Adding Zero-Valent Iron to Enhance Electricity Generation during MFC Start-Up
Abstract
1. Introduction
2. Materials and Methods
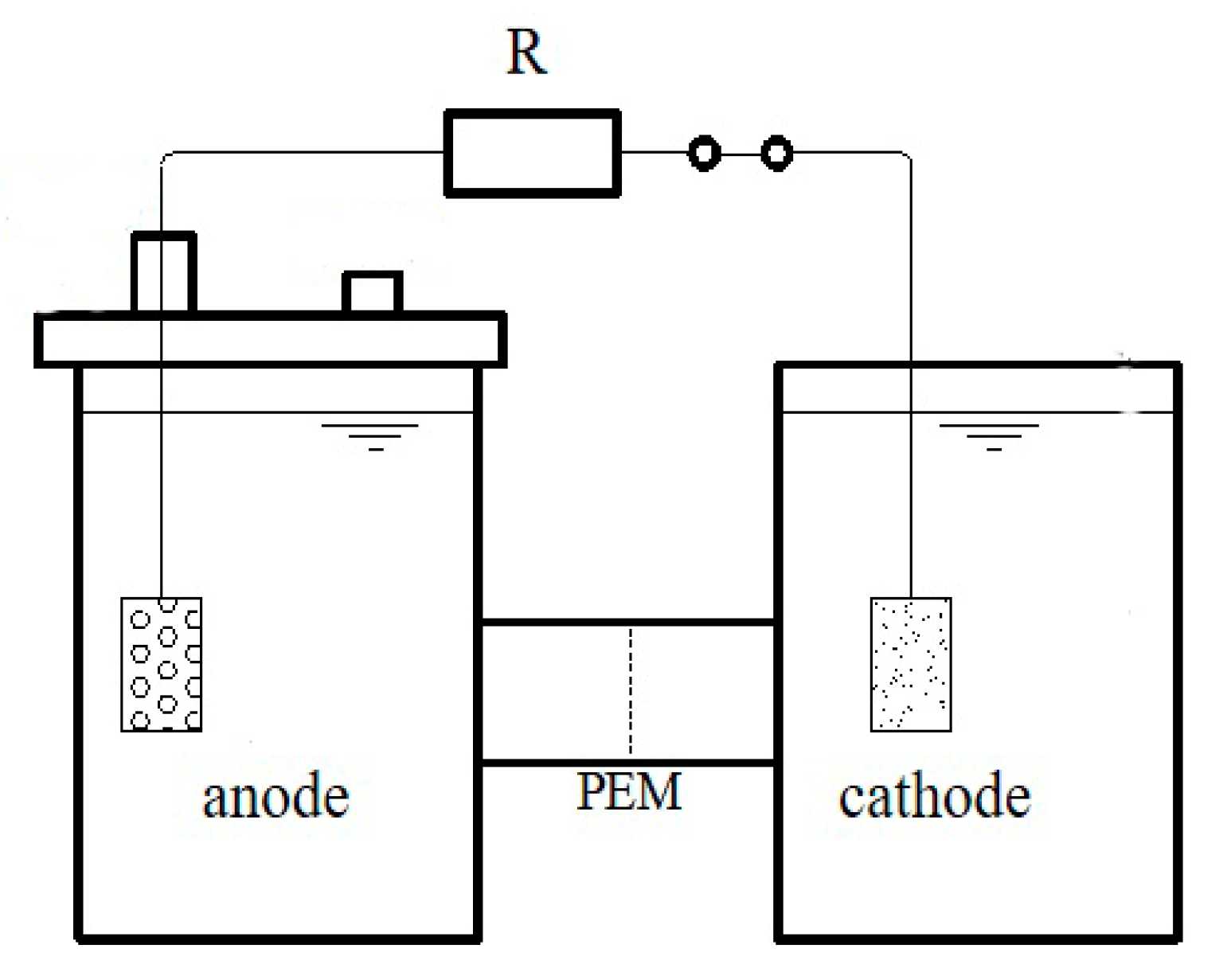
2.1. Experimental Device
2.2. Experimental Methods
2.3. Electrochemical Analysis
2.4. Chemical Analysis
2.5. High-Throughput Sequencing (Miseq)
3. Results and Discussion
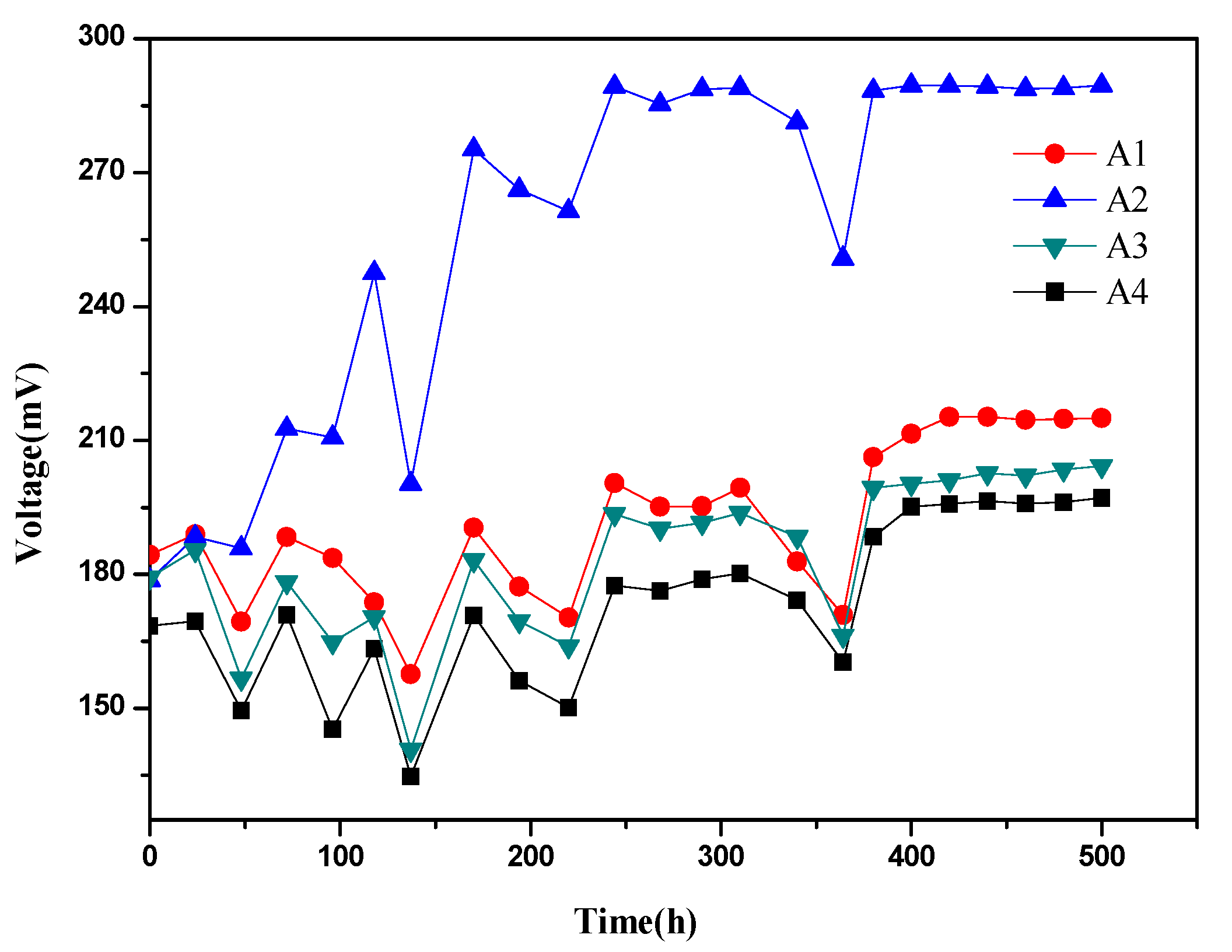
3.1. Effect of Zero-Valent Iron on Voltage of MFCs during Start-Up
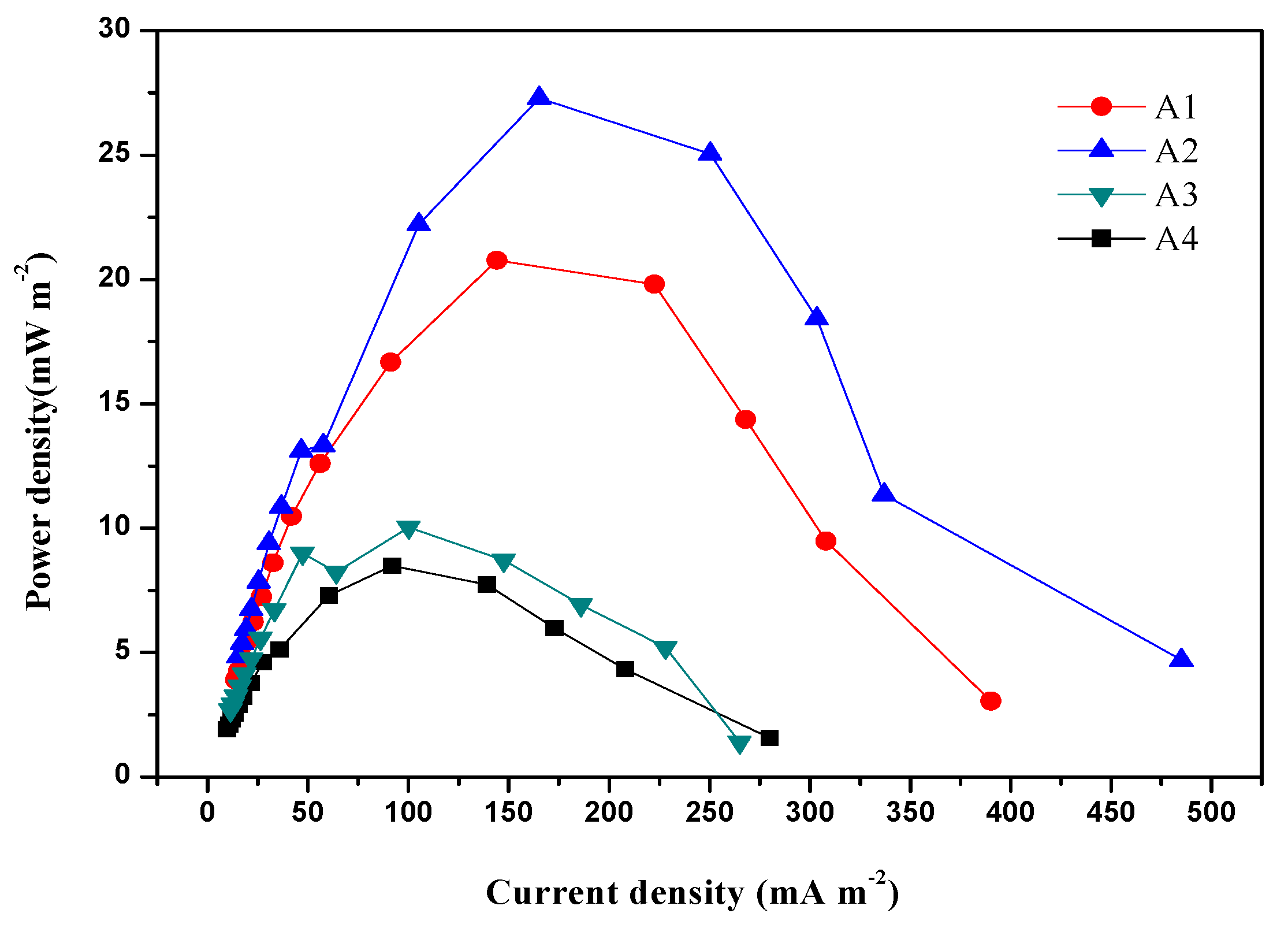
3.2. Effect of Zero-Valent Iron on Power Density of MFCs during Start-Up
3.3. Electrochemical Activity of MFCs
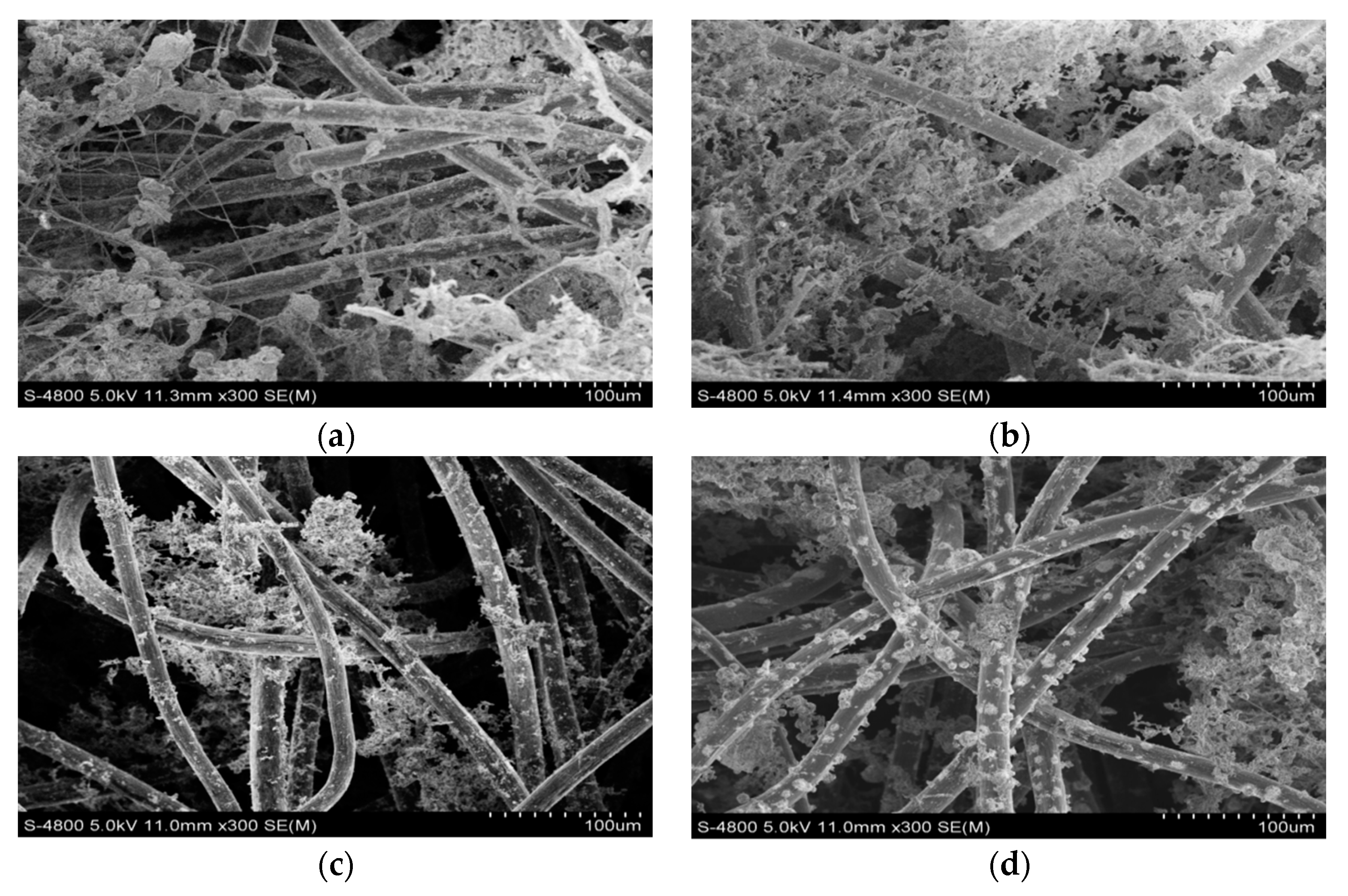
3.4. SEM Images of the Anode Surface of MFCs
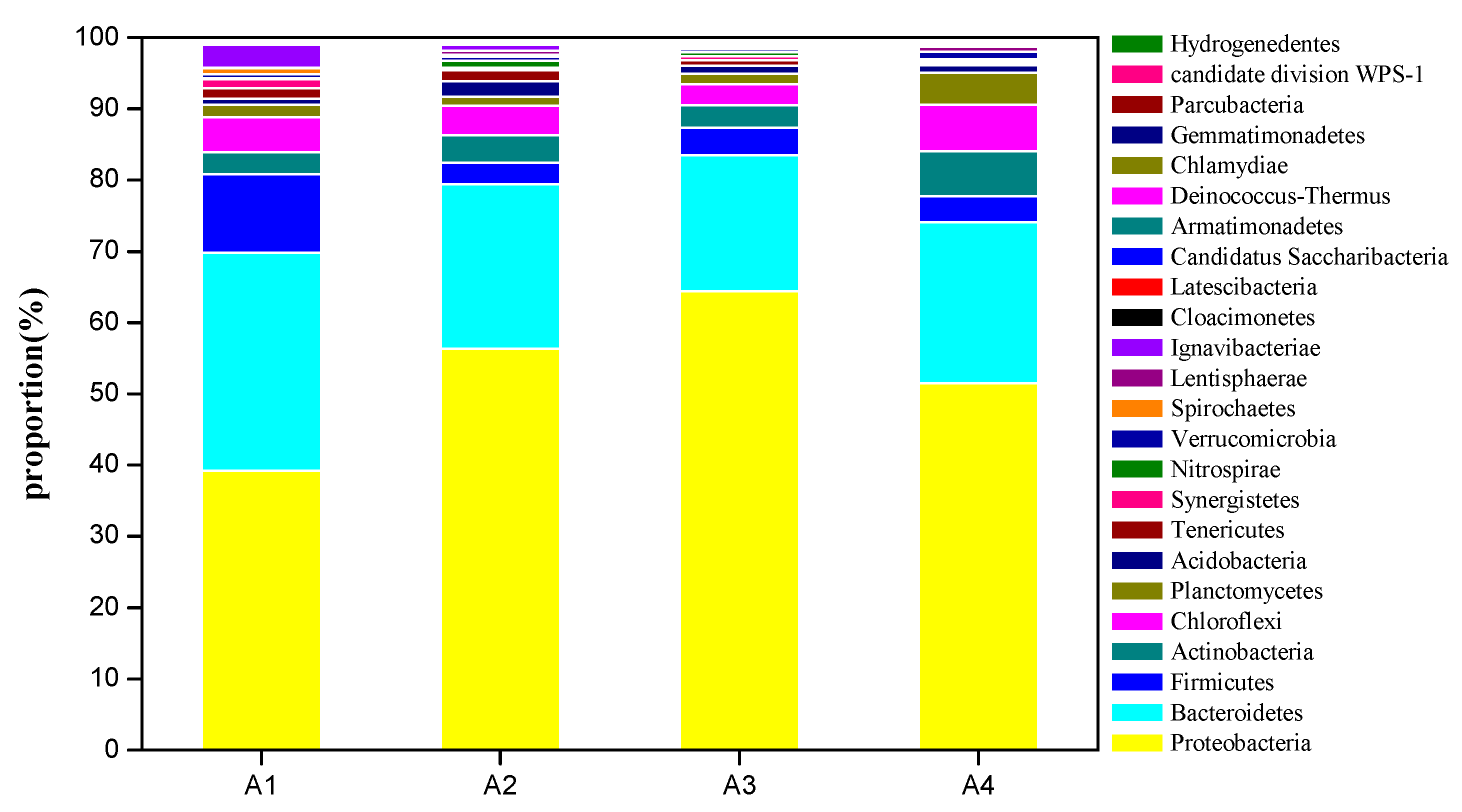
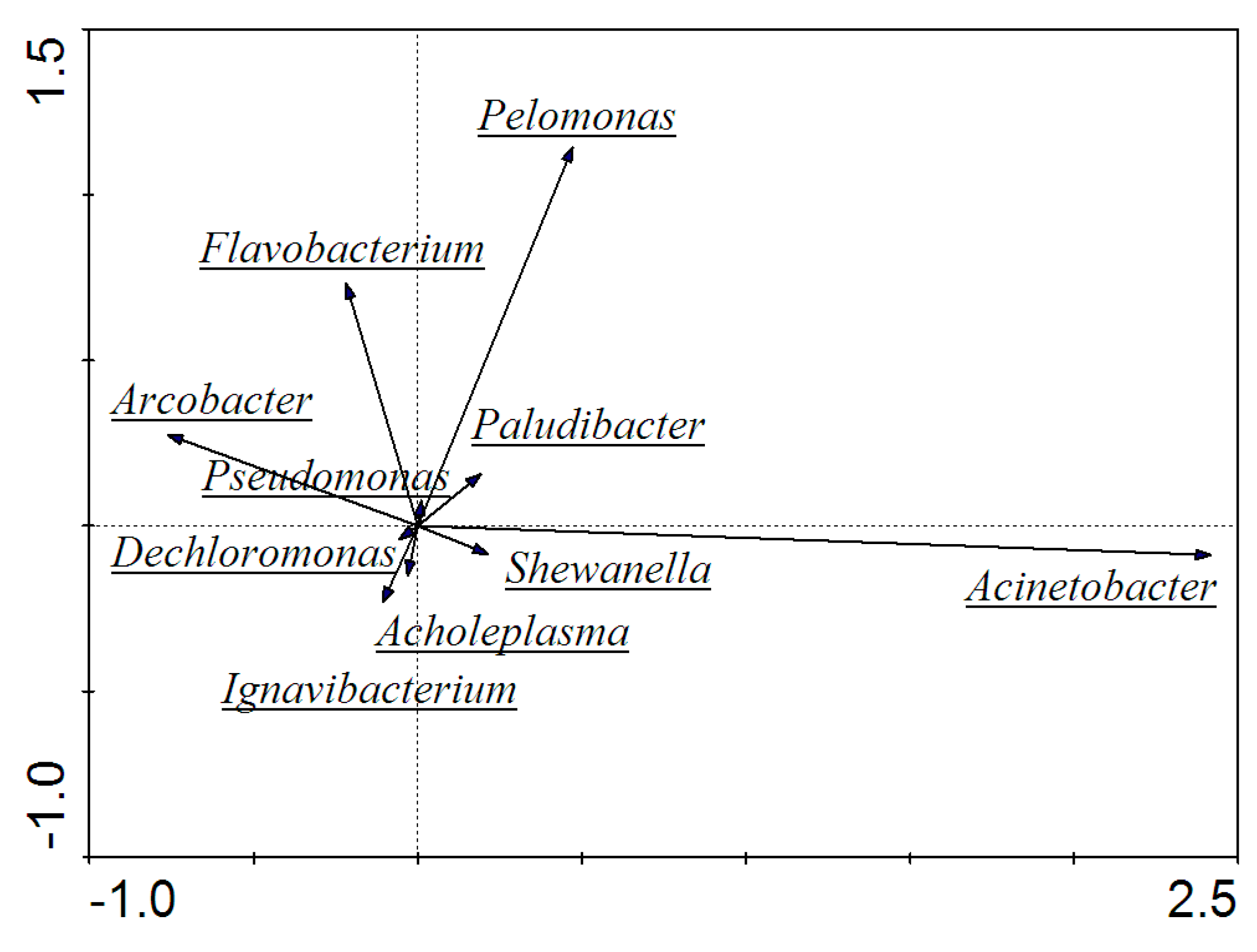
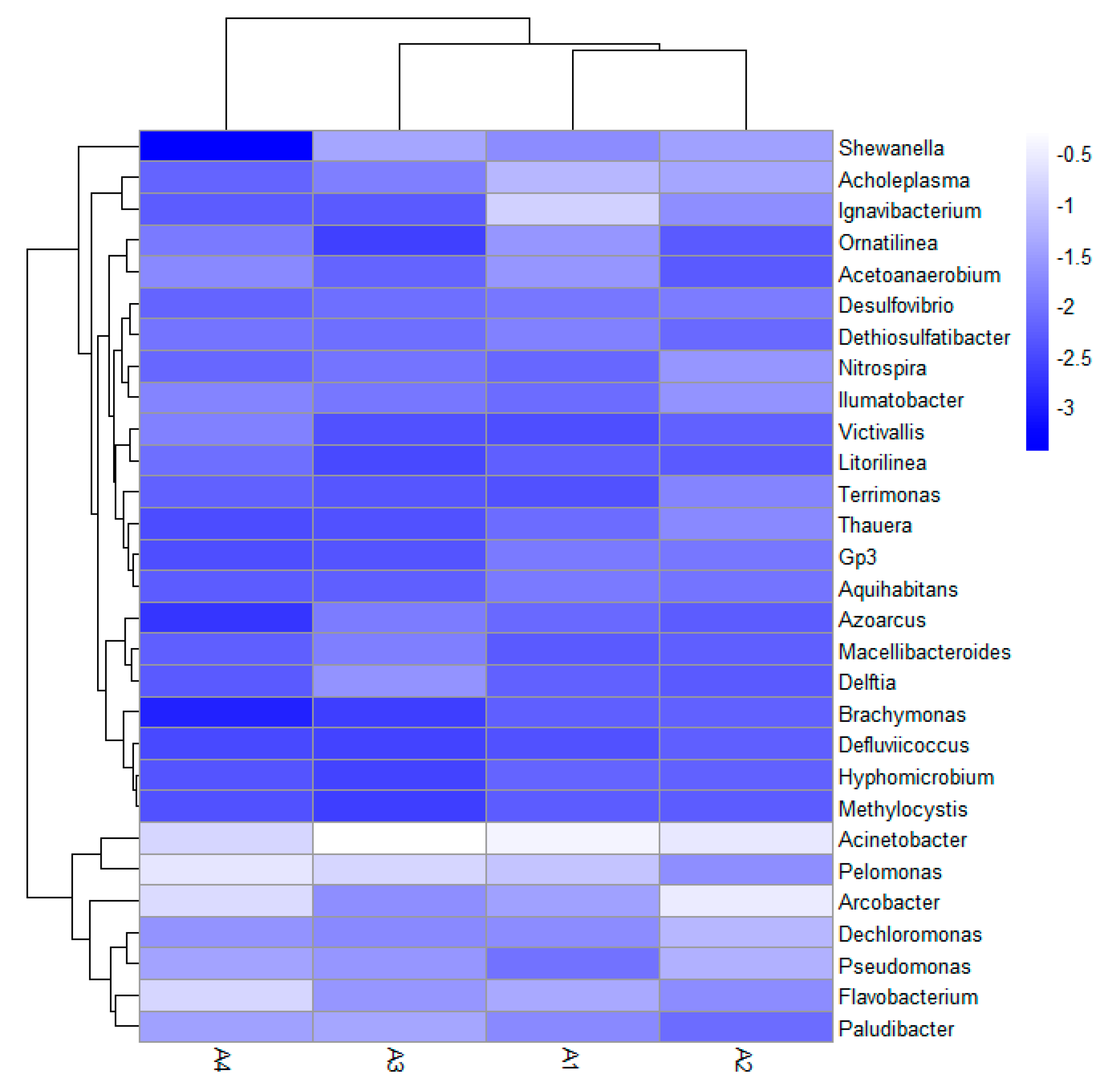
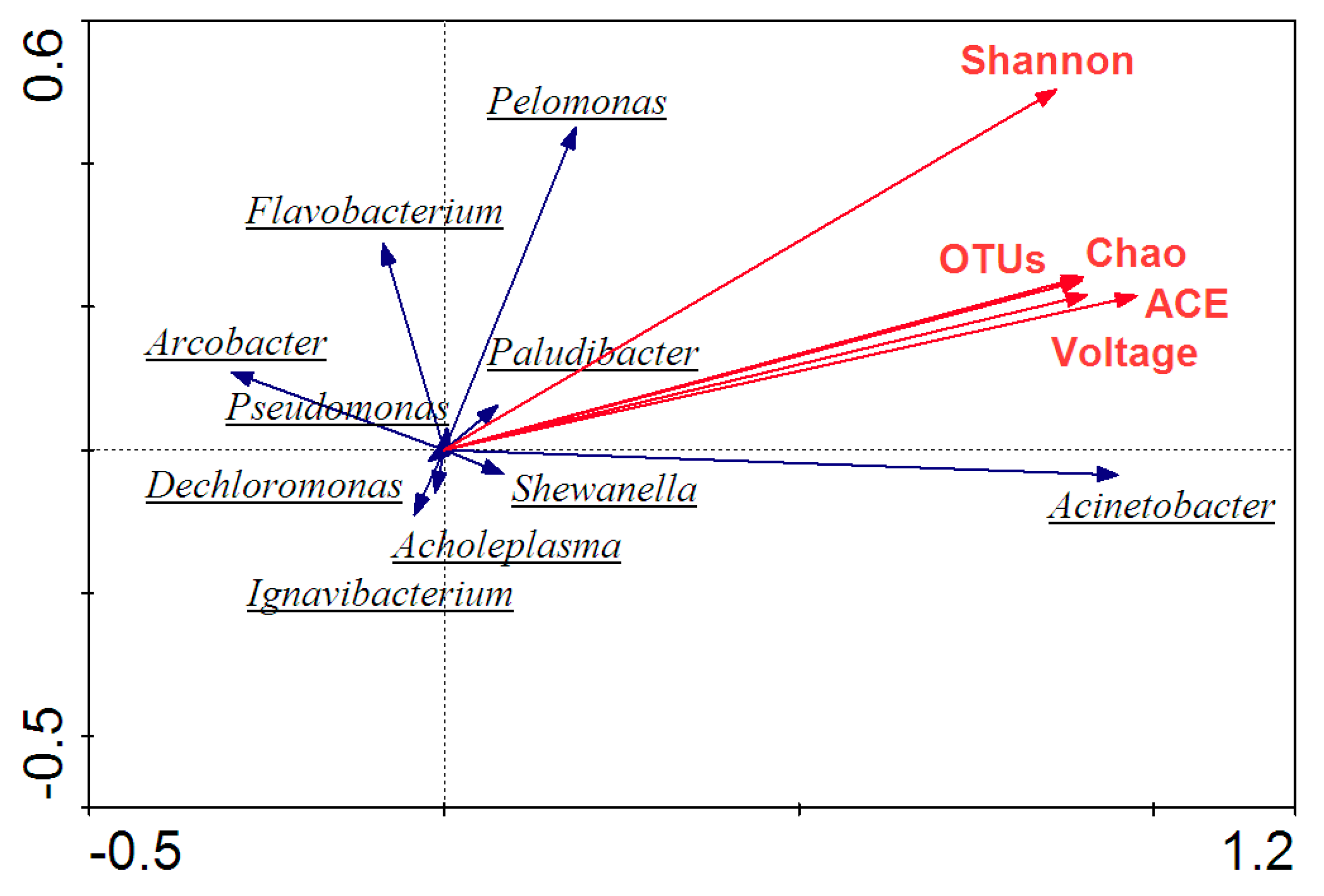
3.5. High-Throughput Sequencing and Microbial Community Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zeng, L.; Zhang, W.; Xia, P.; Tu, W.; Ye, C.; He, M. Porous Ni0.1Mn0.9O1.45 microellipsoids as high-performance anode electrocatalyst for microbial fuel cells. Biosens. Bioelectron. 2017, 102, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Ma, Y.; Liu, Y. Electric energy production from food waste: Microbial fuel cells versus anaerobic digestion. Bioresour. Technol. 2018, 255, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Rago, L.; Zecchin, S.; Marzorati, S.; Goglio, A.; Cavalca, L.; Cristiani, P.; Schievano, A. A study of microbial communities on terracotta separator and on biocathode of air breathing microbial fuel cells. Bioelectrochemistry 2018, 120, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.F.; Li, F.B.; Zhou, S.G.; Huang, D.Y.; Ni, J.R. A study of electron-shuttle mechanism in Klebsiella pneumoniae based-microbial fuel cells. Chin. Sci. Bull. 2010, 55, 99–104. [Google Scholar] [CrossRef]
