Pollution, Particles, and Dementia: A Hypothetical Causative Pathway
Abstract
:1. Introduction
The Original Hypothesis
2. Discussion
2.1. Explaining the Epidemiologic Associations
2.2. From Lung to Other Organs; How Is the Message Sent?
2.3. Biologic Microparticles
2.4. Environmental Risk Factors for Dementia
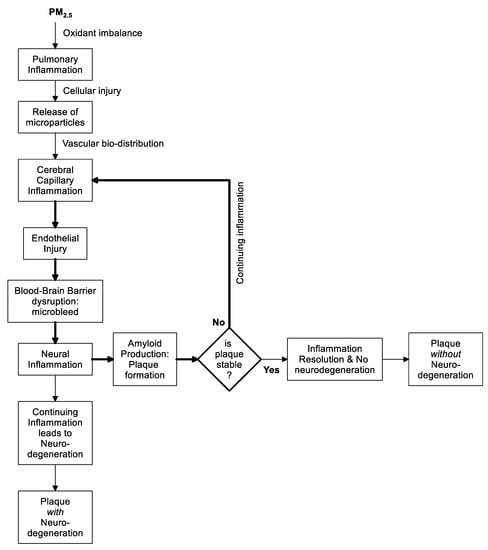
3. Conclusions
The Hypothesis
Author Contributions
Conflicts of Interest
References
- Seaton, A. Farewell King Coal. In From Industrial Triumph to Climate Disaster; Dunedin Academic Press: Edinburgh, UK, 2018. [Google Scholar]
- Schwartz, J.; Marcus, A. Mortality and air pollution in London: a time series analysis. Am. J. Epidemiology 1990, 131, 185–194. [Google Scholar] [CrossRef]
- Dockery, D.W.; Pope, C.A.; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G.; Speizer, F.E. An Association between Air Pollution and Mortality in Six U.S. Cities. New Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef] [Green Version]
- Kilian, J.; Kitazawa, M. The emerging risk of air pollution exposure on cognitive decline and Alzheimer’s disease—evidence from human and animal studies. Biomed. J. 2018, 41, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Carey, I.M.; Anderson, H.R.; Atkinson, R.W.; Beevers, S.D.; Cook, D.G.; Strachan, D.P.; Dajnak, D.; Gulliver, J.; Kelly, F.J. Are noise and air pollution related to the incidence of dementia? A cohort study in London, England. BMJ Open 2018, 8, e022404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderón-Garcidueñas, L.; Villarreal-Ríos, R. Living close to heavy traffic roads, air pollution, and dementia. Lancet 2017, 389, 675–677. [Google Scholar] [CrossRef]
- Clifford, A.; Lang, L.; Chen, R.; Anstey, K.J.; Seaton, A. Exposure to air pollution and cognitive functioning across the life course – A systematic literature review. Environ. Res. 2016, 147, 383–398. [Google Scholar] [CrossRef]
- Seaton, A.; Godden, D.; MacNee, W.; Donaldson, K. Particulate air pollution and acute health effects. Lancet 1995, 345, 176–178. [Google Scholar] [CrossRef]
- Ferin, J.; Oberdörster, G.; Penney, D.P.; Soderholm, S.C.; Gelein, R.; Piper, H.C. Increased pulmonary toxicity of ultra-fine particles? 1. Particle clearance, translocation, morphology. J. Aerosol. Sci. 1990, 21, 381–384. [Google Scholar] [CrossRef]
- Seaton, A.; Soutar, A.; Crawford, V.; Elton, R.; McNerlan, S.; Cherrie, J.; Watt, M.; Agius, R.; Stout, R. Particulate air pollution and the blood. Thorax 1999, 54, 1027–1032. [Google Scholar] [CrossRef] [Green Version]
- Royal Society and the Royal Academy of Engineering. Nanoscience and nanotechnologies: Opportunities and Uncertainties; The Royal Society: London, UK, 2004. [Google Scholar]
- Seaton, A.; Tran, L.; Aitken, R.; Donaldson, K. Nanoparticles, human health hazard and regulation. J. Roy. Soc. Interface 2010, 7, S119–S129. [Google Scholar] [CrossRef] [Green Version]
- Brunekreef, B.; Holgate, S.T. Air pollution and health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef]
- Li, X.Y.; Gilmour, P.S.; Donaldson, K.; McNee, W. Free radical and pro-inflammatory activity of particulate air pollution in vivo and in vitro. Thorax 1996, 51, 1216–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neymar, A.; Hoet, P.; Vanquickenborne, B.; Dinsdale, D.; Thoneer, M.; Hoylaerts, M.F.; Vanbilloen, H.; Mortelmans, L.; Nemery, B. Passage of inhaled particles into the blood in humans. Circulation 2002, 105, 411–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemens, C.; Turner, S.; Dibben, C. Maternal exposure to ambient air pollution and fetal growth in NE Scotland: A population study using routine ultra-sound scans. Environ. Int. 2017, 107, 216–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of Inhaled Ultrafine Particles to the Brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Kermandizadeh, N.V.; Balharry, D.; Wallin, H.; Loft, S.; Moller, P. Nanoparticle translocation—the biokinetics, tissue accumulation, toxicity and fate of materials in secondary organs. A review. Crit. Rev. Toxicol. 2015, 45, 837–872. [Google Scholar] [CrossRef]
- Miller, M.R.; Raftis, J.B.; Langrish, J.P.; McLean, S.G.; Samutrtai, P.; Connell, S.P.; Wilson, S.; Vesey, A.T.; Fokkens, P.H.B.; Boere, A.J.F.; et al. Correction to“Inhaled Nanoparticles Accumulate at Sites of Vascular Disease. ” ACS Nano 2017, 11, 10623–10624. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Semmler, M.; Erbe, F.; Mayer, P.; Takenaka, S.; Schulz, H.; Oberdörster, G.; Ziesenis, A. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J. Toxicol. Environ. Heal. Part A 2002, 65, 1513–1530. [Google Scholar] [CrossRef]
