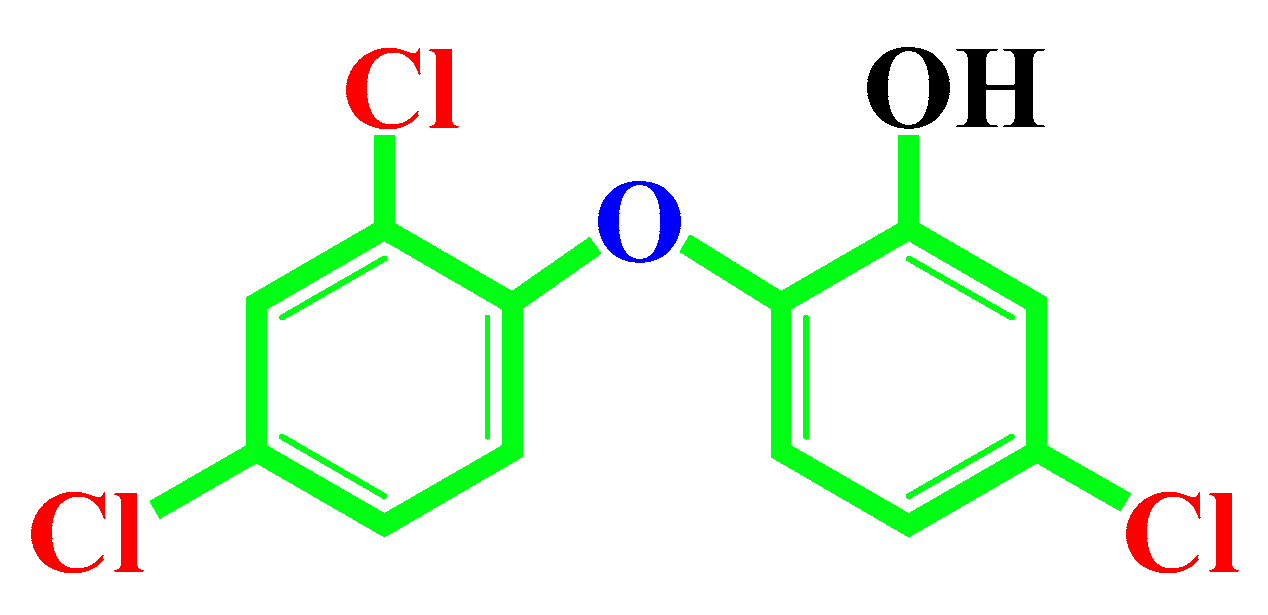
Risk Assessment of Cosmetics Using Triclosan on Future Generation’s Germ Cell Maturation via Lactating Mother Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Dose Selection
2.3. Treated Chemical Doses
2.4. Daily Sperm Production (DSP)
2.5. Immunohistochemistry
2.6. Immunofluorescence Analysis of Testes Tissues
2.7. Image Capture
2.8. RNA Isolation and Semi-Quantitative RT-PCR
2.9. Statistical Analysis
3. Results
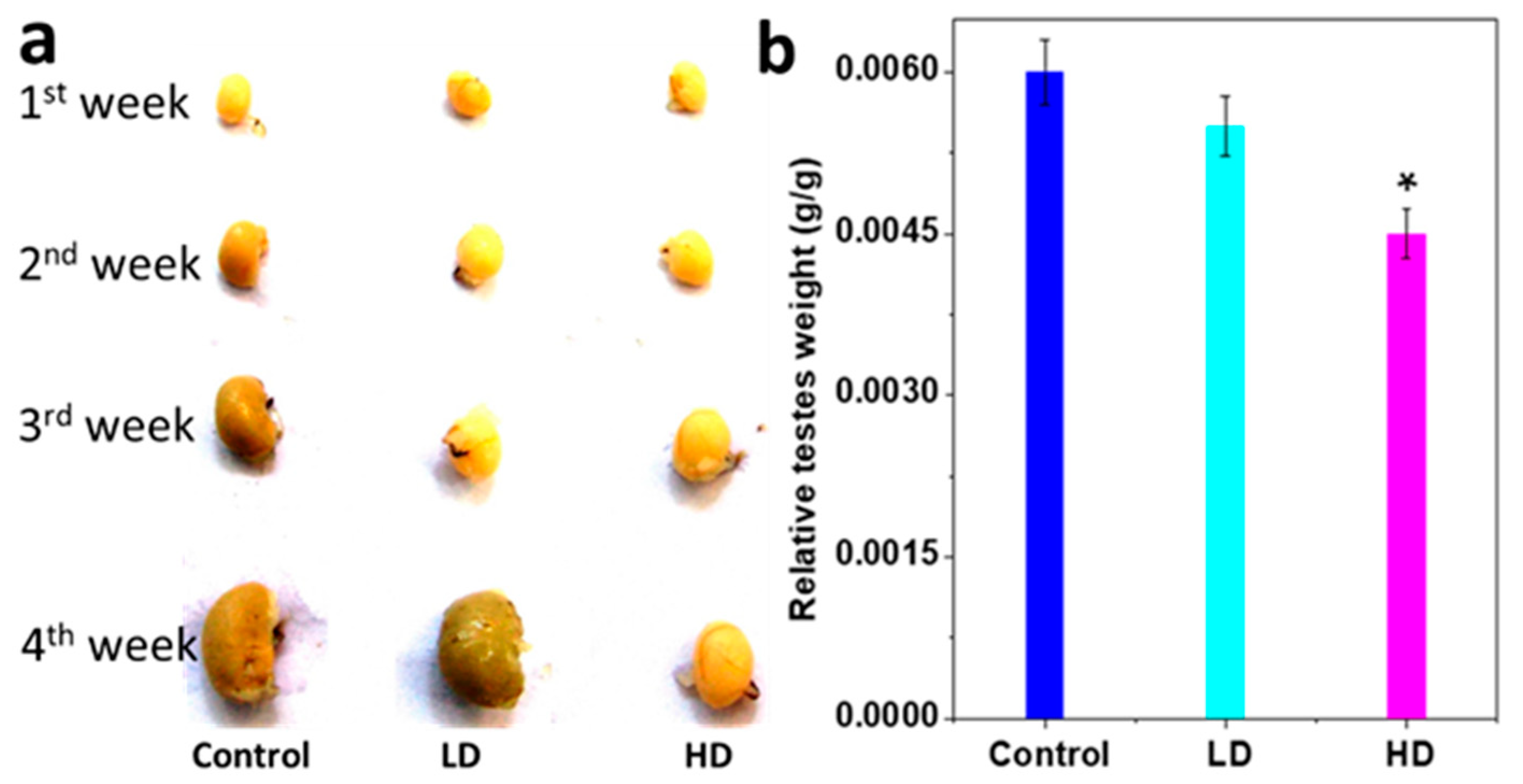
3.1. Body and Testis Growth
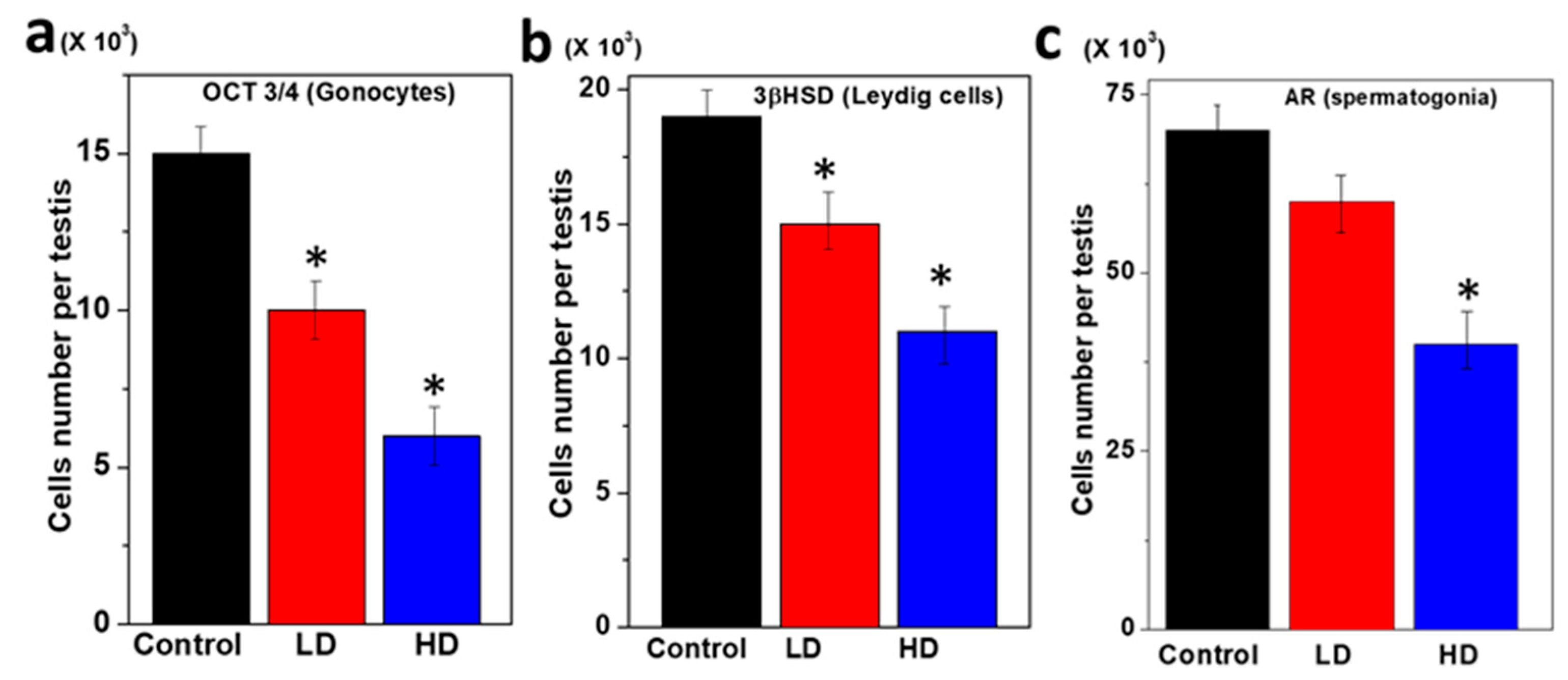
3.2. Immunopositive Staining of Germ Cell Components of Rat Pup Testes
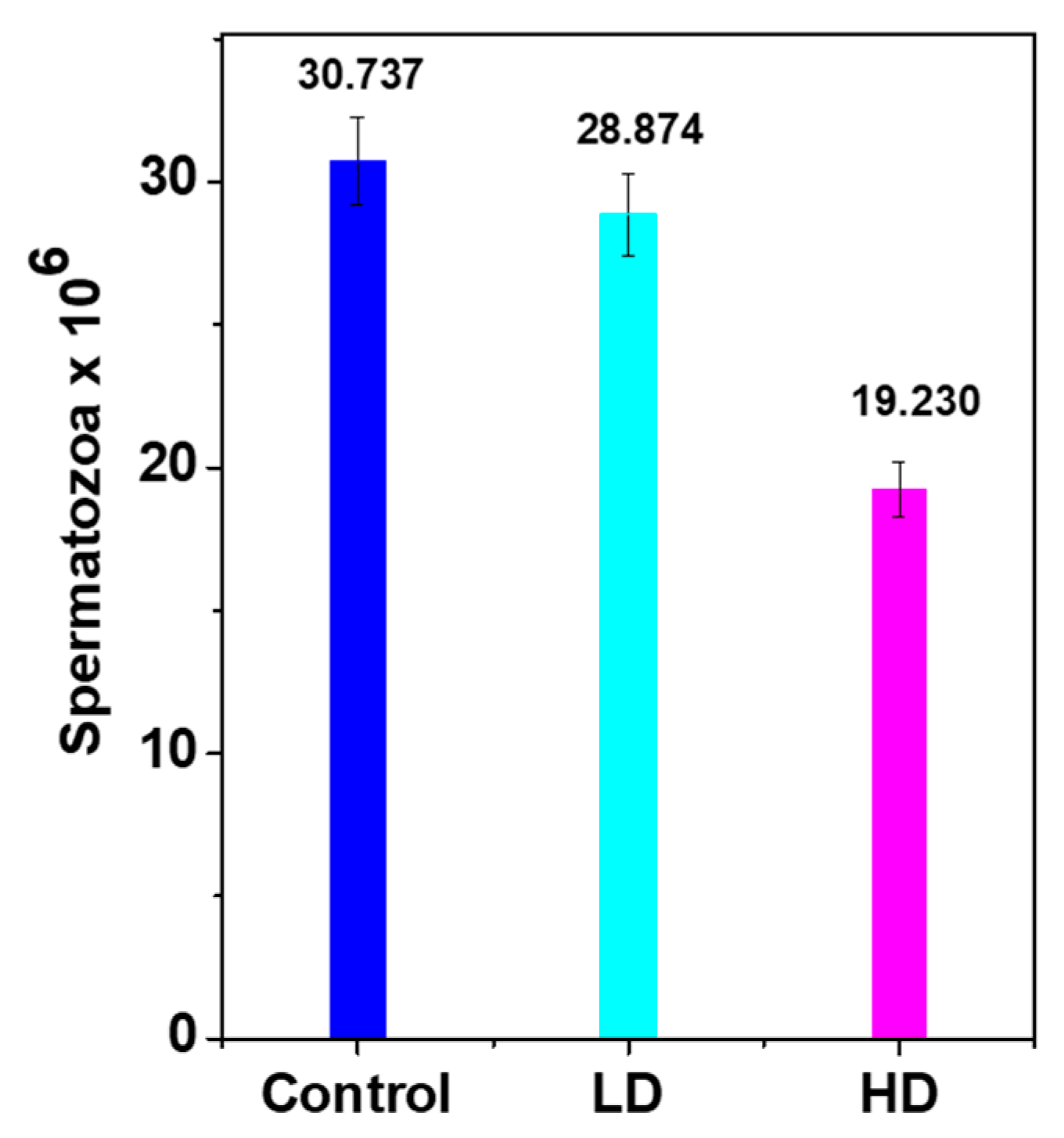
3.3. Daily Sperm Production (DSP)
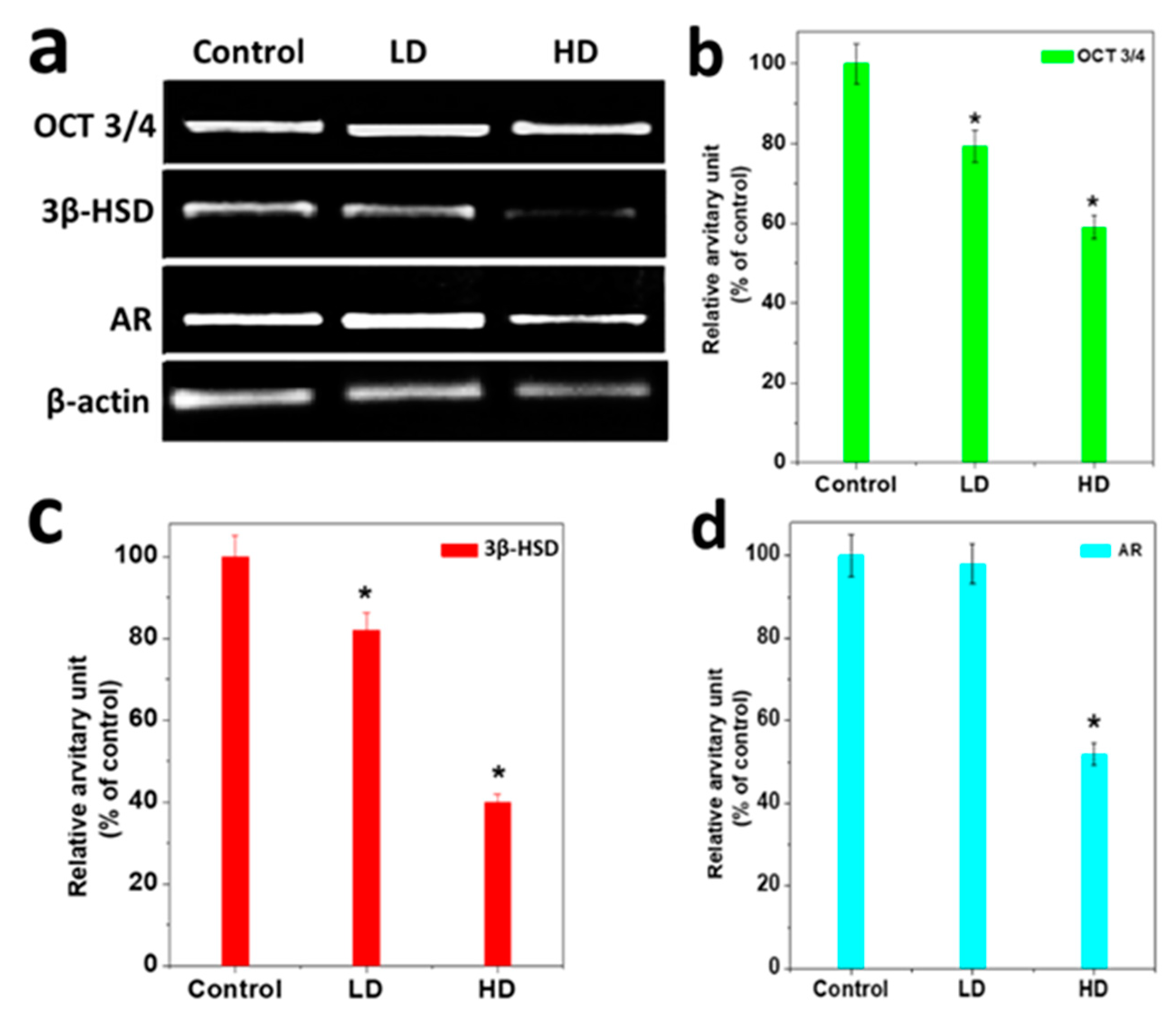
3.4. Analysis of Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, C.; Lee, H.K.; Kong, A.P.S.; Lim, L.L.; Cai, Z.; Chung, A.C. Early-life exposure to endocrine disrupting chemicals associates with childhood obesity. Ann. Pediatr. Endocrinol. Metab. 2018, 23, 182–195. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Pulicharla, R.; Brar, S.K.; Cledón, M.; Verma, M.; Surampalli, R.Y. Triclosan: Current Status, Occurrence, Environmental Risks and Bioaccumulation Potential. Int. J. Environ. Res. Public Health 2015, 12, 5657–5684. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zheng, S.; Zhao, H.; Sun, T. Investigation on the interaction between triclosan and bovine serum albumin by spectroscopic methods. J. Environ. Sci. Health Part B 2019, 55, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Kimberly, P.; Berger, K.; Kogut, R.; Bradman, A.; She, J.; Gavin, Q.; Zahedi, R.; Kimberly, L.; Kim, G. Harley Personal care product use as a predictor of urinary concentrations of certain phthalates, parabens, and phenols in the HERMOSA study. J. Exp. Sci. Environ. Epid. 2019, 29, 21–32. [Google Scholar]
