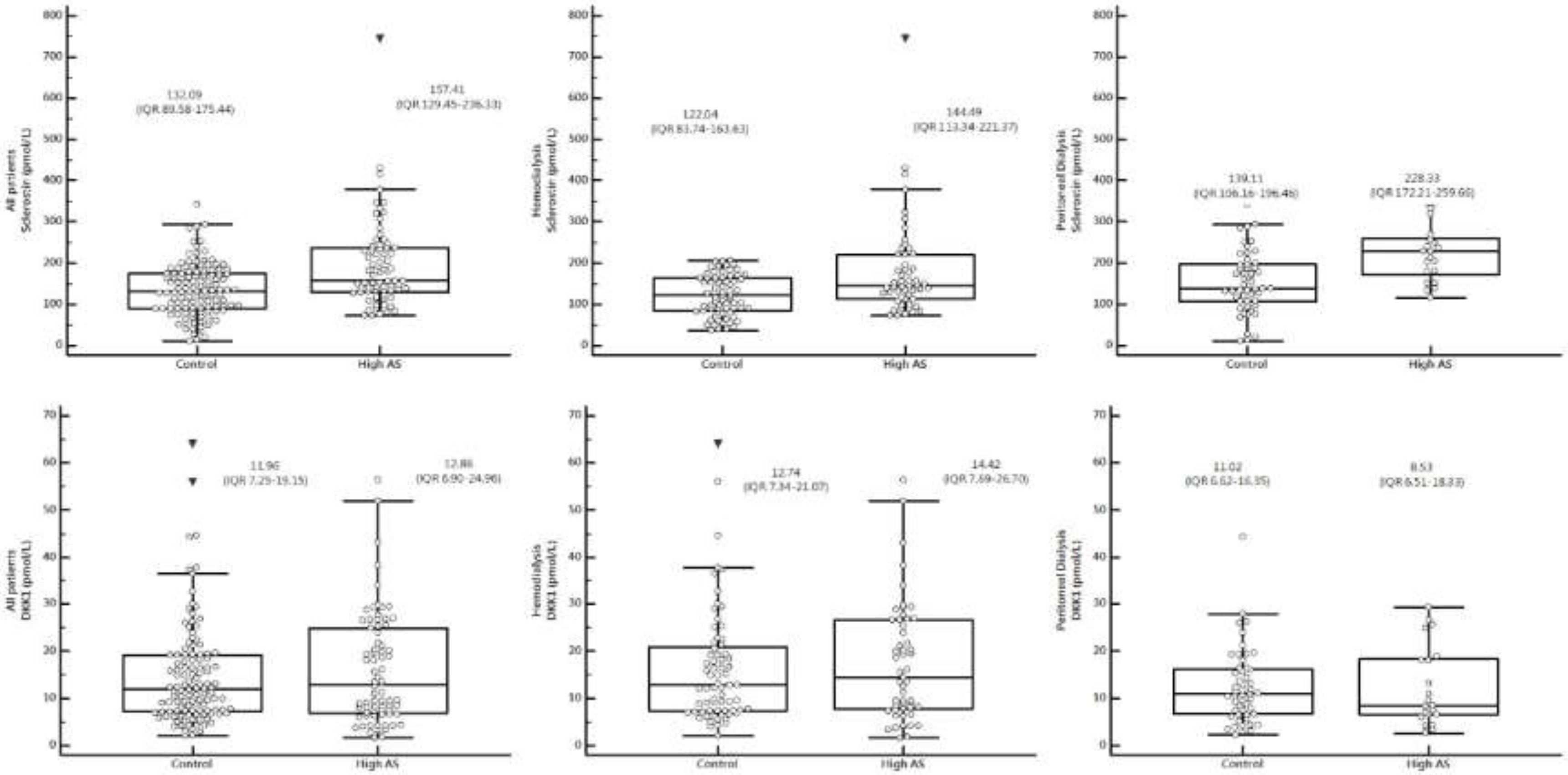
| Sclerostin (pmol/L) | 143.50 (97.21–191.7) | 132.09 (89.58–175.44) | 157.41 (129.45–236.33) | 0.0001 * |
| DKK-1 (pmol/L) | 12.08 (7.15–19.93) | 11.96 (7.25–19.15) | 12.86 (6.90–24.96) | 0.563 |
| Mode, n (%) | <0.001 | |||
| PD | 72 (37.1) | 51 (70.8) | 21 (29.2) | |
| HD | 122 (62.9) | 69 (56.6) | 53 (43.4) | |
| Cormobidity n (%) | 0.003 | |||
| No | 48 (24.7) | 37 (30.8) | 11 (14.9) | |
| Diabetes mellitus | 64 (33.0) | 44 (36.7) | 20 (27.0) | |
| Hypertension | 25 (12.9) | 14 (11.7) | 11 (14.9) | |
| Both | 57 (29.4) | 25 (20.8) | 32 (43.2) | |
| ARB, n (%) | 66 (34.0) | 43 (35.8) | 27 (36.5) | 0.951 |
| β-blocker, n (%) | 63 (32.5) | 38 (31.7) | 25 (33.8) | 0.882 |
| CCB, n (%) | 82 (42.3) | 55 (45.8) | 27 (36.5) | 0.258 |
| Statin, n (%) | 40 (20.6) | 24 (20.0) | 16 (21.6) | 0.930 |
| Characteristics | All Patients (n = 122) | Control Group (n = 69) | High AS Group (n = 53) | p |
|---|---|---|---|---|
| Age (years) | 63.30 ± 12.14 | 60.58 ± 13.00 | 66.83 ± 9.98 | 0.004 * |
| Female, n (%) | 60 (49.2) | 37 (53.6) | 23 (43.4) | 0.263 |
| HD duration (mo) | 57.00 (25.53–119.34) | 58.20 (21.84–131.94) | 56.88 (26.70–104.82) | 0.857 |
| BMI (Kg/m2) | 24.92 ± 5.06 | 24.63 ± 5.28 | 25.29 ± 4.78 | 0.479 |
| DM, n (%) | 52 (42.6) | 19 (27.5) | 33 (62.3) | <0.001 * |
| HTN, n (%) | 59 (48.4) | 27 (39.1) | 32 (60.4) | 0.020 * |
| SBP (mmHg) | 142.47 ± 25.61 | 137.67 ± 26.59 | 148.72 ± 23.05 | 0.018 * |
| DBP (mmHg) | 76.74 ± 16.40 | 76.07 ± 15.63 | 77.60 ± 17.46 | 0.611 |
| cfPWV (m/s) | 10.07 ± 2.98 | 7.88 ± 1.17 | 12.92 ± 2.06 | <0.001 * |
| BUN (mg/dL) | 61.06 ± 15.61 | 60.77 ± 14.94 | 61.43 ± 16.58 | 0.816 |
| Creatinine (mg/dL) | 9.32 ± 2.07 | 9.36 ± 2.08 | 9.28 ± 2.09 | 0.836 |
