Determinants of Vitamin D Supplementation among Individuals with Type 1 Diabetes
Abstract
1. Introduction
2. Methods
3. Results
3.1. General Characteristics
3.2. Comparison of the Study Groups
3.3. Personal Reasons to Use or Not Use Vitamin D Supplementation
3.4. Period and Dose of VD Supplementation
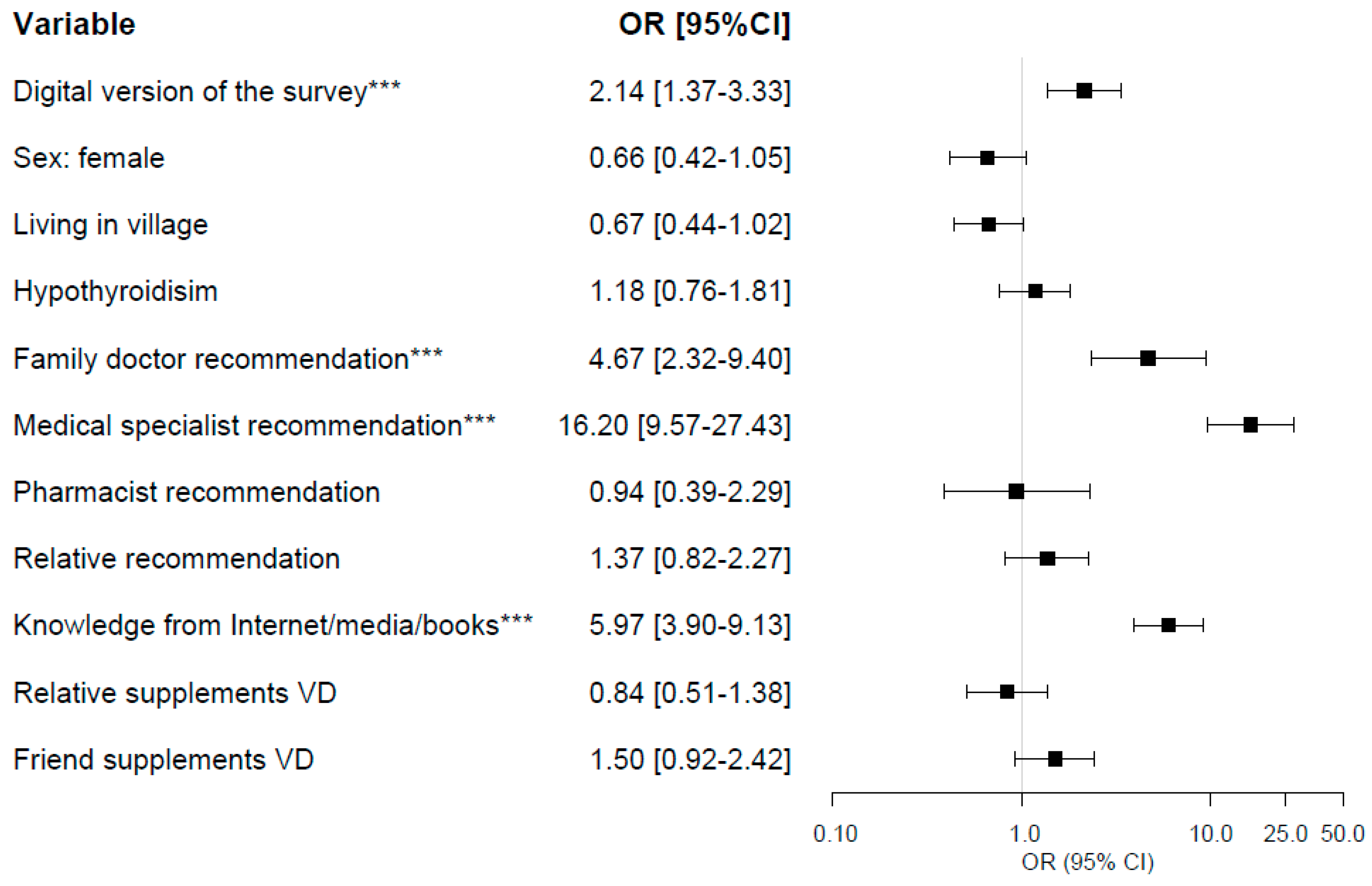
3.5. Logistic Regression Analysis
3.6. Diabetologists Survey
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yeum, K.J.; Song, B.; Joo, N.S. Impact of Geographic Location on Vitamin D Status and Bone Mineral Density. Int. J. Environ. Res. Pub. Health 2016, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Kochhar, A. Interplay of vitamin D and metabolic syndrome: A review. Diabetes Metabo. Syndr. Clin. Res. Rev. 2016, 10, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Skowron, K.; Pawlicka, I.; Gil, K. The role of vitamin D in the pathogenesis of ocular diseases. Folia. Med. Cracov 2018, 58, 103–118. [Google Scholar] [PubMed]
- Płudowski, P.; Ducki, C.; Konstantynowicz, J.; Jaworski, M. Vitamin D status in Poland. Pol. Arch. Med. Wewn. 2016, 126, 530–539. [Google Scholar] [CrossRef]
- Płudowski, P.; Karczmarewicz, E.; Bayer, M.; Carter, G.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dębski, R.; Decsi, T.; Dobrzańska, A.; Franek, E.; et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe—Recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol Pol. 2013, 64, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Upreti, V.; Maitri, V.; Dhull, P.; Handa, A.; Prakash, M.S.; Behl, A. Effect of oral vitamin D supplementation on glycemic control in patients with type 2 diabetes mellitus with coexisting hypovitaminosis D: A parellel group placebo controlled randomized controlled pilot study. Diabetes Metabo. Syndr.Clin. Res. Rev. 2018, 12, 509–512. [Google Scholar] [CrossRef]
- Carmona, R.J.; Adachi, J.D. Calcium and vitamin D for osteoporotic fracture prevention. Pol. Arch. Int. Med. 2007, 117, 441–442. [Google Scholar] [CrossRef]
- Chun, R.F.; Liu, P.T.; Modlin, R.L.; Adams, J.S.; Hewison, M. Impact of vitamin D on immune function: Lessons learned from genome-wide analysis. Front. Physiol. 2014, 5, 151. [Google Scholar] [CrossRef]
- Mathyssen, C.; Gayan-Ramirez, G.; Bouillon, R.; Janssens, W. Vitamin D supplementation in respiratory diseases—Evidence from RCT. Pol. Arch. Int. Med. 2017, 127, 775–784. [Google Scholar] [CrossRef][Green Version]