- Zhang, H.; Shao, X.; Zhao, H.; Li, X.; Wei, J.; Yang, C.; Cai, Z. Integration of Metabolomics and Lipidomics Reveals Metabolic Mechanisms of Triclosan-Induced Toxicity in Human Hepatocytes. Environ. Sci. Technol. 2019, 53, 5406–5415. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Shin, C.; Choi, J.W.; Lee, J.; Lee, S.; Kim, U. Harmacokinetic profile of propyl paraben in humans after oral administration. Environ. Int. 2019, 130, 104917. [Google Scholar] [CrossRef]
- Hubal, E.A.C.; Wetmore, B.A.; Wambaugh, J.F.; El-Masri, H.; Sobus, J.R.; Bahadori, T. Advancing internal exposure and physiologically-based toxicokinetic modeling for 21st-century risk assessments. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 11–20. [Google Scholar] [CrossRef]
- Panico, A.; Serio, F.; Bagordo, F.; Grassi, T.; Idolo, A.; De Giorgi, M.; Guido, M.; Congedo, M.; De Donno, A. Skin safety and health prevention: An overview of chemicals in cosmetic products. J. Prev. Med. Hyg. 2019, 60, E50–E57. [Google Scholar]
- Lee, J.D.; Lee, J.Y.; Kwack, S.J.; Shin, C.Y.; Jang, H.-J.; Kim, H.Y.; Kim, M.K.; Seo, N.-W.; Lee, B.-M.; Kim, K.-B. Risk Assessment of Triclosan, a Cosmetic Preservative. Toxicol. Res. 2019, 35, 137–154. [Google Scholar] [CrossRef]
- Park, J.-S.; Kim, S.; Park, M.; Kim, Y.; Lee, H.; Choi, H.; Lim, S. Relationship between dietary factors and bisphenol a exposure: The second Korean National Environmental Health Survey (KoNEHS 2012–2014). Ann. Occup. Environ. Med. 2017, 29, 42. [Google Scholar] [CrossRef]