| Calcium (mg/dL) | 9.00 ± 0.74 | 8.94 ± 0.71 | 9.07± 0.79 | 0.331 |
| IP (mg/dL) | 4.76 ± 1.26 | 4.75 ± 1.25 | 4.79 ± 1.29 | 0.862 |
| Albumin (mg/dL) | 4.17 ± 0.46 | 4.18 ± 0.47 | 4.16 ± 0.45 | 0.840 |
| TCH (mg/dL) | 144.65 ± 35.32 | 147.45 ± 39.38 | 141.00 ± 29.18 | 0.320 |
| TG (mg/dL) | 113.00 (85.50-187.00) | 106.00 (85.00–192.50) | 127.00 (85.00–184.00) | 0.437 |
| Glucose (mg/dL) | 130.50 (117.75–169.00) | 128.00 (106.50–153.50) | 137.00 (114.00–185.50) | 0.084 |
| CRP (mg/dL) | 0.41 (0.12–0.92) | 0.25 (0.08–0.79) | 0.59 (0.25–1.05) | 0.003 * |
| iPTH (pg/mL) | 204.05 (84.08–416.65) | 244.40 (121.90–445.05) | 157.60 (58.00–392.15) | 0.180 |
| Sclerostin (pmol/L) | 133.54 (90.52–175.17) | 122.04 (83.74–163.63) | 144.49 (113.34–221.37) | 0.002 * |
| DKK-1 (pmol/L) | 13.25 (7.40–22.61) | 12.74 (7.34–21.07) | 14.42 (7.69–26.70) | 0.586 |
| Urea reduction rate | 0.73 ± 0.04 | 0.74 ± 0.04 | 0.73 ± 0.04 | 0.689 |
| Kt/V (Gotch) | 1.34 ± 0.17 | 1.35 ± 0.17 | 1.33 ± 0.16 | 0.658 |
| ARB, n (%) | 36 (29.5) | 18 (26.1) | 18 (34.6) | 0.344 |
| β-blocker, n (%) | 38 (31.1) | 19 (27.5) | 19 (35.8) | 0.326 |
| CCB, n (%) | 47 (38.5) | 30 (43.5) | 17 (32.1) | 0.200 |
| Statin, n (%) | 20 (16.4) | 9 (13.0) | 11 (20.8) | 0.254 |
| Characteristic | All Participants (n = 72) | Control Group (n = 51) | High AS Group (n = 21) | p Value |
|---|---|---|---|---|
| Age (years) | 54.86 ± 16.11 | 51.61 ± 16.37 | 62.76 ± 12.59 | 0.007* |
| Female, n (%) | 41 (56.9) | 32 (62.7) | 9 (42.9) | 0.121 |
| PD vintage (months) | 46.07 ± 38.86 | 39.76 ± 38.66 | 61.38 ± 35.73 | 0.031 * |
| BMI (kg/m2) | 24.91 ± 4.43 | 24.52 ± 4.19 | 25.85 ± 4.92 | 0.249 |
| DM, n (%) | 30 (41.7) | 20 (39.2) | 10 (47.6) | 0.511 |
| HTN, n (%) | 62 (86.1) | 42 (82.4) | 20 (95.2) | 0.151 |