- Santoro, C.; Serov, A.; Gokhale, R.; Rojas-Carbonell, S.; Stariha, L.; Gordon, J.; Artyushkova, K.; Atanassov, P. A family of Fe-N-C oxygen reduction electrocatalysts for microbial fuel cell (MFC) application: Relationships between surface chemistry and performances. Appl. Catal. B Environ. 2017, 205, 24–33. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, L.; Liu, Z.; Hou, J.; Chen, W.; Li, Y.; Sang, L. A novel anode fabricated by three-dimensional printing for use in urine-powered microbial fuel cell. Biochem. Eng. J. 2017, 124, 36–43. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, F.; Zhai, W.; Scott, K.; Wang, X.; Diao, G. Efficient removal of nitrobenzene and concomitant electricity production by single-chamber microbial fuel cells with activated carbon air-cathode. Bioresour. Technol. 2017, 229, 111–118. [Google Scholar] [CrossRef]
- Commault, A.S.; Laczka, O.; Siboni, N.; Tamburic, B.; Crosswell, J.R.; Seymour, J.R.; Ralph, P.J. Electricity and biomass production in a bacteria—Chlorella, based microbial fuel cell treating wastewater. J. Power Sources 2017, 356, 299–309. [Google Scholar] [CrossRef]
- Lovley, D.R. Powering microbes with electricity: Direct electron transfer from electrodes to microbes. Environ. Microbiol. Rep. 2011, 3, 27–35. [Google Scholar] [CrossRef]
- He, C.S.; Mu, Z.X.; Yang, H.Y.; Wang, Y.Z.; Mu, Y.; Yu, H.Q. Electron acceptors for energy generation in microbial fuel cells fed with wastewaters: A mini-review. Chemosphere 2015, 140, 12–17. [Google Scholar] [CrossRef]
- Chen, G.W.; Choi, S.J.; Cha, J.H.; Lee, T.H.; Kim, C.W. Microbial community dynamics and electron transfer of a biocathode in microbial fuel cells. Korean. J. Chem. Eng. 2010, 27, 1513–1520. [Google Scholar]