- Lama, S.; Vanacore, D.; Diano, N.; Nicolucci, C.; Errico, S.; Dallio, M.; Federico, A.; Loguercio, C.; Stiuso, P. Ameliorative effect of Silybin on bisphenol A induced oxidative stress, cell proliferation and steroid hormones oxidation in HepG2 cell cultures. Sci. Rep. 2019, 9, 3228. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yan, Z.; Liu, P.; Fan, J.; Wang, S.; Wang, P.; Zhang, T. Research Progress on Toxic Effects and Water Quality Criteria of Triclosan. Bull. Environ. Contam. Toxicol. 2019, 102, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, A.; Wirt, H.; Patten, A.; Theodore, B.; King-Heiden, T.; Theodore, B. Larval exposure to environmentally relevant concentrations of triclosan impairs metamorphosis and reproductive fitness in zebrafish. Reprod. Toxicol. 2019, 87, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Chakraborty, A.; Kural, M.R.; Roy, P. Alteration of testicular teroidogenesis and histopathology of reproductive system in male rats treated with triclosan. Reprod. Toxicol. 2009, 27, 177–185. [Google Scholar] [CrossRef]
- Zorrilla, L.M.; Gibson, E.K.; Jeffay, S.C.; Crofton, K.M.; Setzer, W.R.; Cooper, R.L.; Stoker, T.E. The Effects of Triclosan on Puberty and Thyroid Hormones in Male Wistar Rats. Toxicol. Sci. 2008, 107, 56–64. [Google Scholar] [CrossRef]
- Jung, E.-M.; An, B.-S.; Choi, K.-C.; Jeung, E.-B. Potential estrogenic activity of triclosan in the uterus of immature rats and rat pituitary GH3 cells. Toxicol. Lett. 2012, 208, 142–148. [Google Scholar] [CrossRef]
