Distribution and Removal of Pharmaceuticals in Liquid and Solid Phases in the Unit Processes of Sewage Treatment Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Target Compounds
2.2. Specifications of Sewage Treatment Plants
2.3. Analytical Methods
2.4. Quality Control
2.5. Calculations of Mass Balance and Standardized Removal Efficiency
2.6. Statistical Analysis
3. Results and Discussion
3.1. Occurrence of Pharmaceuticals in Different Unit Processes of Sewage Treatment Plants
3.1.1. Concentrations of Pharmaceuticals in Sewage
3.1.2. Concentrations of Pharmaceuticals in Reject Water
3.1.3. Concentrations of Pharmaceuticals in Suspended Solids
3.1.4. Concentrations of Pharmaceuticals in Sludge
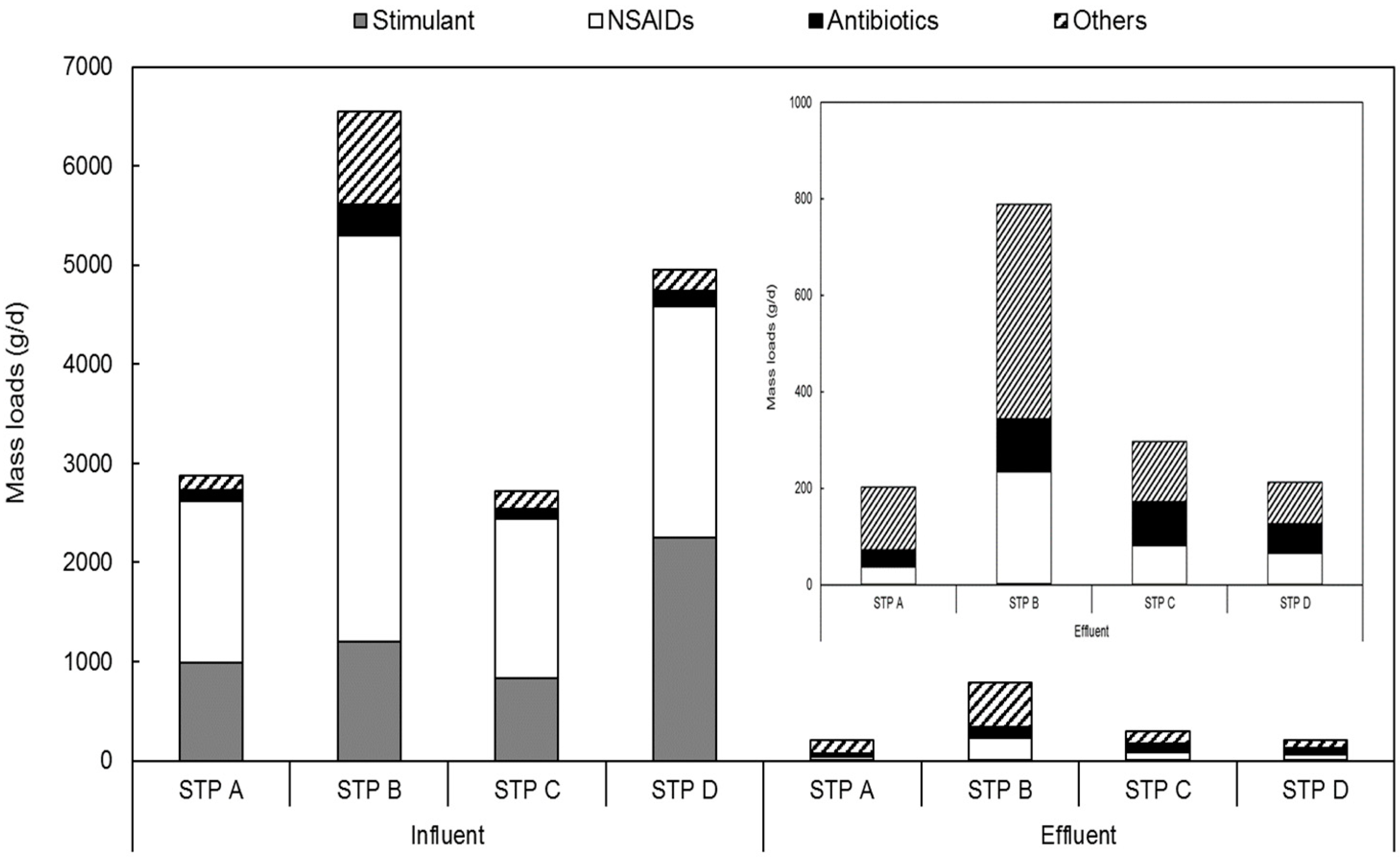
3.2. Mass Loads and Distribution of Pharmaceuticals in Sewage Treatment Plants
3.2.1. Relative Distributions of Each Therapeutic Class of Pharmaceuticals in Sewage Treatment Plants
3.2.2. Mass Loads of Pharmaceuticals in Each Treatment Process
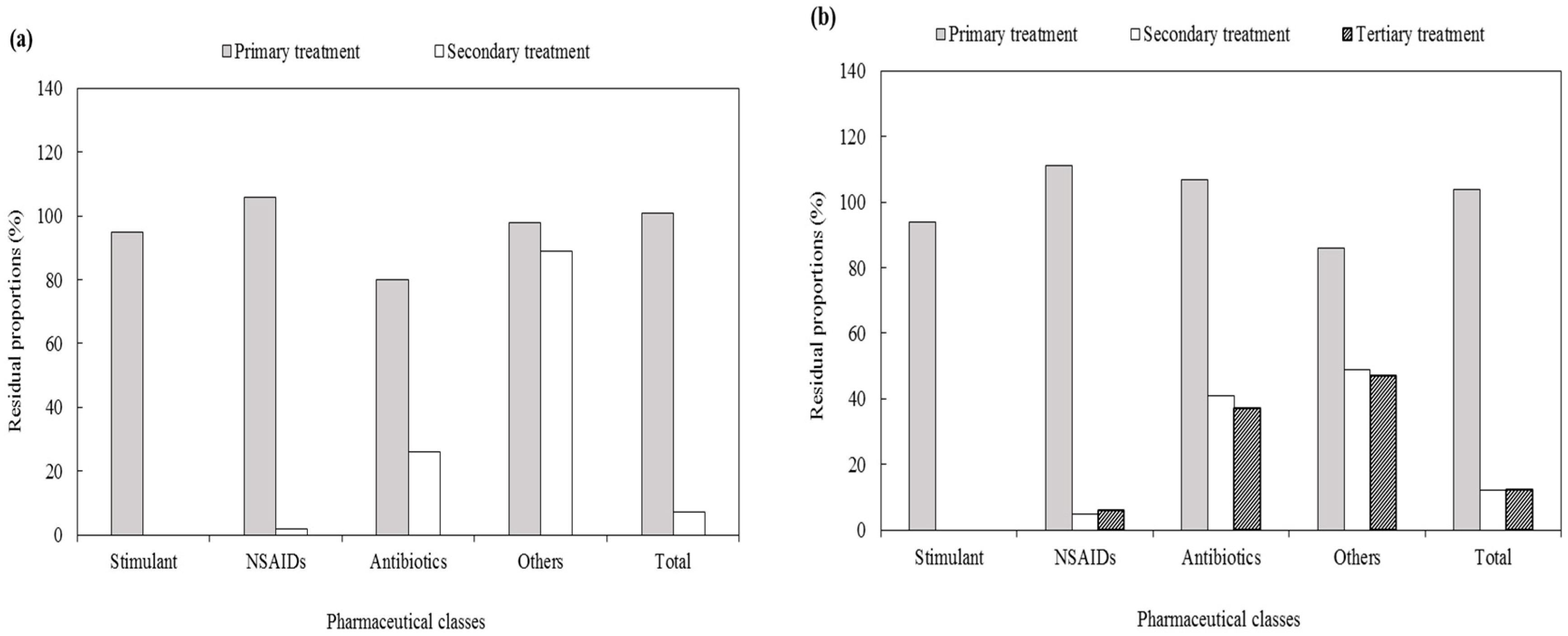
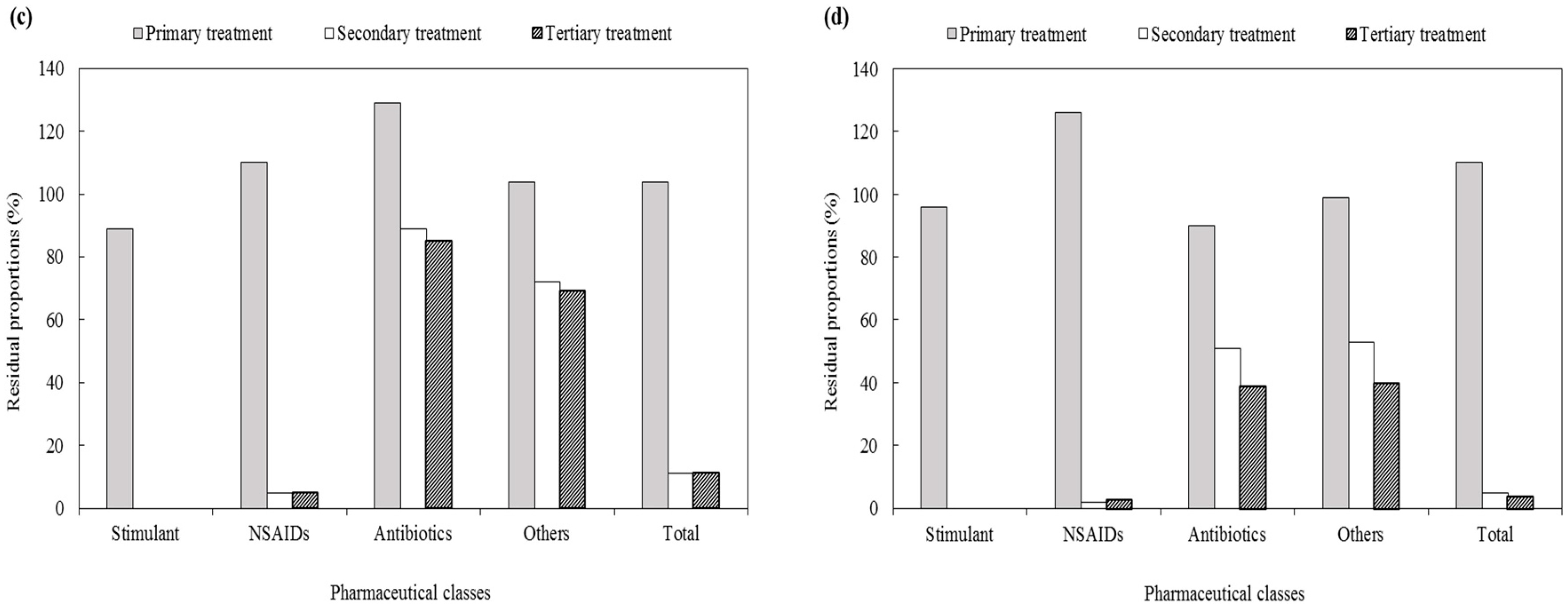
3.2.3. Residual Proportion of Pharmaceuticals in Each Treatment Process
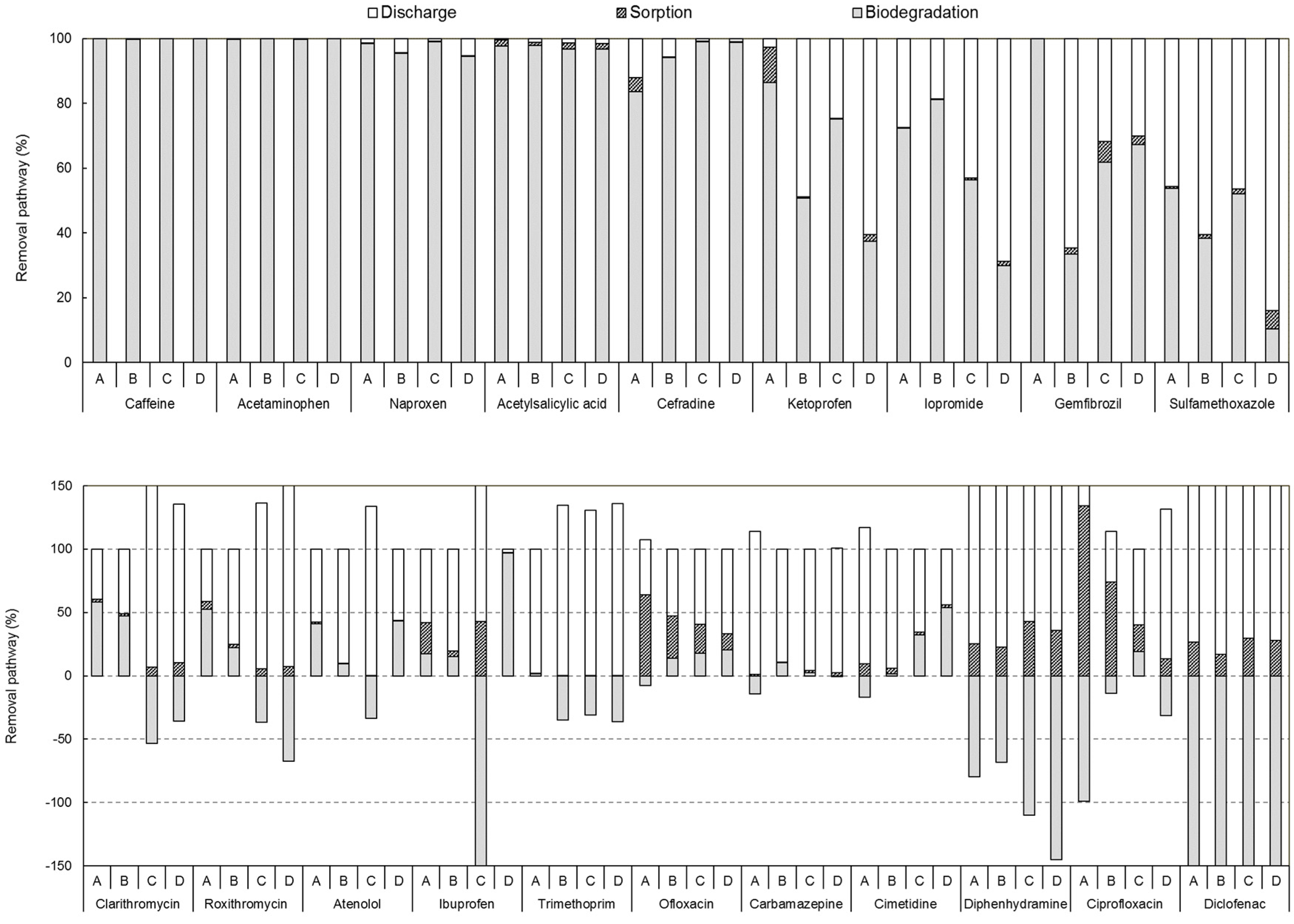
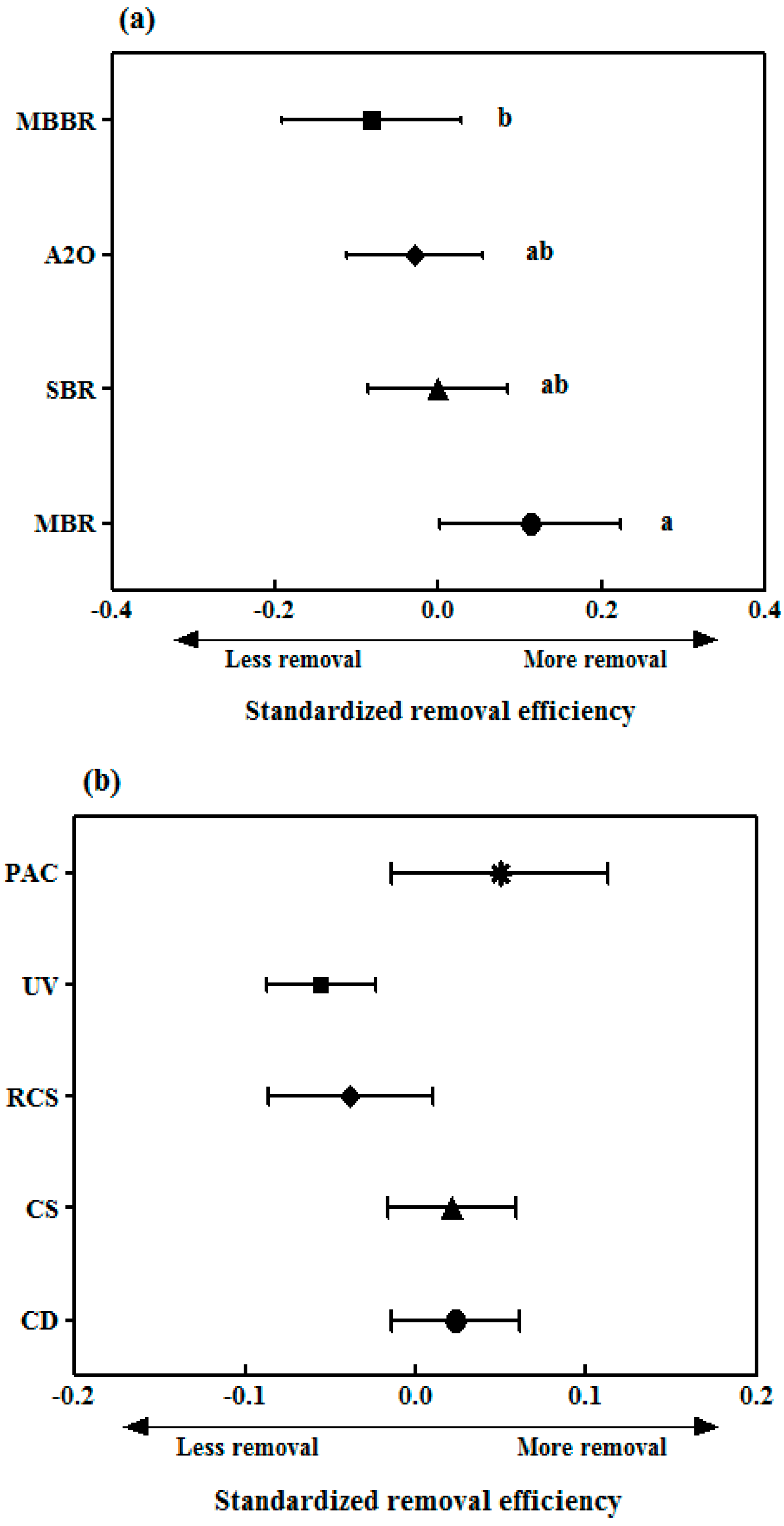
3.3. Comparative Evaluation of Pharmaceutical Removal Efficiencies of Different Treatment Technologies in Sewage Treatment Plants
3.3.1. Removal of Pharmaceuticals by Biological Treatment Processes
3.3.2. Removal of Pharmaceuticals in Tertiary Treatment Processes
3.3.3. Practical Suggestions to Enhance the Pharmaceutical Removal in Sewage Treatment Plants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carballa, M.; Omil, F.; Lema, J.M.; Llompart, M.; García-Jares, C.; Rodríguez, I.; Gómez, M.; Ternes, T. Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res. 2004, 38, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and Personal Care Products in the Environment: Agents of Subtle Change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, R.; Caprio, V.; Ciniglia, C.; De Champdoré, M.; Lo Giudice, R.; Marotta, R.; Zuccato, E. Antibiotics in the environment: Occurrence in Italian STPs, fate, and preliminary assessment on algal toxicity of amoxicillin. Environ. Sci. Technol. 2004, 38, 6832–6838. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.Y.; McGrath, S.P.; Su, J.Q.; Hirsch, P.R.; Clark, I.M.; Shen, Q.; Zhu, Y.G.; Zhao, F.J. Long-term impact of field applications of sewage sludge on soil antibiotic resistome. Environ. Sci. Technol. 2016, 50, 12602–12611. [Google Scholar] [CrossRef] [PubMed]