- Grübler, M.R.; Gaksch, M.; Kienreich, K.; Verheyen, N.; Schmid, J.; Ó Hartaigh, B.W.J.; Richtig, G.; Scharnagl, H.; Meinitzer, A.; Pieske, B.; et al. Effects of Vitamin D Supplementation on Plasma Aldosterone and Renin—A Randomized Placebo-Controlled Trial. J. Clin. Hypertens (Greenwich) 2016, 18, 608–613. [Google Scholar] [CrossRef]
- Chunbin, W.; Han, W.; Lin, C. Efficacy of Vitamin D on Chronic Heart Failure Among Adults. Int. J. Vitam. Nutr. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.; Frankel, J.; Heldt, A.; Grodsky, G. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science 1980, 209, 823–825. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.; Weldon, S.M.; Thompson, T.; Vargo, E.J. The Effect of Vitamin D Supplementation on Glycaemic Control in Women with Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Int. J. Environ. Res. Pub. Health 2019, 16, 1716. [Google Scholar] [CrossRef] [PubMed]
- Wieder-Huszla, S.; Jurczak, A.; Szkup, M.; Barczak, K.; Dołęgowska, B.; Schneider-Matyka, D.; Owsianowska, J.; Grochans, E. Relationships between Vitamin D3 and Metabolic Syndrome. Int. J. Environ. Res. Pub. Health 2019, 16, 175. [Google Scholar] [CrossRef] [PubMed]
- Kiely, M.; Cashman, K. Summary Outcomes of the ODIN Project on Food Fortification for Vitamin D Deficiency Prevention. Int. J. Environ. Res. Pub. Health 2018, 15, 2342. [Google Scholar] [CrossRef] [PubMed]
- Rusińska, A.; Płudowski, P.; Walczak, M.; Borszewska-Kornacka, M.K.; Bossowski, A.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dobrzańska, A.; Franek, E.; Helwich, E.; et al. Vitamin D Supplementation Guidelines for General Population and Groups at Risk of Vitamin D Deficiency in Poland—Recommendations of the Polish Society of Pediatric Endocrinology and Diabetes and the Expert Panel With Participation of National Specialist Consultants and Representatives of Scientific Societies—2018 Update. Front. Endocrinol. 2018, 9, 246. [Google Scholar]
- Hyppönen, E.; Läärä, E.; Reunanen, A.; Järvelin, M.R.; Virtanen, S.M. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet 2001, 358, 1500–1503. [Google Scholar] [CrossRef]
- Cooper, J.D.; Smyth, D.J.; Walker, N.M.; Stevens, H.; Burren, O.S.; Wallace, C.; Greissl, C.; Ramos-Lopez, E.; Hyppönen, E.; Dunger, D.B.; et al. Inherited variation in vitamin D genes is associated with predisposition to autoimmune disease type 1 diabetes. Diabetes 2011, 60, 1624–1631. [Google Scholar] [CrossRef]
