Comparison of Liquid Chromatography Mass Spectrometry and Enzyme-Linked Immunosorbent Assay Methods to Measure Salivary Cotinine Levels in Ill Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Biological Samples
2.2. Measures
2.3. Chemical Analyses
2.4. Statistical Analyses
2.4.1. Mixed Model
2.4.2. Paired Sample t-Test
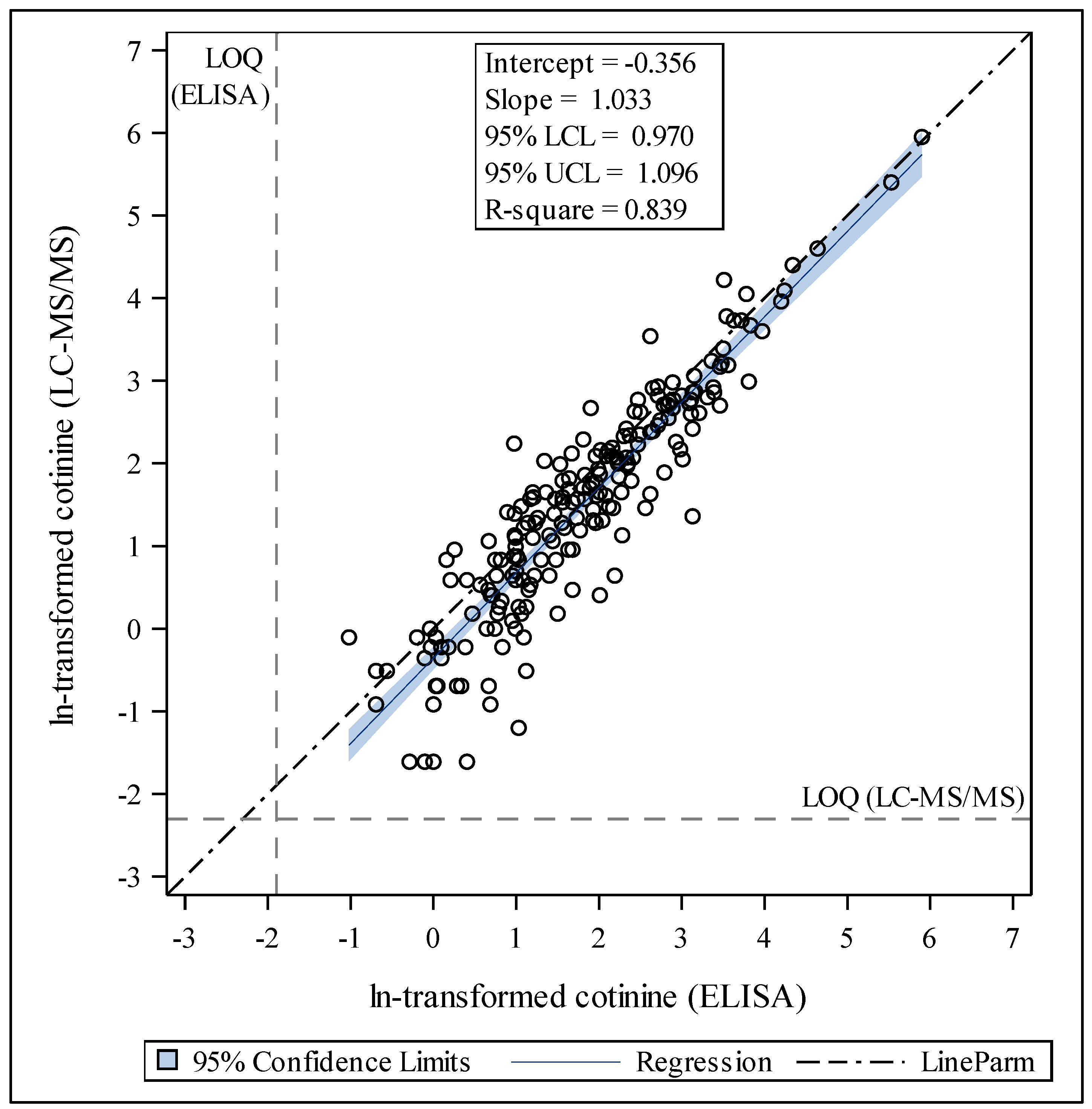
2.4.3. Linear Regression Models
3. Results
3.1. Demographics and TSE Patterns
3.2. Cotinine Measurements and Comparisons of Analyses by LC-MS/MS and ELISA Distribution of Cotinine by LC-MS/MS Compared to ELISA
3.3. Internal Consistency of LC-MS/MS and ELISA
3.4. Differences in Cotinine Results by Demographics, TSE, and Home Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Avila-Tang, E.; Al-Delaimy, W.K.; Ashley, D.L.; Benowitz, N.; Bernert, J.T.; Kim, S.; Samet, J.M.; Hecht, S.S. Assessing secondhand smoke using biological markers. Tob. Control 2013, 22, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Merino, C.; Paton, B.; Correig, X.; Ramirez, N. Biomarkers of Exposure to Secondhand and Thirdhand Tobacco Smoke: Recent Advances and Future Perspectives. Int. J. Environ. Res. Public Health 2018, 15, 2693. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.C.; Chen, S.; Trachtenberg, F.; Rokicki, S.; Adamkiewicz, G.; Levy, D.E. Validity of Self-Reported Tobacco Smoke Exposure among Non-Smoking Adult Public Housing Residents. PLoS ONE 2016, 11, e0155024. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.M.; Cheng, Y.C.; Cho, T.M.; Mishina, E.V.; Del Valle-Pinero, A.Y.; van Bemmel, D.M.; Hatsukami, D.K. Biomarkers of Potential Harm: Summary of an FDA-Sponsored Public Workshop. Nicotine Tob. Res. 2019, 21, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.M.; Edwards, S.H.; Arab, A.; Del Valle-Pinero, A.Y.; Yang, L.; Hatsukami, D.K. Biomarkers of Tobacco Exposure: Summary of an FDA-Sponsored Public Workshop. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 291–302. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Bernert, J.T.; Foulds, J.; Hecht, S.S.; Jacob, P.; Jarvis, M.J.; Joseph, A.; Oncken, C.; Piper, M.E. Biochemical Verification of Tobacco Use and Abstinence: 2019 Update. Nicotine Tob. Res. 2019. [Google Scholar] [CrossRef]