- Lienert, J.; Güdel, K.; Escher, B.I. Screening method for ecotoxicological hazard assessment of 42 pharmaceuticals considering human metabolism and excretory routes. Environ. Sci. Technol. 2007, 41, 4471–4478. [Google Scholar] [CrossRef]
- Gracia-Lor, E.; Sancho, J.V.; Serrano, R.; Hernández, F. Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia. Chemosphere 2012, 87, 453–462. [Google Scholar] [CrossRef]
- Joss, A.; Zabczynski, S.; Göbel, A.; Hoffmann, B.; Löffler, D.; McArdell, C.S.; Ternes, T.A.; Thomsen, A.; Siegrist, H. Biological degradation of pharmaceuticals in municipal wastewater treatment: Proposing a classification scheme. Water Res. 2006, 40, 1686–1696. [Google Scholar] [CrossRef]
- Vieno, N.; Tuhkanen, T.; Kronberg, L. Elimination of pharmaceuticals in sewage treatment plants in Finland. Water Res. 2007, 41, 1001–1012. [Google Scholar] [CrossRef]
- Gao, P.; Ding, Y.; Li, H.; Xagoraraki, I. Occurrence of pharmaceuticals in a municipal wastewater treatment plant: Mass balance and removal processes. Chemosphere 2012, 88, 17–24. [Google Scholar] [CrossRef]
- Trinh, T.; van den Akker, B.; Coleman, H.M.; Stuetz, R.M.; Drewes, J.E.; Le-Clech, P.; Khan, S.J. Seasonal variations in fate and removal of trace organic chemical contaminants while operating a full-scale membrane bioreactor. Sci. Total Environ. 2016, 550, 176–183. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment-A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Yamashita, N.; Tanaka, H. Membrane fouling control and enhanced removal of pharmaceuticals and personal care products by coagulation-MBR. Chemosphere 2018, 197, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Yamashita, N.; Park, C.; Shimono, T.; Takeuchi, D.M.; Tanaka, H. Removal characteristics of pharmaceuticals and personal care products: Comparison between membrane bioreactor and various biological treatment processes. Chemosphere 2017, 179, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lee, I.; Lee, W.; Kim, H. Mass-balance-model-based evaluation of sewage treatment plant contribution to residual pharmaceuticals in environmental waters. Chemosphere 2019, 225, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Sharma, V.K.; Chiang, P.; Kim, H. Fast-target analysis and hourly variation of 60 pharmaceuticals in wastewater using UPLC-high resolution mass spectrometry. Arch. Environ. Contam. Toxicol. 2015, 69, 525–534. [Google Scholar] [CrossRef]