- Stoker, T.E.; Gibson, E.K.; Zorrilla, L.M. Triclosan Exposure Modulates Estrogen-Dependent Responses in the Female Wistar Rat. Toxicol. Sci. 2010, 117, 45–53. [Google Scholar] [CrossRef]
- Naseer, A.B.; Panigrahi, M.; Verma, A.D.; Sadam, A.; Sulabh, S.; Chhotaray, S.; Parida, S.; Krishnaswamy, N.; Bhushan, B. Endometrial transcript profile of progesterone-regulated genes during early pregnancy of Water Buffalo (Bubalusbubalis). Reprod. Dom. Anim. 2019, 54, 100–107. [Google Scholar]
- Lorenz, V.; Milesi, M.M.; Schimpf, M.G.; Luque, E.H.; Varayoud, J. Epigenetic disruption of estrogen receptor alpha is induced by a glyphosate-based herbicide in the preimplantation uterus of rats. Mol. Cell. Endocrinol. 2018, 480, 133–141. [Google Scholar] [CrossRef]
- Li, X.; Zeng, C.; Shang, J.; Wang, S.; Gao, X.-L.; Xue, Q. Association between serum estradiol level on the human chorionic gonadotrophin administration day and clinical outcome. Chin. Med. J. 2019, 132, 1194–1201. [Google Scholar] [CrossRef]
- Decatanzaro, D. Sex steroids as pheromones in mammals: The exceptional role of estradiol. Horm. Behav. 2015, 68, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Goblet, C.; Lewis, B.; Jacobsen, V.; Jarboe, M.; Silva, D.; Penfold, L.; Newell-Fugate, A.E. Exposure of managed red river hogs (Potamochoerusporcus) to urine from males stimulates estrous cycling and modulates fecal sex steroid metabolites in males and females. Gen. Comp. Endocrinol. 2019, 24, 113262. [Google Scholar] [CrossRef]
- Ena, L.; Lim, J.S.; Son, J.Y.; Park, Y.J.; Lee, Y.H.; Kim, J.Y.; Kwack, S.J.; Lee, B.M.; Ahn, M.-Y.; Kim, H.S. Evaluation of subchronic exposure to triclosan on hepatorenal and reproductive toxicities in prepubertal male rats. J. Toxicol. Environ. Health Part A 2018, 81, 421–431. [Google Scholar] [CrossRef] [PubMed]