- Picioreanu, C.; Katuri, K.P.; Loosdrecht, M.C.M.V.; Head, I.M.; Scott, K. Modelling microbial fuel cells with suspended cells and added electron transfer mediator. J. Appl. Electrochem. 2010, 40, 151–162. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, L.; Zularisam, A.W. Exoelectrogens: Recent advances in molecular drivers involved in extracellular electron transfer and strategies used to improve it for microbial fuel cell applications. Renew. Sustain. Energy Rev. 2016, 56, 1322–1336. [Google Scholar] [CrossRef]
- Pinto, D.; Coradin, T.; Labertyrobert, C. Effect of anode polarization on biofilm formation and electron transfer in Shewanella oneidensis/graphite felt microbial fuel cells. Bioelectrochemistry 2018, 120, 1–9. [Google Scholar] [CrossRef]
- Sarma, D.; Barua, P.B.; Dey, N.; Nath, S.; Thakuria, M.; Mallick, S. Investigation and taguchi optimization of microbial fuel cell salt bridge dimensional parameters. J. Inst. Eng. 2018, 1–10. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Quan, X.; Chen, S. Effects of ferric iron on the anaerobic treatment and microbial biodiversity in a coupled microbial electrolysis cell (MEC)—Anaerobic reactor. Water Res. 2013, 47, 5719–5728. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Xing, D.F.; Lu, L.; Wei, M.; Liu, B.; Ren, N. Ferric iron enhances electricity generation by Shewanella oneidensis MR-1 in MFCs. Bioresour. Technol. 2013, 135, 630. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.J.; Muchowska, K.B.; Chatelain, P.; Moran, J. Native iron reduces CO2 to intermediates and end-products of the acetyl-coa pathway. Nat. Ecol. Evol. 2018, 2, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Quan, X.; Li, Y.; Zhao, Z.; Meng, X.; Chen, S. Optimization of anaerobic acidogenesis by adding Fe0, powder to enhance anaerobic wastewater treatment. Chem. Eng. J. 2012, 192, 179–185. [Google Scholar] [CrossRef]
- Yakunin, A.F.; Hallenbeck, P.C. Purification and characterization of pyruvate oxidoreductase from the photosynthetic bacterium Rhodobacter capsulatus. Biochim. Biophys. Acta 1998, 1409, 39–49. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Dolfing, J.; Wade, M.J.; Sloan, W.J.; Quince, C.; Curtis, T.P. Temperature, inocula and substrate: Contrasting electroactive consortia, diversity and performance in microbial fuel cells. Bioelectrochemistry 2017, 119, 43–50. [Google Scholar] [CrossRef]