- Ashfaq, M.; Li, Y.; Wang, Y.; Chen, W.; Wang, H.; Chen, X.; Wu, W.; Huang, Z.; Yu, C.-P.; Sun, Q. Occurrence, fate, and mass balance of different classes of pharmaceuticals and personal care products in an anaerobic-anoxic-oxic wastewater treatment plant in Xiamen, China. Water Res. 2017, 123, 655–667. [Google Scholar] [CrossRef]
- Baalbaki, Z.; Sultana, T.; Maere, T.; Vanrolleghem, P.A.; Metcalfe, C.D.; Yargeau, V. Fate and mass balance of contaminants of emerging concern during wastewater treatment determined using the fractionated approach. Sci. Total Environ. 2016, 573, 1147–1158. [Google Scholar] [CrossRef]
- Guerra, P.; Kim, M.; Shah, A.; Alaee, M.; Smyth, S.A. Occurrence and fate of antibiotic, analgesic/anti-inflammatory, and antifungal compounds in five wastewater treatment processes. Sci. Total Environ. 2014, 473–474, 235–243. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Hu, A.; Rashid, A.; Ashfaq, M.; Wang, Y.; Wang, H.; Luo, H.; Yu, C.P.; Sun, Q. Monitoring, mass balance and fate of pharmaceuticals and personal care products in seven wastewater treatment plants in Xiamen City, China. J. Hazard. Mater. 2018, 354, 81–90. [Google Scholar] [CrossRef]
- Statistics of Sewerage; Ministry of Environment: Sejong, Korea, 2016; [Korean].
- Shrivastava, A.; Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Melvin, S.D.; Leusch, F.D.L. Removal of trace organic contaminants from domestic wastewater: A meta-analysis comparison of sewage treatment technologies. Environ. Int. 2016, 92–93, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Ben, W.; Zhu, B.; Yuan, X.; Zhang, Y.; Yang, M.; Qiang, Z. Occurrence, removal and risk of organic micropollutants in wastewater treatment plants across China: Comparison of wastewater treatment processes. Water Res. 2018, 130, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef]
- Tewari, S.; Jindal, R.; Kho, Y.L.; Eo, S.; Choi, K. Major pharmaceutical residues in wastewater treatment plants and receiving waters in Bangkok, Thailand, and associated ecological risks. Chemosphere 2013, 91, 697–704. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Klein, E.Y.; Boeckel, T.P.V.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef]
- Kim, Y.A.; Park, Y.S.; Youk, T.; Lee, H.; Lee, K. Changes in antimicrobial usage patterns in Korea: 12-yeal analysis based on database of the national health insurance service-national sample cohort. Sci. Rep. 2018, 8, 12210. [Google Scholar] [CrossRef]
- Harrison, E.Z.; Oakes, S.R.; Hysell, M.; Hay, A. Organic chemicals in sewage sludges. Sci. Total Environ. 2006, 367, 481–497. [Google Scholar] [CrossRef]