- Jacob, P., 3rd; Benowitz, N.L.; Destaillats, H.; Gundel, L.; Hang, B.; Martins-Green, M.; Matt, G.E.; Quintana, P.J.; Samet, J.M.; Schick, S.F.; et al. Thirdhand Smoke: New Evidence, Challenges, and Future Directions. Chem. Res. Toxicol. 2017, 30, 270–294. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Hukkanen, J.; Jacob, P., 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharmacol. 2009, 192, 29–60. [Google Scholar] [CrossRef]
- Benowitz, N.L. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol. Rev. 1996, 18, 188–204. [Google Scholar] [CrossRef]
- Leong, J.W.; Dore, N.D.; Shelley, K.; Holt, E.J.; Laing, I.A.; Palmer, L.J.; LeSouef, P.N. The elimination half-life of urinary cotinine in children of tobacco-smoking mothers. Pulm. Pharmacol. Ther. 1998, 11, 287–290. [Google Scholar] [CrossRef]
- Dempsey, D.A.; Sambol, N.C.; Jacob, P., 3rd; Hoffmann, E.; Tyndale, R.F.; Fuentes-Afflick, E.; Benowitz, N.L. CYP2A6 genotype but not age determines cotinine half-life in infants and children. Clin. Pharmacol. Ther. 2013, 94, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Ino, T.; Ohta, M.; Otani, T.; Hanada, S.; Sakuraoka, A.; Matsumoto, A.; Ichiba, M.; Hara, M. Enzyme-linked immunosorbent assay of nicotine metabolites. Environ. Health Prev. Med. 2010, 15, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Irvine, L.; Crombie, I.K.; Clark, R.A.; Slane, P.W.; Feyerabend, C.; Goodman, K.E.; Cater, J.I. Advising parents of asthmatic children on passive smoking: Randomised controlled trial. BMJ 1999, 318, 1456–1459. [Google Scholar] [CrossRef] [PubMed]
- Behbod, B.; Sharma, M.; Baxi, R.; Roseby, R.; Webster, P. Family and carer smoking control programmes for reducing children’s exposure to environmental tobacco smoke. Cochrane Database Syst. Rev. 2018, 1, CD001746. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.N.; Nair, U.S.; Hovell, M.F.; DiSantis, K.I.; Jaffe, K.; Tolley, N.M.; Wileyto, E.P.; Audrain-McGovern, J. Reducing Underserved Children’s Exposure to Tobacco Smoke: A Randomized Counseling Trial With Maternal Smokers. Am. J. Prev. Med. 2015, 49, 534–544. [Google Scholar] [CrossRef]
- Lepore, S.J.; Collins, B.N.; Coffman, D.L.; Winickoff, J.P.; Nair, U.S.; Moughan, B.; Bryant-Stephens, T.; Taylor, D.; Fleece, D.; Godfrey, M. Kids Safe and Smokefree (KiSS) Multilevel Intervention to Reduce Child Tobacco Smoke Exposure: Long-Term Results of a Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2018, 15, 1239. [Google Scholar] [CrossRef]