- De Campos, P.; Oliveira, I.M.; De Souza, J.S.; Da Conceição, R.R.; Giannocco, G.; Chiamolera, M.I.; Silva, M.R.-D.; Romano, M.A.; Romano, R.M. Maternal bisphenol A exposure disrupts spermatogenesis in adult rat offspring. J. Toxicol. Environ. Health Part A 2019, 82, 163–175. [Google Scholar] [CrossRef]
- Migone, F.F.; Hung, P.-H.; Cowan, R.G.; Selvaraj, V.; Suarez, S.S.; Quirk, S.M. Overactivation of hedgehog signaling in the developing Müllerian duct interferes with duct regression in males and causes subfertility. Reproduction 2017, 153, 481–492. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Migrenne, S.; Moreau, E.; Pakarinen, P.; Dierich, A.; Merlet, J.; Habert, R.; Racine, C. Mouse Testis Development and Function Are Differently Regulated by Follicle-Stimulating Hormone Receptors Signaling During Fetal and Prepubertal Life. PLoS ONE 2012, 7, e53257. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Cao, M.; Chen, S.; Zhao, Y.; Chen, L.; Li, C.; Zhou, X. Effective and economical column-based method for RNA isolation from animal cells. Biotechnol. Lett. 2019, 41, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Pfaffa, W.D.; Baum, M.J. Hormone-dependent medial preoptic/lumbar spinal cord/autonomiccoordination supporting male sexual behaviors. Mol. Cell. Endocrinol. 2018, 467, 21–30. [Google Scholar] [CrossRef]