- Kumirska, J.; Migowska, N.; Caban, M.; Łukaszewicz, P.; Stepnowski, P. Simultaneous determination of non-steroidal anti-inflammatory drugs and oestrogenic hormones in environmental solid samples. Sci. Total Environ. 2015, 508, 498–505. [Google Scholar] [CrossRef]
- Lin, H.; Li, H.; Chen, L.; Li, L.; Yin, L.; Lee, H.; Yang, Z. Mass loading and emission of thirty-seven pharmaceuticals in a typical municipal wastewater treatment plant in Hunan Province, Southern China. Ecotoxicol. Environ. Saf. 2018, 147, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Thiebault, T.; Boussafir, M.; Le Milbeau, C. Occurrence and removal efficiency of pharmaceuticals in an urban wastewater treatment plant: Mass balance, fate and consumption assessment. J. Environ. Chem. Eng. 2017, 5, 2894–2902. [Google Scholar] [CrossRef]
- Gao, J.; Huang, J.; Chen, W.; Wang, B.; Wang, Y.; Deng, S.; Yu, G. Fate and removal of typical pharmaceutical and personal care products in a wastewater treatment plant from Beijing: A mass balance study. Front. Environ. Sci. Eng. 2016, 10, 491–501. [Google Scholar] [CrossRef]
- Behera, S.K.; Kim, H.W.; Oh, J.E.; Park, H.S. Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Sci. Total Environ. 2011, 409, 4351–4360. [Google Scholar] [CrossRef]
- Conkle, J.L.; White, J.R.; Metcalfe, C.D. Reduction of pharmaceutically active compounds by a lagoon wetland wastewater treatment system in Southeast Louisiana. Chemosphere 2008, 73, 1741–1748. [Google Scholar] [CrossRef]
- Kumar, R.; Sarmah, A.K.; Padhye, L.P. Fate of pharmaceuticals and personal care products in a wastewater treatment plant with parallel secondary wastewater treatment train. J. Environ. Manag. 2019, 233, 649–659. [Google Scholar] [CrossRef]
- Golet, E.M.; Xifra, I.; Siegrist, H.; Alder, A.C.; Giger, W. Environmental exposure assessment of fluoroquinolone antibacterial agents from sewage to soil. Environ. Sci. Technol. 2003, 37, 3243–3249. [Google Scholar] [CrossRef]
- Tran, N.H.; Chen, H.; Reinhard, M.; Mao, F.; Gin, K.Y.H. Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes. Water Res. 2016, 104, 461–472. [Google Scholar] [CrossRef]
- Su, J.Q.; Wei, B.; Ou-Yang, W.Y.; Huang, F.Y.; Zhao, Y.; Xu, H.J.; Zhu, Y.G. Antibiotic Resistome and Its Association with Bacterial Communities during Sewage Sludge Composting. Environ. Sci. Technol. 2015, 49, 7356–7363. [Google Scholar] [CrossRef]
- Yi, K.; Wang, D.; Yang, Q.; Li, X.; Chen, H.; Sun, J.; An, H.; Wang, L.; Deng, Y.; Liu, J.; et al. Effect of ciprofloxacin on biological nitrogen and phosphorus removal from wastewater. Sci. Total Environ. 2017, 605–606, 368–375. [Google Scholar] [CrossRef]