| CAPD, n (%) | 52 (72.2) | 36 (70.6) | 16 (76.2) | 0.630 |
| SBP (mmHg) | 142.00 ± 23.94 | 138.67 ± 24.31 | 150.10 ± 21.42 | 0.065 |
| DBP (mmHg) | 84.63 ± 13.25 | 82.51 ± 12.95 | 89.76 ± 12.87 | 0.034 * |
| cfPWV (m/s) | 9.08 ± 3.12 | 7.47 ± 1.69 | 12.98 ± 2.18 | < 0.001* |
| BUN (mg/dL) | 59.01 ± 18.65 | 58.57 ± 18.91 | 60.10 ± 18.39 | 0.755 |
| Creatinine (mg/dL) | 10.92 ± 3.24 | 10.62 ± 3.49 | 11.63 ± 2.45 | 0.235 |
| Calcium (mg/dL) | 9.04 ± 0.80 | 8.98 ± 0.73 | 9.18 ± 0.95 | 0.339 |
| IP (mg/dL) | 5.19 ± 1.43 | 5.20 ± 1.50 | 5.18 ± 1.28 | 0.964 |
| Albumin (mg/dL) | 3.74 ± 0.38 | 3.74 ± 0.42 | 3.73 ± 0.28 | 0.913 |
| TCH (mg/dL) | 171.81 ± 39.58 | 171.22 ± 38.46 | 173.24 ± 43.12 | 0.845 |
| TG (mg/dL) | 147.00 (91.50–212.75) | 139.00 (91.00–200.00) | 159.00 (108.00–232.50) | 0.511 |
| Glucose (mg/dL) | 105.00 (94.25–126.75) | 101.00 (91.00–120.00) | 117.00 (100.00–165.50) | 0.011 * |
| iPTH (pg/mL) | 250.05 (121.64–558.70) | 259.70 (120.20–585.90) | 229.20 (111.71–503.42) | 0.706 |
| CRP (mg/dL) | 0.26 (0.07–0.80) | 0.14 (0.06–0.69) | 0.32 (0.24–1.07) | 0.015 * |
| Sclerostin (pmol/L) | 173.91 (122.32–229.02) | 139.11 (106.16–196.46) | 228.33 (172.21–259.66) | <0.001 * |
| DKK-1 (pmol/L) | 10.55 (6.63–17.30) | 11.02 (6.62–16.35) | 8.53 (6.51–18.33) | 0.733 |
| Weekly Kt/V | 2.09 ± 0.43 | 2.15 ± 0.45 | 1.95 ± 0.33 | 0.070 |
| Peritoneal Kt/V | 1.74 ± 0.45 | 1.76 ± 0.46 | 1.71 ± 0.42 | 0.654 |
| Total CCr (l/week) | 59.66 ± 24.23 | 61.54 ± 25.66 | 55.09 ± 20.20 | 0.308 |
| Peritoneal CCr (l/week) | 42.33 ± 16.34 | 42.33 ± 16.88 | 42.35 ± 15.35 | 0.995 |
| ARB, n (%) | 30 (41.7) | 22 (43.1) | 8 (38.1) | 0.693 |
| β-blocker, n (%) | 25 (34.7) | 19 (37.3) | 6 (28.6) | 0.482 |
| CCB, n (%) | 35 (48.6) | 25 (49.0) | 10 (47.6) | 0.914 |