- Alfhili, M.A.; Lee, M.-H. Triclosan: An Update on Biochemical and Molecular Mechanisms. Oxidative Med. Cell. Longev. 2019, 2019, 1607304. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.P.A.; Ramos, J.G.; Campos, M.S.; Lima, D.D.S.; Brito, P.V.D.A.; Mendes, E.P.; Taboga, S.R.; Biancardi, M.F.; Ghedini, P.C.; Santos, F.C.A. Bisphenol-S promotes endocrine-disrupting effects similar to those promoted by bisphenol-A in the prostate of adult gerbils. Reprod. Toxicol. 2019, 85, 83–92. [Google Scholar] [CrossRef]
- Elsheikh, A.S.; Fadul, T.F.; Aboagla, E.M.-E.; Gameel, A.A.R. Effects of potassium bromate on male rat growth and testicular histology. Asian Pac. J. Reprod. 2016, 5, 376–380. [Google Scholar] [CrossRef]
- Reynolds, R.W.; Bryson, G. Effect of estradoil on the hypothalamic regulation of body weight in the rat. Res. Commun. Chem. Pathol. Pharmacol. 1974, 7, 715–724. [Google Scholar] [PubMed]
- Hond, E.D.; Tournaye, H.; De Sutter, P.; Ombelet, W.; Baeyens, W.; Covaci, A.; Cox, B.; Nawrot, T.S.; Van Larebeke, N.; D’Hooghe, T. Human exposure to endocrine disrupting chemicals and fertility: A case–control study in male subfertility patients. Environ. Int. 2015, 84, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Toft, G.; Jonsson, B.A.; Lindh, C.H.; Giwercman, A.; Spano, M.; Heederik, D.; Lenters, V.; Vermeulen, R.; Rylander, L.; Pedersen, H.S.; et al. Exposure to perfluorinated compounds and human semen quality in Arctic and European populations. Hum. Reprod. 2014, 27, 2532–2540. [Google Scholar] [CrossRef]
- Zoeller, R.T.; Doan, L.; Demeneix, B.; Gore, A.C.; Nadal, A.; Tan, S. Update on Activities in Endocrine Disruptor Research and Policy. Endocrinol. 2019, 160, 1681–1683. [Google Scholar] [CrossRef]