- Blaakman, S.W.; Borrelli, B.; Wiesenthal, E.N.; Fagnano, M.; Tremblay, P.J.; Stevens, T.P.; Halterman, J.S. Secondhand Smoke Exposure Reduction After NICU Discharge: Results of a Randomized Trial. Acad. Pediatr. 2015, 15, 605–612. [Google Scholar] [CrossRef]
- Halterman, J.S.; Szilagyi, P.G.; Fisher, S.G.; Fagnano, M.; Tremblay, P.; Conn, K.M.; Wang, H.; Borrelli, B. Randomized controlled trial to improve care for urban children with asthma: Results of the School-Based Asthma Therapy trial. Arch. Pediatr. Adolesc. Med. 2011, 165, 262–268. [Google Scholar] [CrossRef]
- Schoedel, K.A.; Hoffmann, E.B.; Rao, Y.; Sellers, E.M.; Tyndale, R.F. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics 2004, 14, 615–626. [Google Scholar] [CrossRef]
- Matt, G.E.; Quintana, P.J.; Liles, S.; Hovell, M.F.; Zakarian, J.M.; Jacob, P., 3rd; Benowitz, N.L. Evaluation of urinary trans-3’-hydroxycotinine as a biomarker of children’s environmental tobacco smoke exposure. Biomarkers 2006, 11, 507–523. [Google Scholar] [CrossRef]
- Hukkanen, J.; Jacob, P., 3rd; Benowitz, N.L. Metabolism and disposition kinetics of nicotine. Pharmacol. Rev. 2005, 57, 79–115. [Google Scholar] [CrossRef]
- Matt, G.E.; Quintana, P.J.; Hovell, M.F.; Bernert, J.T.; Song, S.; Novianti, N.; Juarez, T.; Floro, J.; Gehrman, C.; Garcia, M.; et al. Households contaminated by environmental tobacco smoke: Sources of infant exposures. Tob. Control 2004, 13, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Matt, G.E.; Quintana, P.J.; Destaillats, H.; Gundel, L.A.; Sleiman, M.; Singer, B.C.; Jacob, P.; Benowitz, N.; Winickoff, J.P.; Rehan, V.; et al. Thirdhand tobacco smoke: Emerging evidence and arguments for a multidisciplinary research agenda. Environ. Health Perspect. 2011, 119, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Mahabee-Gittens, E.M.; Merianos, A.L.; Hoh, E.; Quintana, P.J.; Matt, G.E. Nicotine on Children’s Hands: Limited Protection of Smoking Bans and Initial Clinical Findings. Tob. Use Insights 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Mahabee-Gittens, E.M.; Merianos, A.L.; Matt, G.E. Preliminary evidence that high levels of nicotine