| Statin, n (%) | 20 (27.8) | 15 (29.4) | 5 (23.8) | 0.630 |
| Variables | OR | 95% CI | p | aOR | 95% CI | p |
|---|---|---|---|---|---|---|
| Age (years) | 1.052 | 1.025–1.079 | 0.0001 * | 1.064 | 1.032–1.098 | 0.0001 |
| Female | 0.563 | 0.314–1.011 | 0.055 | |||
| Dialysis duration | 1.002 | 0.997–1.007 | 0.448 | |||
| BMI | 1.038 | 0.977–1.102 | 0.226 | |||
| SBP (mmHg) | 1.019 | 1.006–1.031 | 0.003 | 1.017 | 1.003–1.032 | 0.018 |
| DBP (mmHg) | 1.009 | 0.991–1.028 | 0.335 | |||
| BUN (mg/dL) | 1.004 | 0.987–1.022 | 0.622 | |||
| Creatinine (mg/dL) | 1.007 | 0.904–1.122 | 0.900 | |||
| Calcium (mg/dL) | 1.286 | 0.877–1.885 | 0.198 | |||
| IP (mg/dL) | 0.978 | 0.787–1.215 | 0.841 | |||
| Albumin (mg/dL) | 1.228 | 0.669–2.257 | 0.508 | |||
| TCH (mg/dL) | 0.995 | 0.988–1.003 | 0.201 | |||
| TG (mg/dL) | 0.9996 | 0.997–1.002 | 0.764 | |||
| Glucose (mg/dL) | 1.010 | 1.004–1.016 | 0.001 | 1.008 | 1.002–1.015 | 0.016 |
| iPTH (pg/mL) | 0.9995 | 0.98–1.001 | 0.322 | |||
| CRP (mg/dL) | 1.915 | 1.248–2.937 | 0.003 | 2.109 | 1.196–3.717 | 0.001 |
| Sclerostin (pmol/L) | 1.010 | 1.005–1.014 | <0.0001 | 1.012 | 1.006–1.017 | 0.0001 |
| DKK–1 (pmol/L) | 1.011 | 0.985–1.038 | 0.409 | |||
| Mode n (%) | ||||||
| PD | 1 | |||||
| HD | 1.865 | 1.002–3.473 | 0.049 | |||
| Comorbidity | ||||||
| No | 1 | |||||
| DM | 1.529 | 0.650–3.598 | 0.331 | |||
| HTN | 2.643 | 0.936–7.460 | 0.066 | |||
| Both | 4.306 | 1.836–10.099 | 0.0008 | |||
| ARB | 1.029 | 0.563–1880 | 0.927 | |||
| β-blocker | 1.101 | 0.594–2.039 | 0.760 | |||
| CCB | 0.679 | 0.374–1.230 | 0.201 | |||
| Statin | 1.103 | 0.542–2.248 | 0.786 | |||
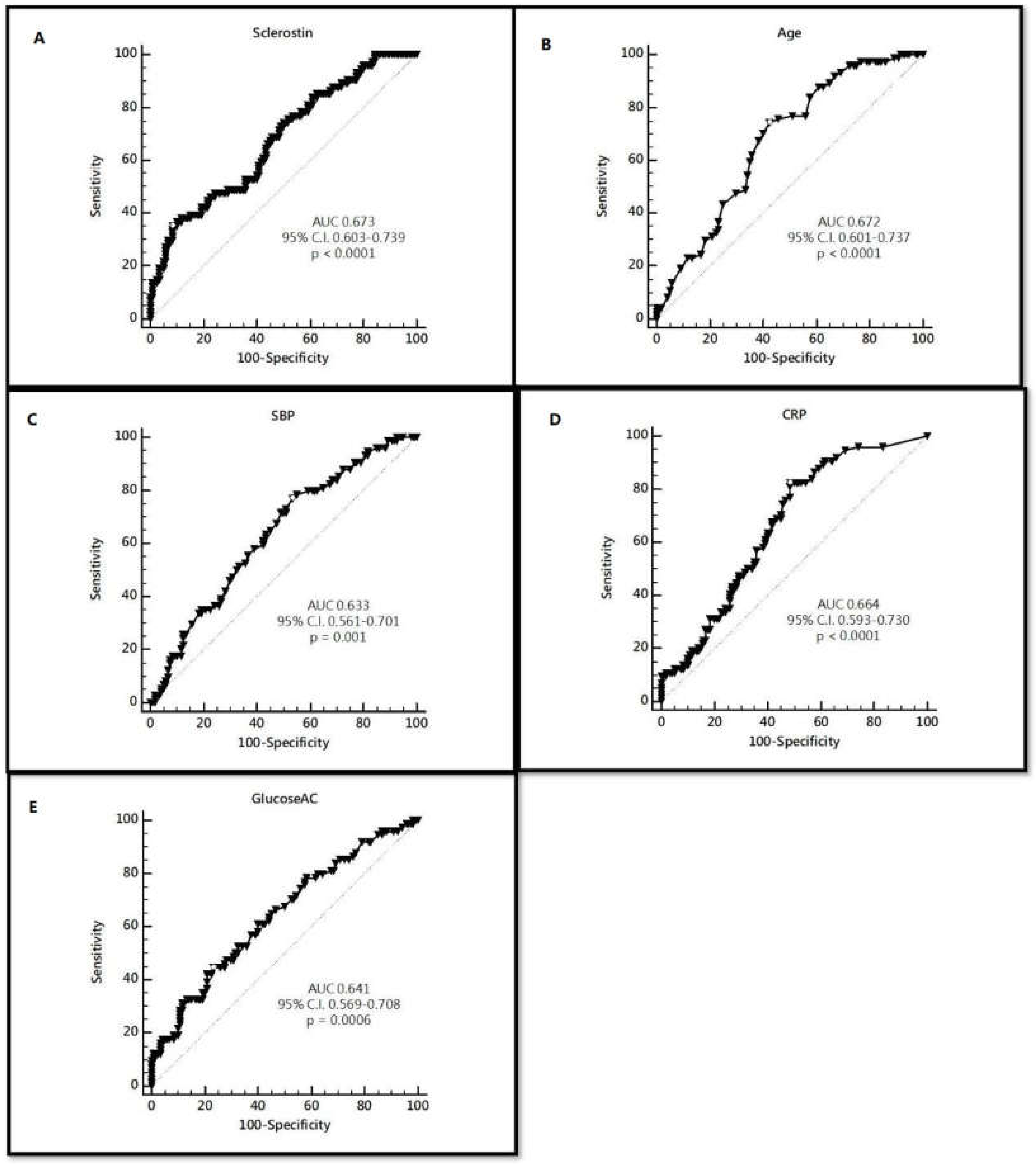
| Variables | Criterion | Sensitivity | 95% CI | Specificity | 95% CI |