- Sarkar, D.; Singh, S.K. Inhibition of testicular steroidogenesis and impaired differentiation of Sertoli cells in peripubertal mice offspring following maternal exposure to BDE-209 during lactation suppress germ cell proliferation. Toxicol. Lett. 2018, 290, 83–96. [Google Scholar] [CrossRef]
- Hummitzsch, K.; Hatzirodos, N.; Irving-Rodgers, H.F.; Hartanti, M.D.; Perry, V.E.A.; Anderson, R.A.; Rodgers, R.J. Morphometric analyses and gene expression related to germ cells, gonadal ridge epithelial-like cells and granulosa cells during development of the bovine fetal ovary. PLoS ONE 2019, 14, e0214130. [Google Scholar] [CrossRef]
- Hildorf, S.; Dong, L.; Thorup, J.; Clasen-Linde, E.; Andersen, C.Y.; Cortes, D. Sertoli Cell Number Correlates with Serum Inhibin B in Infant Cryptorchid Boys. Sex. Dev. 2019, 13, 74–82. [Google Scholar] [CrossRef]
| Antigen | Supplier | Species | Retrieval | Dilution |
|---|---|---|---|---|
| 3βHSD | Santa Cruz Biotechnology | Rabbit | No | 1:1000 |
| Androgen receptor | Santa Cruz Biotechnology | Rabbit | Yes | 1:1000 |
| OCT 3/4 | Santa Cruz Biotechnology | Rabbit | Yes | 1:1000 |
| Gene | Primer Sequence | Product Size (bp) | Gene Bank Accession No. |
|---|---|---|---|
| 3β HSD | F-CCGCAAGTATTCATGACAGA R-CCGCAAGTATCATGACAGA | 547 | M38178 |
| AR | F-TTACGAAGTGGGCATGATGA R-ATCTTGTCCAGGACTCGGTG | 570 | M10133 |
| OCT3/4 | F:TCACTCACATCGCCAATCAG R:CCTGTAGCCTCATACTCTTCTC | 305 | M10022 |
| β-Actin | F-TCACCCACACTGTGCCCCATCTACGA R-CAGCGGAACCGCTCATTGCCAATGG | 300 | NM001101.2 |
| Groups | Body Weight (g ± S.E.M) | Testes Weight (g ± S.E.M) | |
|---|---|---|---|
| Initial | Final | ||
| Control (0 mg/kg/day) | 7 ± 0.70711 | 43 ± 0.85391 | 0.26 ± 0.01813 |
| LD (3 mg/kg/day) | 8 ± 0.44721 | 39 ± 0.91287 * | 0.22 ± 0.02225 |