- Yu, Z.; Yan, M.; Ting, L.; Zhishuai, D.; Yuxue, W. Modification of carbon felt anodes using double-oxidant HNO3/H2O2 for application in microbial fuel cells. RSC. Adv. 2018, 8, 2059–2064. [Google Scholar]
- Miner, G. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Jia, H.; Yang, G.; Ngo, H.; Guo, W.; Zhang, H.; Gao, F.; Wang, J. Enhancing simultaneous response and amplification of biosensor in microbial fuel cell-based upflow anaerobic sludge bed reactor supplemented with zero-valent iron. Chem. Eng. J. 2017, 327, 1117–1127. [Google Scholar] [CrossRef]
- Ling, R.; Chen, J.P.; Shao, J.; Reinhard, M. Degradation of organic compounds during the corrosion of ZVI by hydrogen peroxide at neutral pH: Kinetics, mechanisms and effect of corrosion promoting and inhibiting ions. Water Res. 2018, 134, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhu, L.; Xu, X.; Zang, L.; Kong, Y. Reductive transformation and dechlorination of chloronitrobenzenes in UASB reactor enhanced with zero-valent iron addition. J. Chem. Technol. Biotechnol. 2011, 86, 290–298. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Y.; Li, Q.; Quan, X. Adding Fe0, powder to enhance the anaerobic conversion of propionate to acetate. Biochem. Eng. J. 2013, 73, 80–85. [Google Scholar] [CrossRef]
- Han, T.H.; Cho, M.H.; Lee, J. Indole oxidation enhances electricity production in an E. coli -catalyzed microbial fuel cell. Biotechnol. Bioprocess Eng. 2014, 19, 126–131. [Google Scholar] [CrossRef]
- Roy, S.; Saswati, L.S.; Lima, S.; Dhaka, S.; Dinda, R. Synthesis, structural studies and catalytic activity of a series of dioxidomolybdenum(VI)-thiosemicarbazone complexes. Inorg. Chim. Acta 2018, 474, 134–143. [Google Scholar] [CrossRef]