- Kim, M.; Guerra, P.; Shah, A.; Parsa, M.; Alaee, M.; Smyth, S.A. Removal of pharmaceuticals and personal care products in a membrane bioreactor wastewater treatment plant. Water Sci. Technol. 2014, 69, 2221–2229. [Google Scholar] [CrossRef] [PubMed]
- Gurung, K.; Ncibi, M.C.; Sillanpää, M. Removal and fate of emerging organic micropollutants (EOMs) in municipal wastewater by a pilot-scale membrane bioreactor (MBR) treatment under varying solid retention times. Sci. Total Environ. 2019, 667, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Joss, A.; Keller, E.; Alder, A.C.; Göbel, A.; McArdell, C.S.; Ternes, T.; Siegrist, H. Removal of pharmaceuticals and fragrances in biological wastewater treatment. Water Res. 2005, 39, 3139–3152. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, M.; de Alda, M.J.L.; Diaz-Cruz, S.; Postigo, C.; Radjenovic, J.; Gros, M.; Barcelo, D. Fate and removal of pharmaceuticals and illicit drugs in conventional and membrane bioreactor wastewater treatment plants and by riverbank filtration. Philos. Trans. R. Soc. A 2009, 367, 3979–4003. [Google Scholar] [CrossRef]
- Rodayan, A.; Majewsky, M.; Yargeau, V. Impact of approach used to determine removal levels of drugs of abuse during wastewater treatment. Sci. Total Environ. 2014, 487, 731–739. [Google Scholar] [CrossRef]
- Salgado, R.; Marques, R.; Noronha, J.P.; Carvalho, G.; Oehmen, A.; Reis, M.A.M. Assessing the removal of pharmaceuticals and personal care products in a full-scale activated sludge plant. Environ. Sci. Pollut. Res. 2012, 19, 1818–1827. [Google Scholar] [CrossRef]
- Batt, A.L.; Kim, S.; Aga, D.S. Enhanced biodegradation of lopromide and trimethoprim in nitrifying activated sludge. Environ. Sci. Technol. 2006, 40, 7367–7373. [Google Scholar] [CrossRef]
- Clara, M.; Strenn, B.; Gans, O.; Martinez, E.; Kreuzinger, N.; Kroiss, H. Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants. Water Res. 2005, 39, 4797–4807. [Google Scholar] [CrossRef]
- Park, J.; Yamashita, N.; Wu, G.; Tanaka, H. Removal of pharmaceuticals and personal care products by ammonia oxidizing bacteria acclimated in a membrane bioreactor: Contributions of cometabolism and endogenous respiration. Sci. Total Environ. 2017, 605–606, 18–25. [Google Scholar] [CrossRef]
- Tran, N.H.; Urase, T.; Ngo, H.H.; Hu, J.; Ong, S.L. Insight into metabolic and cometabolic activities of autotrophic and heterotrophic microorganisms in the biodegradation of emerging trace organic contaminants. Bioresour. Technol. 2013, 146, 721–731. [Google Scholar] [CrossRef]