|---|---|---|---|---|---|
| Sclerostin | 208.64 | 35.14 | 24.4–47.1 | 91.67 | 85.2–95.9 |
| Age | 59 | 74.32 | 62.8–83.8 | 57.50 | 48.1–66.5 |
| SBP | 132 | 77.03 | 65.8–86.0 | 46.67 | 37.5–56.0 |
| CRP | 0.2 | 82.43 | 71.8–90.3 | 51.67 | 42.4–60.9 |
| Glucose | 142 | 44.59 | 33.0–56.6 | 76.67 | 68.1–83.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-F.; Hou, J.-S.; Wang, C.-H.; Lin, Y.-L.; Lai, Y.-H.; Kuo, C.-H.; Liou, H.-H.; Tsai, J.-P.; Hsu, B.-G. Serum Sclerostin But Not DKK-1 Correlated with Central Arterial Stiffness in End Stage Renal Disease Patients. Int. J. Environ. Res. Public Health 2020, 17, 1230. https://doi.org/10.3390/ijerph17041230
Wu C-F, Hou J-S, Wang C-H, Lin Y-L, Lai Y-H, Kuo C-H, Liou H-H, Tsai J-P, Hsu B-G. Serum Sclerostin But Not DKK-1 Correlated with Central Arterial Stiffness in End Stage Renal Disease Patients. International Journal of Environmental Research and Public Health. 2020; 17(4):1230. https://doi.org/10.3390/ijerph17041230
Chicago/Turabian StyleWu, Chun-Feng, Jia-Sian Hou, Chih-Hsien Wang, Yu-Li Lin, Yu-Hsien Lai, Chiu-Huang Kuo, Hung-Hsiang Liou, Jen-Pi Tsai, and Bang-Gee Hsu. 2020. "Serum Sclerostin But Not DKK-1 Correlated with Central Arterial Stiffness in End Stage Renal Disease Patients" International Journal of Environmental Research and Public Health 17, no. 4: 1230. https://doi.org/10.3390/ijerph17041230
APA StyleWu, C.-F., Hou, J.-S., Wang, C.-H., Lin, Y.-L., Lai, Y.-H., Kuo, C.-H., Liou, H.-H., Tsai, J.-P., & Hsu, B.-G. (2020). Serum Sclerostin But Not DKK-1 Correlated with Central Arterial Stiffness in End Stage Renal Disease Patients. International Journal of Environmental Research and Public Health, 17(4), 1230. https://doi.org/10.3390/ijerph17041230