- Moyo, B.; Momodu, D.; Fasakin, O.; Bello, A.; Dangbegnon, J.; Manyala, N. Electrochemical analysis of nanoporous carbons derived from activation of polypyrrole for stable supercapacitors. J. Mater. Sci. 2018, 53, 5229–5241. [Google Scholar] [CrossRef]
- Lu, L.; Liang, L.; Teh, K.S.; Xie, Y.; Wan, Z.; Tang, Y. The Electrochemical Behavior of Carbon Fiber Microelectrodes Modified with Carbon Nanotubes Using a Two-Step Electroless Plating/Chemical Vapor Deposition Process. Sensors 2017, 17, 725. [Google Scholar] [CrossRef]
- Choi, T.S.; Song, Y.C.; Timothy, H. A facile method for preparation of efficient oxygen reduction catalyst for a microbial fuel cell cathode. KSCE J. Civ. Eng. 2017, 1–9. [Google Scholar] [CrossRef]
- Sumisha, A.; Haribabu, K. Modification of graphite felt using nano polypyrrole and polythiophene for microbial fuel cell applications-a comparative study. Int. J. Hydrogen Energy 2018, 43, 3308–3316. [Google Scholar] [CrossRef]
- Rosenbaum, M.; He, Z.; Angenent, L.T. Light energy to bioelectricity: Photosynthetic microbial fuel cells. Curr. Opin. Biotechnol. 2010, 21, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Mateo, S.; Gonzalez, D.C.A.; Cañizares, P.; Lobato, J.; Rodrigo, M.A.; Fernandez, F.J. Bioelectricity generation in a self-sustainable Microbial Solar Cell. Bioresour. Technol. 2014, 159, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, R.; Uskaikar, H.P.; Berchmans, S. Electrochemically Prepared Manganese Oxide as a Cathode Material for a Microbial Fuel Cell. Anal. Lett. 2012, 45, 1645–1657. [Google Scholar] [CrossRef]
- In, S.I.; Kim, H.W.; Lee, K.S.; Razzaq, A.; Lee, S.H.; Grimes, C.A. Photocoupled Bioanode: A New Approach for Improved Microbial Fuel Cell Performance. Energy Technol. 2017, 6, 257–262. [Google Scholar]
- Kim, K.Y.; Yang, E.; Lee, M.Y.; Chae, K.J.; Kim, C.M.; Kim, I.S. Polydopamine coating effects on ultrafiltration membrane to enhance power density and mitigate biofouling of ultrafiltration microbial fuel cells (UF-MFCs). Water Res. 2014, 54, 62–68. [Google Scholar] [CrossRef]