- Sipma, J.; Osuna, B.; Collado, N.; Monclús, H.; Ferrero, G.; Comas, J.; Rodriguez-Roda, I. Comparison of removal of pharmaceuticals in MBR and activated sludge systems. Desalination 2010, 250, 653–659. [Google Scholar] [CrossRef]
- Sui, Q.; Huang, J.; Deng, S.; Chen, W.; Yu, G. Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in different biological wastewater treatment processes. Environ. Sci. Technol. 2011, 45, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.E.; Chhetri, R.K.; Ooi, G.; Hansen, K.M.S.; Litty, K.; Christensson, M.; Kragelund, C.; Andersen, H.R.; Bester, K. Biodegradation of pharmaceuticals in hospital wastewater by staged Moving Bed Biofilm Reactors (MBBR). Water Res. 2015, 83, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Shreve, M.J.; Brennan, R.A. Trace organic contaminant removal in six full-scale integrated fixed-film activated sludge (IFAS) systems treating municipal wastewater. Water Res. 2019, 151, 318–331. [Google Scholar] [CrossRef]
- Stadler, L.B.; Vela, J.D.; Jain, S.; Dick, G.J.; Love, N.G. Elucidating the impact of microbial community biodiversity on pharmaceutical biotransformation during wastewater treatment. Microb. Biotechnol. 2018, 11, 995–1007. [Google Scholar] [CrossRef]
- Wolff, D.; Krah, D.; Dotsch, A.; Ghattas, A.; Wick, A.; Ternes, T.A. Insights into the variability of microbial community composition and micropollutant degradation in diverse biological wastewater treatment systems. Water Res. 2018, 143, 313–324. [Google Scholar] [CrossRef]
- Gallardo-Altamirano, M.J.; Maza-Marquez, P.; Montemurro, N.; Rodelas, B.; Osorio, F.; Pozo, C. Linking microbial diversity and population dynamics to the removal efficiency of pharmaceutically active compounds (PhACs) in an anaerobic/anoxic/aerobic (A2O) system. Chemosphere 2019, 233, 828–842. [Google Scholar] [CrossRef]
- Vuono, D.C.; Regnery, J.; Li, D.; Jones, Z.L.; Holloway, R.W.; Drewes, J.E. rRNA gene expression of abundant and rare activated-sludge microorganisms and growth rate induced micropollutant removal. Environ. Sci. Technol. 2016, 50, 6299–6309. [Google Scholar] [CrossRef]
- Alexander, J.T.; Hai, F.I.; Al-aboud, T.M. Chemical coagulation-based processes for trace organic contaminant removal: Current state and future potential. J. Environ. Manag. 2012, 111, 195–207. [Google Scholar] [CrossRef]