on children’s hands may contribute to overall tobacco smoke exposure. Tob. Control 2018, 27, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Matt, G.E.; Quintana, P.J.; Zakarian, J.M.; Fortmann, A.L.; Chatfield, D.A.; Hoh, E.; Uribe, A.M.; Hovell, M.F. When smokers move out and non-smokers move in: Residential thirdhand smoke pollution and exposure. Tob. Control 2011, 20, e1. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, D.A.; Meyers, M.J.; Oh, S.S.; Nguyen, E.A.; Fuentes-Afflick, E.; Wu, A.H.; Jacob, P.; Benowitz, N.L. Determination of tobacco smoke exposure by plasma cotinine levels in infants and children attending urban public hospital clinics. Arch. Pediatr. Adolesc. Med. 2012, 166, 851–856. [Google Scholar] [CrossRef]
- Kalkbrenner, A.E.; Hornung, R.W.; Bernert, J.T.; Hammond, S.K.; Braun, J.M.; Lanphear, B.P. Determinants of serum cotinine and hair cotinine as biomarkers of childhood secondhand smoke exposure. J. Expo. Sci. Environ. Epidemiol. 2010, 20, 615–624. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Perez-Stable, E.J.; Fong, I.; Modin, G.; Herrera, B.; Jacob, P., 3rd. Ethnic differences in N-glucuronidation of nicotine and cotinine. J. Pharmacol. Exp. Ther. 1999, 291, 1196–1203. [Google Scholar]
- Wilson, S.E.; Kahn, R.S.; Khoury, J.; Lanphear, B.P. Racial differences in exposure to environmental tobacco smoke among children. Environ. Health Perspect. 2005, 113, 362–367. [Google Scholar] [CrossRef]
- Mahabee-Gittens, E.M.; Gordon, J.S.; Krugh, M.E.; Henry, B.; Leonard, A.C. A smoking cessation intervention plus proactive quitline referral in the pediatric emergency department: A pilot study. Nicotine Tob. Res. 2008, 10, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- High Sensitivity Salivary Cotinine Quantitative Enzyme Immunoassay Kit; Salimetrics, LLC: State College, PA, USA, 2015.
- Murphy, S.E.; Wickham, K.M.; Lindgren, B.R.; Spector, L.G.; Joseph, A. Cotinine and trans 3’-hydroxycotinine in dried blood spots as biomarkers of tobacco exposure and nicotine metabolism. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 513–518. [Google Scholar] [CrossRef]
- Data Quality Assessment and Data Usability Evaluation Technical Guidance; New Jersey Department of Environmental Protection: Trenton, NJ, USA, 2014; pp. 1–132.