- Kouzuma, A.; Ishii, S.; Watanabe, K. Metagenomic insights into the ecology and physiology of microbes in bioelectrochemical systems. Bioresour. Technol. 2018, 255, 302–307. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, P.; Shang, W.; Zhang, H.; Li, Y.; Zhang, W.; Zhang, Z.; Liu, F. Enrichment culture of electroactive microorganisms with high magnetic susceptibility enhances the performance of microbial fuel cells. Bioelectrochemistry 2018, 121, 65–73. [Google Scholar] [CrossRef]
- Antwi, P.; Li, J.; Boadi, P.O.; Shi, E.; Meng, J.; Chi, X.; Deng, K.; Ayivi, F. Dosing effect of zero valent iron (ZVI) on biomethanation and microbial community distribution as revealed by 16S rRNA high-throughput sequencing. Int. Biodeterior. Biodegrad. 2017, 123, 191–199. [Google Scholar] [CrossRef]
- Patil, S.A.; Surakasi, V.P.; Koul, S.; Ijmulwar, S.; Vivek, A.; Shouche, Y.S.; Kapadnis, B.P. Electricity generation using chocolate industry wastewater and its treatment in activated sludge based microbial fuel cell and analysis of developed microbial community in the anode chamber. Bioresour. Technol. 2009, 100, 5132–5139. [Google Scholar] [CrossRef] [PubMed]
- Borole, A.P.; Mielenz, J.R.; Vishnivetskaya, T.A.; Hamilton, C.Y. Controlling accumulation of fermentation inhibitors in biorefinery recycle water using microbial fuel cells. Biotechnol. Biofuels 2009, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Rago, L.; Cristiani, P.; Villa, F.; Zecchin, S.; Colombo, A.; Cavalca, L.; Schievano, A. Influences of dissolved oxygen concentration on biocathodic microbial communities in microbial fuel cells. Bioelectrochemistry 2017, 116, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Montpart, N.; Rago, L.; Baeza, J.A.; Guisasola, A. Hydrogen production in single chamber microbial electrolysis cells with different complex substrates. Water Res. 2015, 68, 601–615. [Google Scholar] [CrossRef]