- Boulanger, C.M.; Amabile, N.; Tedgui, A. Circulating microparticles: A potential prognostic marker for atherosclerotic vascular disease. Hypertension 2006, 48, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Amabile, N.; Rautou, P.-E.; Tedgui, A.; Boulanger, C.M. Microparticles: Key Protagonists in Cardiovascular Disorders. Semin. Thromb. Hemost. 2010, 36, 907–916. [Google Scholar] [CrossRef]
- Reid, V.; Webster, N.R. Role of microparticles in sepsis. Br. J. Anaesth. 2012, 109, 503–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pope, C.A.; Bhatnagar, A.; McCracken, J.P.; Abplanalp, W.; Conklin, D.J.; O’Toole, T. Exposure to Fine Particulate Air Pollution Is Associated with Endothelial Injury and Systemic Inflammation. Circ. Res. 2016, 119, 1204–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morel, O.; Toti, F.; Morel, M.; Freyssinet, J.-M. Microparticles in endothelial cell and vascular homeostasis: Are they really noxious? Haematologica 2009, 94, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Whalley, L.J. Understanding Brain Aging and Dementia; Columbia University Press: New York, NY, SUA, 2015. [Google Scholar]
- Robertson, M.; Seaton, A.; Whalley, L.J. Can we reduce the risk of dementia? QJM 2015, 108, 93–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Hu, Z.; Wei, L.; Ma, Y.; Liu, Z.; Copeland, J.R. Incident Dementia in a Defined Older Chinese Population. PLoS ONE 2011, 6, 24817. [Google Scholar] [CrossRef] [Green Version]
- Jick, H.; Zornberg, G.L.; Jick, S.S.; Seshadri, S.; A Drachman, D. Statins and the risk of dementia. Lancet 2000, 356, 1627–1631. [Google Scholar] [CrossRef]
- Shah, A.S.V.; Lee, K.K.; A McAllister, D.; Hunter, A.; Nair, H.; Whiteley, W.; Langrish, J.P.; E Newby, D.; Mills, N.L. Short term exposure to air pollution and stroke: systematic review and meta-analysis. BMJ 2015, 350, h1295. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.-T.; Beiser, A.S.; Breteler, M.M.B.; Fratiglioni, L.; Helmer, C.; Hendrie, H.C.; Honda, H.; Ikram, M.A.; Langa, K.M.; Lobo, A.; et al. The changing prevalence and incidence of dementia over time — current evidence. Nat. Rev. Neurol. 2017, 13, 327–339. [Google Scholar] [CrossRef] [Green Version]
- Hosseinzadeh, S.; Noroozian, M.; Mousavizadeh, K. Plasma microparticles in Alzheimer’s disease: The role of vascular dysfunction. Metab. Brain Dis. 2018, 33, 293–299. [Google Scholar] [CrossRef]
- Xue, S.; Cai, X.; Li, W.; Zhang, Z.; Dong, W.; Hui, G. Elevated Plasma Endothelial Microparticles in Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. 2012, 34, 174–180. [Google Scholar] [CrossRef]
- LaHousse, L.; Vernooij, M.W.; Darweesh, S.K.L.; Akoudad, S.; Loth, D.W.; Joos, G.F.; Hofman, A.; Stricker, B.H.; Ikram, M.A.; Brusselle, G.G. Chronic Obstructive Pulmonary Disease and Cerebral Microbleeds. The Rotterdam Study. Am. J. Respir. Crit. Care Med. 2013, 188, 783–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubo, H.; Takahashi, T. The role of microparticles in chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 303–314. [Google Scholar]
- LaFerla, F.M.; Green, K.N.; Oddo, S. Intracellular amyloid-β in Alzheimer’s disease. Nature Rev. Neurosci. 2007, 8, 499–509. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seaton, A.; Tran, L.; Chen, R.; Maynard, R.L.; Whalley, L.J. Pollution, Particles, and Dementia: A Hypothetical Causative Pathway. Int. J. Environ. Res. Public Health 2020, 17, 862. https://doi.org/10.3390/ijerph17030862
Seaton A, Tran L, Chen R, Maynard RL, Whalley LJ. Pollution, Particles, and Dementia: A Hypothetical Causative Pathway. International Journal of Environmental Research and Public Health. 2020; 17(3):862. https://doi.org/10.3390/ijerph17030862
Chicago/Turabian StyleSeaton, Anthony, Lang Tran, Ruoling Chen, Robert L. Maynard, and Lawrence J. Whalley. 2020. "Pollution, Particles, and Dementia: A Hypothetical Causative Pathway" International Journal of Environmental Research and Public Health 17, no. 3: 862. https://doi.org/10.3390/ijerph17030862
APA StyleSeaton, A., Tran, L., Chen, R., Maynard, R. L., & Whalley, L. J. (2020). Pollution, Particles, and Dementia: A Hypothetical Causative Pathway. International Journal of Environmental Research and Public Health, 17(3), 862. https://doi.org/10.3390/ijerph17030862