- Mahabee-Gittens, E.M.; Merianos, A.L.; Gordon, J.S.; Stone, L.; Semenova, O.; Matt, G.E. Electronic Health Record Classification of Tobacco Smoke Exposure and Cotinine Levels in Hospitalized Pediatric Patients. Hosp. Pediatr. 2019, 9, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Merianos, A.L.; Jandarov, R.A.; Choi, K.; Mahabee-Gittens, E.M. Tobacco smoke exposure disparities persist in U.S. children: NHANES 1999–2014. Prev. Med. 2019, 123, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Mahabee-Gittens, E.M.; Merianos, A.L.; Stone, L.; Tabangin, M.E.; Khoury, J.C.; Gordon, J.S. Tobacco Use Behaviors and Perceptions of Parental Smokers in the Emergency Department Setting. Tob. Use Insights 2019, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shenassa, E.D.; Rossen, L.M.; Cohen, J.; Morello-Frosch, R.; Payne-Sturges, D.C. Income Inequality and US Children’s Secondhand Smoke Exposure: Distinct Associations by Race-Ethnicity. Nicotine Tob. Res. 2017, 19, 1292–1299. [Google Scholar] [CrossRef]
- Tsai, J.; Homa, D.M.; Gentzke, A.S.; Mahoney, M.; Sharapova, S.R.; Sosnoff, C.S.; Caron, K.T.; Wang, L.; Melstrom, P.C.; Trivers, K.F. Exposure to Secondhand Smoke Among Nonsmokers—United States, 1988–2014. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1342–1346. [Google Scholar] [CrossRef]
- Howrylak, J.A.; Spanier, A.J.; Huang, B.; Peake, R.W.; Kellogg, M.D.; Sauers, H.; Kahn, R.S. Cotinine in children admitted for asthma and readmission. Pediatrics 2014, 133, e355–e362. [Google Scholar] [CrossRef]
- Murphy, S.E. Nicotine Metabolism and Smoking: Ethnic Differences in the Role of P450 2A6. Chem. Res. Toxicol. 2017, 30, 410–419. [Google Scholar] [CrossRef]
- Parzynski, C.S.; Jaszyna-Gasior, M.; Franken, F.H.; Moolchan, E.T. Measuring nicotine intake among highly-dependent adolescent smokers: Comparability of saliva and plasma cotinine concentrations. Pharmacol. Biochem. Behav. 2008, 89, 145–149. [Google Scholar] [CrossRef][Green Version]
- Jarvis, M.J.; Primatesta, P.; Erens, B.; Feyerabend, C.; Bryant, A. Measuring nicotine intake in population surveys: Comparability of saliva cotinine and plasma cotinine estimates. Nicotine Tob. Res. 2003, 5, 349–355. [Google Scholar] [CrossRef] [PubMed]