- Matamoros, V.; Salvadó, V. Evaluation of a coagulation/flocculation-lamellar clarifier and filtration-UV-chlorination reactor for removing emerging contaminants at full-scale wastewater treatment plants in Spain. J. Environ. Manag. 2013, 117, 96–102. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, H.; Cicek, N. Treatment of organic micropollutants in water and wastewater by UV-based processes: A literature review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1443–1476. [Google Scholar] [CrossRef]
- Guillossou, R.; Le Roux, J.; Mailler, R.; Vulliet, E.; Morlay, C.; Nauleau, F.; Gasperi, J.; Rocher, V. Organic micropollutants in a large wastewater treatment plant: What are the benefits of an advanced treatment by activated carbon adsorption in comparison to conventional treatment? Chemosphere 2019, 218, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Margot, J.; Schärer, M.; Weil, M.; Magnet, A.; Kienle, C.; Thonney, D.; Rossi, L.; Abegglen, C.; Barry, D.A.; de Alencastro, L.F.; et al. Treatment of micropollutants in municipal wastewater: Ozone or powdered activated carbon? Sci. Total Environ. 2013, 461–462, 480–498. [Google Scholar] [CrossRef] [PubMed]
- Zietzschmann, F.; Worch, E.; Altmann, J.; Ruhl, A.S.; Sperlich, A.; Meinel, F.; Jekel, M. Impact of EfOM size on competition in activated carbon adsorption of organic micro-pollutants from treated wastewater. Water Res. 2014, 65, 297–306. [Google Scholar] [CrossRef]
- Dai, Y.D.; Shah, K.J.; Huang, C.P.; Kim, H.; Chiang, P.C. Adsorption of nonylphenol to multi-walled carbon nanotubes: Kinetics and isotherm study. Appl. Sci. 2018, 8, 2295. [Google Scholar] [CrossRef]
- Wong, K.T.; Eu, N.C.; Ibrahim, S.; Kim, H.; Yoon, Y.; Jang, M. Recyclable magnetite-loaded palm shell-waste based activated carbon for the effective removal of methylene blue from aqueous solution. J. Clean. Prod. 2018, 115, 337–342. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Y.; Si, F.; Yao, C.; Du, M.; Hussain, I.; Kim, H.; Huang, S.; Zhang, L.; Hayat, W. Persulfate non-radical activation by nano-CuO for efficient removal of chlorinated organic compounds: Reduced graphene oxide-assisted and CuO (0 0 1) facet-dependent. Chem. Eng. J. 2019, 356, 178–189. [Google Scholar] [CrossRef]
- Sharma, V.K.; McDonald, T.J.; Kim, H.; Garg, V.K. Magnetic graphene–carbon nanotube iron nanocomposites as adsorbents and antibacterial agents for water purification. Adv. Colloid Interface Sci. 2015, 225, 229–240. [Google Scholar] [CrossRef]
- Li, P.; Liu, L.; Wu, J.; Cheng, R.; Shi, L.; Zheng, X.; Zhang, Z. Identify driving forces of MBR applications in China. Sci. Total Environ. 2019, 647, 627–638. [Google Scholar] [CrossRef]
- Bui, X.T.; Vo, T.P.T.; Ngo, H.H.; Guo, W.S.; Nguyen, T.T. Multricriteria assessment of advanced treatment technologies for micropollutatns removal at large-scale applications. Sci. Total Environ. 2016, 563–564, 1050–1067. [Google Scholar] [CrossRef]