- Kamiński, M.; Uruska, A.; Rogowicz-Frontczak, A.; Lipski, D.; Niedźwiecki, P.; Różańska, O.; Skonieczna, P.; Michalska, A.; Flotyńska, J.; Araszkiewicz, A.; et al. Insulin Resistance in Adults with Type 1 Diabetes is Associated with Lower Vitamin D Serum Concentration. Exp. Clin. Endocrinol. Diabetes 2019. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Szalecki, M.; Pludowski, P.; Jaworski, M.; Brzozowska, A. Vitamin D status, body composition and glycemic control in Polish adolescents with type 1 diabetes. Minerva Endocrinol. 2016, 41, 445–455. [Google Scholar]
- Engelen, L.; Schalkwijk, C.G.; Eussen, S.J.P.M.; Scheijen, J.L.J.M.; Soedamah-Muthu, S.S.; Chaturvedi, N.; Fuller, J.H.; Stehouwer, C.D.A. Low 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 levels are independently associated with macroalbuminuria, but not with retinopathy and macrovascular disease in type 1 diabetes: The EURODIAB prospective complications study. Cardiovasc. Diabetol. 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Ziaei-Kajbaf, T.; Aminzadeh, M.; Fatahinezhad, E.; Aletayeb, S.M.H. Vitamin D status in diabetic children and adolescents. Diabetes Metabol. Syndr. Clin. Res. Rev. 2018, 12, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Windrim, C.M.; Crosby, D.A.; Mitchell, K.; Brophy, C.; Mahony, R.; Higgins, M. Vitamin D supplementation in pregnancy—A survey of compliance with recommendations. Ir. J. Med. Sci. 2018, 187, 709–712. [Google Scholar] [CrossRef]
- Zadka, K.; Pałkowska-Goździk, E.; Rosołowska-Huszcz, D. The State of Knowledge about Nutrition Sources of Vitamin D, Its Role in the Human Body, and Necessity of Supplementation among Parents in Central Poland. Int. J. Environ. Res. Pub. Health 2018, 15, 1489. [Google Scholar] [CrossRef] [PubMed]
- de Nooijer, J.; Onnink, M.; van Assema, P. Vitamin D supplementation in young children: Associations with Theory of Planned Behaviour variables, descriptive norms, moral norms and habits. Pub. Health Nutr. 2010, 13, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Buckinx, F.; Reginster, J.Y.; Cavalier, E.; Petermans, J.; Ricour, C.; Dardenne, C.; Bruyère, O. Determinants of vitamin D supplementation prescription in nursing homes: A survey among general practitioners. Osteoporos. Int. 2016, 27, 881–886. [Google Scholar] [CrossRef]
- Bonevski, B.; Girgis, A.; Magin, P.; Horton, G.; Brozek, I.; Armstrong, B. Prescribing sunshine: A cross-sectional survey of 500 Australian general practitioners’ practices and attitudes about vitamin D. Int. J. Cancer 2012, 130, 2138–2145. [Google Scholar] [CrossRef]
- Package ‘Forestplot’. Available online: https://cran.r-project.org/web/packages/forestplot/forestplot.pdf (accessed on 21 January 2020).