- SRNT Subcommittee on Biochemical Verification, Biochemical verification of tobacco use and cessation. Nicotine Tob. Res. 2002, 4, 149–159. [CrossRef] [PubMed]
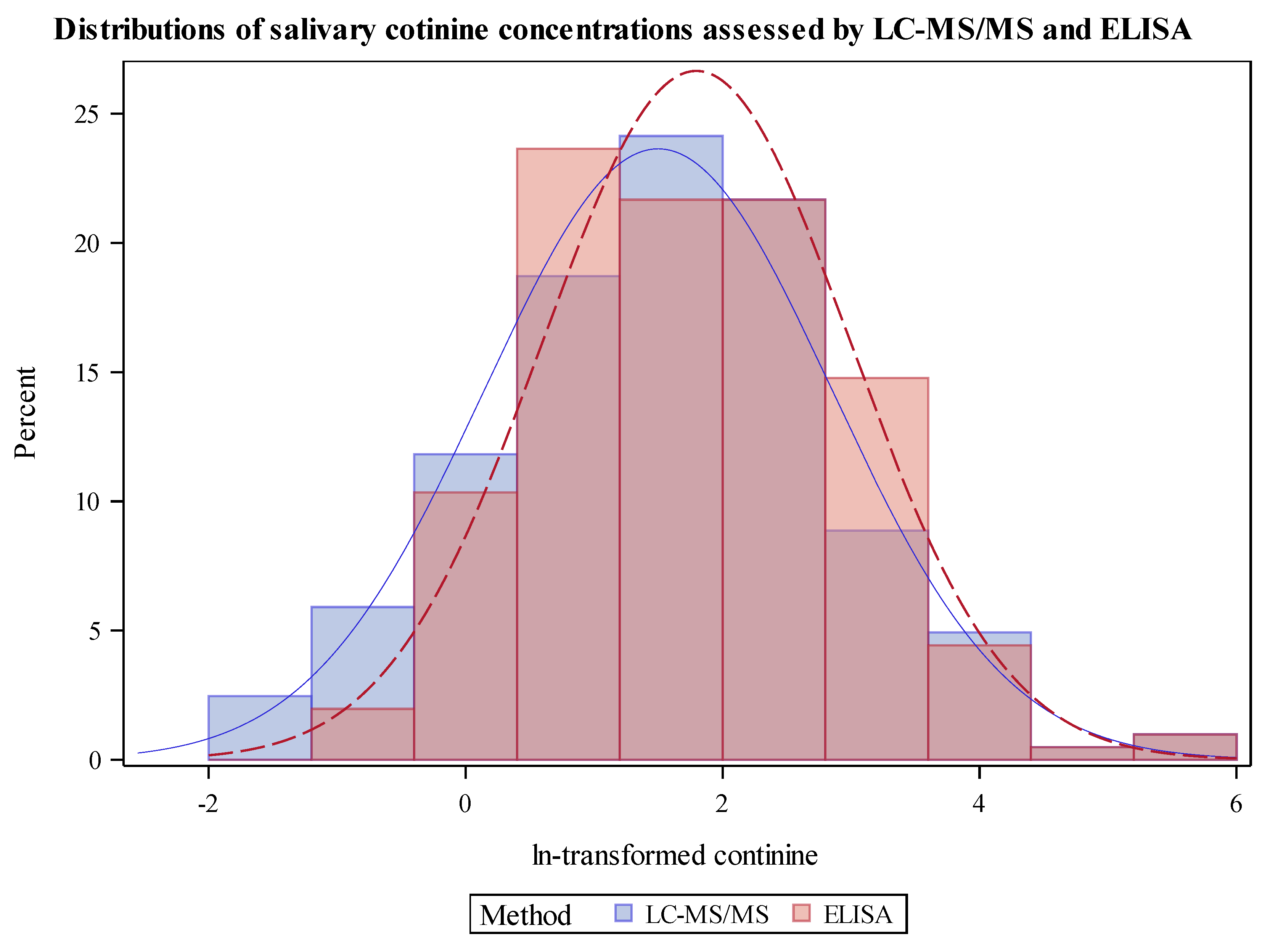
| Analyte | Method | Total Samples Analyzed | Total Samples Detected | Percent Detected | LOQ | GeoM | Geometric SD | Min | P25 | P50 | P75 | P95 | Max |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cotinine (ng/mL) | LC-MS/MS | 218 | 211 | 97% | 0.10 | 4.1 | 4.1 | <LOQ | 1.3 | 4.3 | 10.3 | 41.5 | 382 |
| ELISA | 218 | 208 | 95% | 0.15 | 5.7 | 3.5 | <LOQ | 2.2 | 5.1 | 12.2 | 41.2 | 364 |
| Internal ELISA (N = 55) | Internal LC-MS/MS (N = 20) | |||||
|---|---|---|---|---|---|---|
| Across methods (N = 203) | ICC | Median RPD * | ICC | Median RPD | ||
| Overall | 0.884 | 0.993 | 0.113 | 0.991 | 0.061 | |
| Age | 0–6 yrs (n = 122) | 0.899 | 0.992 | 0.118 | 0.996 | 0.057 |
| 7–17 yrs (n = 81) | 0.824 | 0.994 | 0.099 | 0.983 | 0.073 | |
| Sex | Male (n = 100) | 0.890 | 0.993 | 0.087 | 0.995 | 0.056 |
| Female (n = 103) | 0.869 | 0.993 | 0.156 | 0.985 | 0.073 | |
| LC-MS/MS Based Cotinine | ELISA Based Cotinine | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | % of Cohort | 95% LCL | 95% UCL | p-Value | 95% LCL | 95% UCL | p-Value | ||
| Age of child | N/A | −0.11 | −0.15 | −0.08 | < 0.0001 | −0.09 | −0.13 | −0.06 | < 0.0001 |
| # cigarettes/day smoked-caregiver | N/A | 0.09 | 0.05 | 0.12 | < 0.0001 | 0.07 | 0.04 | 0.11 | < 0.0001 |
| # cigarettes/day smoked around child | N/A | 0.01 | −0.00 | 0.02 | 0.17 | 0.01 | −0.01 | 0.02 | 0.34 |
| Sex of child | |||||||||
| Female | 51.3% | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Male | 48.7% | 0.38 | 0.02 | 0.75 | 0.04 | 0.30 | −0.06 | 0.65 | 0.10 |
| Race/ethnicity | |||||||||
| Non-Hispanic White | 36.6% | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Non-Hispanic Black | 54.3% | 0.52 | 0.10 | 0.95 | 0.04 | 0.38 | −0.03 | 0.80 | 0.16 |
| Other | 9.1% | 0.07 | −0.61 | 0.75 | 0.04 | 0.06 | −0.61 | 0.73 | 0.16 |
| Caregiver’s Annual Income | |||||||||
| Less than $5,000 | 35.5% | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| $5,001 to $15,000 | 26.9% | 0.10 | −0.35 | 0.55 | 0.01 | 0.04 | −0.40 | 0.47 | 0.02 |
| $15,001 to $30,000 | 21.3% | −0.44 | −0.93 | 0.05 | 0.01 | −0.49 | −0.97 | −0.01 | 0.02 |