- Lu, L.; Huggins, T.; Jin, S.; Zuo, Y.; Ren, Z.J. Microbial metabolism and community structure in response to bioelectrochemically enhanced remediation of petroleum hydrocarbon-contaminated soil. Environ. Sci. Technol. 2014, 48, 4021–4029. [Google Scholar] [CrossRef]
- Yusoff, M.Z.; Hu, A.; Feng, C.; Maeda, T.; Shirai, Y.; Hassan, M.A. Influence of pretreated activated sludge for electricity generation in microbial fuel cell application. Bioresour. Technol. 2013, 145, 90–96. [Google Scholar] [CrossRef]
- Embree, M.; Nagarajan, H.; Movahedi, N.; Chitsaz, H.; Zengler, K. Single-cell genome and metatranscriptome sequencing reveal metabolic interactions of an alkane-degrading methanogenic community. ISME J. 2013, 8, 757. [Google Scholar] [CrossRef]
- Toffin, L.; Bidault, A.; Pignet, P.; Tindall, B.J.; Prieur, D. Shewanella profunda sp. nov. isolated from deep marine sediment of the Nankai Trough. Int. J. Syst. Evolut. Microbiol. 2004, 54 Pt 6, 1943. [Google Scholar] [CrossRef]
- Freguia, S.; Tsujimura, S.; Kano, K. Electron transfer pathways in microbial oxygen biocathodes. Electrochim. Acta 2010, 55, 813–818. [Google Scholar] [CrossRef]
- Zhao, H.; Kong, C.H. Elimination of pyraclostrobin by simultaneous microbial degradation coupled with the Fenton process in microbial fuel cells and the microbial community. Bioresour. Technol. 2018, 258, 227. [Google Scholar] [CrossRef]
- Kumar, G.; Bakonyi, P.; Kobayashi, T.; Xu, K.Q.; Sivagurunathan, P.; Kim, S.H.; Buitron, G. Enhancement of biofuel production via microbial augmentation: The case of dark fermentative hydrogen. Renew. Sustain. Energy Rev. 2016, 57, 879–891. [Google Scholar] [CrossRef]
- Newton, G.J.; Mori, S.; Nakamura, R.; Hashimoto, K.; Watanabe, K. Analyses of current-generating mechanisms of Shewanella loihica PV-4 and Shewanella oneidensis MR-1 in microbial fuel cells. Appl. Environ. Microb. 2009, 75, 7674. [Google Scholar] [CrossRef] [PubMed]