- Ewers, B.; Trolle, E.; Jacobsen, S.S.; Vististen, D.; Almdal, T.P.; Vilsbøll, T.; Bruun, J.M. Data on the use of dietary supplements in Danish patients with type 1 and type 2 diabetes. Data Brief 2019, 22, 241–244. [Google Scholar] [CrossRef]
- Hansen, L.; Tjønneland, A.; Køster, B.; Brot, C.; Andersen, R.; Cohen, A.; Frederiksen, K.; Olsen, A. Vitamin D Status and Seasonal Variation among Danish Children and Adults: A Descriptive Study. Nutrients 2018, 10, 1801. [Google Scholar] [CrossRef]
- Moon, R.J.; Curtis, E.M.; Davies, J.H.; Cooper, C.; Harvey, N.C. Seasonal variation in Internet searches for vitamin D. Arch Osteoporos 2017, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Broussard, D.L. Public health in pharmacy: Improving vitamin D status in the U.S. population. J. Am. Pharm. Assoc. 2013, 53, 206–209. [Google Scholar] [CrossRef]
- Calvo, M.S.; Whiting, S.J. Public health strategies to overcome barriers to optimal vitamin D status in populations with special needs. J. Nutr. 2006, 136, 1135–1139. [Google Scholar] [CrossRef]
- Goodman, S.; Morrongiello, B.; Meckling, K. A randomized, controlled trial evaluating the efficacy of an online intervention targeting vitamin D intake, knowledge and status among young adults. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Beck, F.; Richard, J.B.; Nguyen-Thanh, V.; Montagni, I.; Parizot, I.; Renahy, E. Use of the internet as a health information resource among French young adults: Results from a nationally representative survey. J. Med. Internet Res. 2014, 16, e128. [Google Scholar] [CrossRef] [PubMed]
- McCormack, D.; Mai, X.; Chen, Y. Determinants of vitamin D supplement use in Canadians. Pub. Health Nutr. 2017, 20, 1768–1774. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Feature | Total n = 734 (100%) | VD Supplementation = YES n = 455 (62.0%) | VD Supplementation = NO n = 279 (38%) | p-Value |
|---|---|---|---|---|
| General characteristics and diabetes history | ||||
| Paper survey | 184 (25.1) | 79 (17.4) | 105 (37.6) | <0.001 |
| Sex: female | 551 (75.1) | 352 (77.4) | 199 (71.3) | 0.07 |
| Age [years] | 31 (24–39) | 32 (24–40) | 30 (23–39) | 0.05 |
| Age > 65 years | 11 (1.5) | 5 (1.1) | 6 (2.2) | 0.26 |
| Diabetes duration [years] | 12 (5–20) | 12 (5–21) | 12 (5–19) | 0.55 |
| Weight [kg] | 68 (60–79) | 68 (59–80) | 69 (60–78) | 0.63 |
| Height [m] | 1.69 (1.64–1.74) | 1.68 (1.64–1.74) | 1.69 (1.64–1.75) | 0.08 |
| BMI [kg/m2] | 23.9 (21.5–26.7) | 24.1 (21.4–26.8) | 23.7 (21.6–26.5) | 0.68 |
| Overweight (BMI 25–30 [kg/m2]) | 193 (26.3) | 116 (25.5) | 77 (27.6) | 0.53 |
| Obesity (BMI ≥ 30 [kg/m2]) | 83 (11.3) | 57 (12.5) | 26 (9.3) | 0.18 |
| Living place: village | 193 (26.3) | 100 (22.0) | 93 (33.3) | <0.001 |
| Living place: city < 50,000 citizens | 186 (25.3) | 110 (24.2) | 76 (27.2) | 0.35 |
| Living place: city > 50,000 citizens | 355 (48.4) | 245 (53.8) | 110 (39.4) | <0.001 |
| At least one diabetic complication | 156 (21.3) | 97 (21.3) | 59 (21.1) | 0.96 |
| Retinopathy | 106 (14.4) | 54 (11.9) | 52 (18.6) | 0.01 |
| Nephropathy | 28 (3.8) | 14 (3.1) | 14 (5.0) | 0.18 |
| Neuropathy | 87 (11.9) | 57 (12.5) | 30 (10.8) | 0.47 |
| Diabetic Foot Syndrome | 20 (2.7) | 10 (2.2) | 10 (3.6) | 0.26 |
| Ischemic Heart Disease | 24 (3.3) | 18 (4.0) | 6 (2.2) | 0.18 |