| $30,001 to $50,000 | 9.1% | −1.07 | −1.74 | −0.41 | 0.01 | −0.91 | −1.56 | −0.27 | 0.02 |
| $50,001 to $75,000 | 5.6% | −0.48 | −1.29 | 0.34 | 0.01 | −0.43 | −1.23 | 0.36 | 0.02 |
| More than $75,000 | 1.5% | −0.98 | −2.50 | 0.55 | 0.01 | −1.55 | −3.04 | −0.06 | 0.02 |
| Type of home | |||||||||
| Single family house | 48.2% | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Multi-family house | 20.3% | 0.49 | 0.02 | 0.96 | 0.06 | 0.22 | −0.24 | 0.68 | 0.19 |
| Apartment | 30.0% | 0.53 | 0.10 | 0.97 | 0.06 | 0.45 | 0.02 | 0.87 | 0.19 |
| Other | 1.5% | 0.66 | −0.82 | 2.14 | 0.06 | −0.37 | −1.81 | 1.08 | 0.19 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahabee-Gittens, E.M.; Mazzella, M.J.; Doucette, J.T.; Merianos, A.L.; Stone, L.; Wullenweber, C.A.; A. Busgang, S.; Matt, G.E. Comparison of Liquid Chromatography Mass Spectrometry and Enzyme-Linked Immunosorbent Assay Methods to Measure Salivary Cotinine Levels in Ill Children. Int. J. Environ. Res. Public Health 2020, 17, 1157. https://doi.org/10.3390/ijerph17041157
Mahabee-Gittens EM, Mazzella MJ, Doucette JT, Merianos AL, Stone L, Wullenweber CA, A. Busgang S, Matt GE. Comparison of Liquid Chromatography Mass Spectrometry and Enzyme-Linked Immunosorbent Assay Methods to Measure Salivary Cotinine Levels in Ill Children. International Journal of Environmental Research and Public Health. 2020; 17(4):1157. https://doi.org/10.3390/ijerph17041157
Chicago/Turabian StyleMahabee-Gittens, E. Melinda, Matthew J. Mazzella, John T. Doucette, Ashley L. Merianos, Lara Stone, Chase A. Wullenweber, Stefanie A. Busgang, and Georg E. Matt. 2020. "Comparison of Liquid Chromatography Mass Spectrometry and Enzyme-Linked Immunosorbent Assay Methods to Measure Salivary Cotinine Levels in Ill Children" International Journal of Environmental Research and Public Health 17, no. 4: 1157. https://doi.org/10.3390/ijerph17041157
APA StyleMahabee-Gittens, E. M., Mazzella, M. J., Doucette, J. T., Merianos, A. L., Stone, L., Wullenweber, C. A., A. Busgang, S., & Matt, G. E. (2020). Comparison of Liquid Chromatography Mass Spectrometry and Enzyme-Linked Immunosorbent Assay Methods to Measure Salivary Cotinine Levels in Ill Children. International Journal of Environmental Research and Public Health, 17(4), 1157. https://doi.org/10.3390/ijerph17041157