| HD (5 mg/kg/day) | 7 ± 0.6 | 28 ± 1.47196 * | 0.13 ± 0.01633 * |
| No | Name of Germ Cells | Morphology | Antibodies and Their Nature of Binding | ||
|---|---|---|---|---|---|
| OCT 3/4 | 3βHSD | AR | |||
| 1 | Gonocytes | Small, round, single | positive | Negative | Mostly Negative |
| 2 | Leydig cells | Large, elongated, single | Negative | positive | Negative |
| 3 | Spermatogonia | Round, single, bids like | Mostly Negative | Negative | Positive |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandal, T.K.; Parvin, N.; Joo, S.W.; Roy, P. Risk Assessment of Cosmetics Using Triclosan on Future Generation’s Germ Cell Maturation via Lactating Mother Rats. Int. J. Environ. Res. Public Health 2020, 17, 1143. https://doi.org/10.3390/ijerph17041143
Mandal TK, Parvin N, Joo SW, Roy P. Risk Assessment of Cosmetics Using Triclosan on Future Generation’s Germ Cell Maturation via Lactating Mother Rats. International Journal of Environmental Research and Public Health. 2020; 17(4):1143. https://doi.org/10.3390/ijerph17041143
Chicago/Turabian StyleMandal, Tapas K., Nargish Parvin, Sang Woo Joo, and Partha Roy. 2020. "Risk Assessment of Cosmetics Using Triclosan on Future Generation’s Germ Cell Maturation via Lactating Mother Rats" International Journal of Environmental Research and Public Health 17, no. 4: 1143. https://doi.org/10.3390/ijerph17041143
APA StyleMandal, T. K., Parvin, N., Joo, S. W., & Roy, P. (2020). Risk Assessment of Cosmetics Using Triclosan on Future Generation’s Germ Cell Maturation via Lactating Mother Rats. International Journal of Environmental Research and Public Health, 17(4), 1143. https://doi.org/10.3390/ijerph17041143