| Hypothyroidism | 226 (30.8) | 160 (35.2) | 66 (23.7) | <0.01 |
| Coeliac disease | 32 (4.4) | 23 (5.1) | 9 (3.2) | 0.24 |
| Asthma | 39 (5.3) | 25 (5.5) | 14 (5.0) | 0.78 |
| Influence of the respondent’s environment | ||||
| Family doctor recommendation: YES | 109 (14.9) | 96 (21.1) | 13 (4.7) | <0.001 |
| Medical specialist recommendation: YES | 262 (35.7) | 239 (52.5) | 23 (8.2) | <0.001 |
| Pharmacist recommendation: YES | 53 (7.2) | 41 (9.0) | 12 (4.3) | 0.02 |
| Relative recommendation: YES | 190 (25.9) | 139 (30.5) | 51 (18.3) | <0.001 |
| Friend recommendation: YES | 148 (20.2) | 98 (21.5) | 50 (17.9) | 0.24 |
| Knowledge acquired from Internet/media/books: YES | 288 (39.2) | 227 (49.9) | 61 (21.9) | <0.001 |
| My relative supplements VD: YES | 287 (39.1) | 199 (43.7) | 88 (31.5) | <0.01 |
| My friend supplements VD: YES | 243 (33.1) | 176 (38.7) | 67 (24.0) | <0.001 |
| Number of positive responses [n] | 2 (1–3) | 2 (1–4) | 1 (0–2) | <0.001 |
| Variables | Total n = 38 (100%) | Declared VD Supplementation n = 30 (79%) | Denied VD Supplementation n = 8 (21%) | p-Value |
|---|---|---|---|---|
| Sex: female [n] | 28 (74) | 20 (67) | 8 (100) | 0.057 |
| Practice [years] | 26 (18–31) | 29 (17–32) | 20 (18–23) | 0.21 |
| Percentage of patients which talk about VD supplementation [n]: | ||||
| >75% | 12 (32) | 12 (40) | 0 (0) | 0.03 |
| 25–75% | 13 (34) | 10 (33) | 3 (38) | 0.83 |
| <25% | 9 (24) | 7 (23) | 2 (25) | 0.92 |
| 0% | 4 (11) | 1 (3) | 3 (38) | <0.01 |
| Agree with the sentence | ||||
| “I have not enough time to counsel VD supplementation” | 14 (37) | 10 (33) | 4 (50) | 0.39 |
| “I believe that VD supplementation does not provide significant benefits to my patients” | 4 (11) | 0 (0) | 4 (50) | <0.001 |
| “I think that is makes no sense to recommend VD supplementation because patients will not use it regularly” | 1 (3) | 0 (0) | 1 (13) | 0.049 |
| “I do not recommend VD supplementation to not overload the patient with additional costs” | 0 (0) | 0 (0) | 0 (0) | - |
| “I believe that every medical professional should discuss VD supplementation with the patient” | 32 (84) | 28 (93) | 4 (50) | <0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamiński, M.; Molenda, M.; Banaś, A.; Uruska, A.; Zozulińska-Ziółkiewicz, D. Determinants of Vitamin D Supplementation among Individuals with Type 1 Diabetes. Int. J. Environ. Res. Public Health 2020, 17, 715. https://doi.org/10.3390/ijerph17030715
Kamiński M, Molenda M, Banaś A, Uruska A, Zozulińska-Ziółkiewicz D. Determinants of Vitamin D Supplementation among Individuals with Type 1 Diabetes. International Journal of Environmental Research and Public Health. 2020; 17(3):715. https://doi.org/10.3390/ijerph17030715
Chicago/Turabian StyleKamiński, Mikołaj, Magdalena Molenda, Agnieszka Banaś, Aleksandra Uruska, and Dorota Zozulińska-Ziółkiewicz. 2020. "Determinants of Vitamin D Supplementation among Individuals with Type 1 Diabetes" International Journal of Environmental Research and Public Health 17, no. 3: 715. https://doi.org/10.3390/ijerph17030715
APA StyleKamiński, M., Molenda, M., Banaś, A., Uruska, A., & Zozulińska-Ziółkiewicz, D. (2020). Determinants of Vitamin D Supplementation among Individuals with Type 1 Diabetes. International Journal of Environmental Research and Public Health, 17(3), 715. https://doi.org/10.3390/ijerph17030715