- Pentráková, L.; Su, K.; Pentrák, M.; Stucki, J.W. A review of microbial redox interactions with structural Fe in clay minerals. Clay Miner. 2013, 48, 543–560. [Google Scholar] [CrossRef]

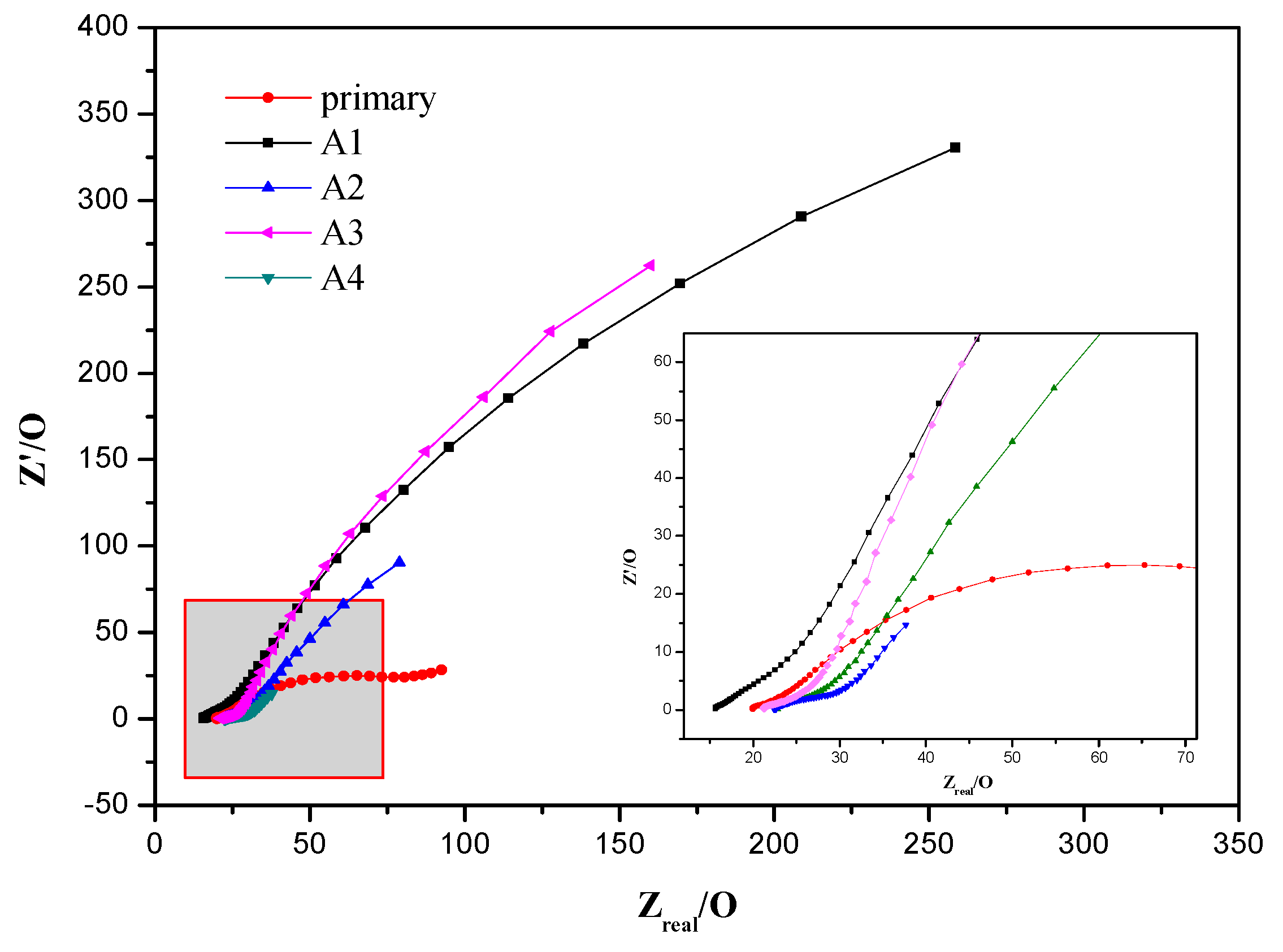
| RS/Ω | RCT/Ω × 10−3 | C/F × 10−3 | W/(S•s−0.5) × 10−3 | |
|---|---|---|---|---|
| A1 | 18.44 | 25.600 | 0.0290 | 1.53 |
| A2 | 24.71 | 10.000 | 27.9 | 11.9 |
| A3 | 22.76 | 32.470 | 2.26 | 0.330 |
| A4 | 23.98 | 33,840 | 1.12 | 23.8 |
| primary | 22.24 | 35,730 | 0.360 | 70.2 |
| OTUs | Chao | Shannon | ACE | Simpson | |
|---|---|---|---|---|---|
| A1 | 3495 | 10,386.96 | 6.104079 | 17,856.5333 | 0.011626 |
| A2 | 4578 | 15,520.60 | 6.492959 | 29,822.3437 | 0.013034 |
| A3 | 3539 | 10,659.00 | 5.786235 | 18,800.1165 | 0.026910 |
| A4 | 2762 | 6914.500 | 5.765082 | 10,409.8210 | 0.020122 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Zhou, K.; He, H.; Cao, J.; Zhou, S. Adding Zero-Valent Iron to Enhance Electricity Generation during MFC Start-Up. Int. J. Environ. Res. Public Health 2020, 17, 806. https://doi.org/10.3390/ijerph17030806
Li C, Zhou K, He H, Cao J, Zhou S. Adding Zero-Valent Iron to Enhance Electricity Generation during MFC Start-Up. International Journal of Environmental Research and Public Health. 2020; 17(3):806. https://doi.org/10.3390/ijerph17030806
Chicago/Turabian StyleLi, Chao, Kang Zhou, Hanyue He, Jiashun Cao, and Shihua Zhou. 2020. "Adding Zero-Valent Iron to Enhance Electricity Generation during MFC Start-Up" International Journal of Environmental Research and Public Health 17, no. 3: 806. https://doi.org/10.3390/ijerph17030806
APA StyleLi, C., Zhou, K., He, H., Cao, J., & Zhou, S. (2020). Adding Zero-Valent Iron to Enhance Electricity Generation during MFC Start-Up. International Journal of Environmental Research and Public Health, 17(3), 806. https://doi.org/10.3390/ijerph17030806