- Adenaert, W.T.M.; Chys, M.; Auvinen, H.; Dumoulin, A.; Rousseau, D.; Van Hulle, S.W.H. (Future) Regulation of Trace Organic Compounds in WWTP Effluents as a Driver for Advanced Wastewater Treatment. Ozone News 2014, 42, 17–23. [Google Scholar]

| Pharmaceuticals | Molecular Formula | Molecular Weight (g/mol) | Octanol-Water Partition Coefficient (Log Kow) | Acid Dissociation Constant (pKa) | Water Solubility (mg/mL) |
|---|---|---|---|---|---|
| Analgesics/Non-steroidal anti-inflammatory drugs (NSAIDs) | |||||
| Acetaminophen | C8H9NO2 | 151.2 | 0.5 | 9.4 | 30.4 |
| Acetylsalicylic acid | C9H8O4 | 180.2 | 1.2 | 3.5 | 4.6 |
| Diclofenac | C14H11Cl2NO2 | 296.2 | 3.9 | 4.2 | 4.5 × 10−3 |
| Ibuprofen | C13H18O2 | 206.3 | 3.6 | 4.9 | 4.1 × 10−2 |
| Ketoprofen | C16H14O3 | 254.3 | 3.1 | 4.5 | 0.1 |
| Naproxen | C14H14O3 | 230.3 | 3.2 | 4.2 | 0.1 |
| Antibiotics | |||||
| Cefradine | C16H19N3O4S | 349.4 | −0.3 | 2.6/7.3 | 2.8 |
| Ciprofloxacin | C17H18FN3O3 | 331.4 | 0.3 | 6.1/8.7 | 11.5 |
| Clarithromycin | C38H69NO13 | 748 | 3.2 | 9 | 3.4 × 10−4 |
| Erythromycin | C37H67NO13 | 734 | 3.1 | 8.9 | 5.2 × 10−4 |
| Ofloxacin | C18H20FN3O4 | 361.4 | −0.4 | 6.3/7.9 | 28.3 |
| Oxolinic acid | C13H11NO5 | 261.2 | 0.9 | 6.9 | 8 |
| Roxithromycin | C41H76N2O15 | 837.1 | 2.8 | 9.2 | 1.9×10−5 |
| Sulfadimethoxine | C12H14N4O4S | 310.3 | 1.6 | 2.1/6.1 | 0.4 |
| Sulfamethazine | C12H14N4O2S | 278.3 | 0.6 | 2.1/7.5 | 11.3 |
| Sulfamethoxazole | C10H11N3O3S | 253.3 | 0.9 | 1.6/5.7 | 3.9 |
| Trimethoprim | C14H18N4O3 | 290.3 | 0.9 | 7.1 | 2.3 |
| Antiarrhythmic agents | |||||
| Atenolol | C14H22N2O3 | 266.3 | 0.2 | 9.6 | 0.7 |
| Propranolol | C16H21NO2 | 259.4 | 0.7 | 9.4 | 0.2 |
| Antihistamines | |||||
| Cimetidine | C10H16N6S | 252.3 | 0.4 | 6.8 | 10.5 |
| Diphenhydramine | C17H21NO | 255.4 | 3.3 | 9 | 0.4 |
| Hormone | |||||
| Testosterone | C19H28O2 | 288.4 | 3.3 | - | 0.1 |
| Stimulant | |||||
| Caffeine | C8H10N4O2 | 194.2 | −0.1 | 14 | 2.6 |
| Others | |||||
| Carbamazepine | C15H12N2O | 236.3 | 2.5 | 13.9 | 1.8 × 10−2 |
| Gemfibrozil | C15H22O3 | 250.3 | 4.8 | 4.5 | 5.0 × 10−3 |
| Iopromide | C18H24I3N3O8 | 791.1 | −2.1 | 10.6 | 2.4 × 10−2 |
| Sildenafil | C22H30N6O4S | 474.6 | 2.8 | 5.9 | 3.5 |
| STPs | Class | Influent | Primary Effluent | Secondary Effluent | Tertiary Effluent | Activated Sludge | Return Sludge | Waste Sludge | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | S | T | L | S | T | L | S | T | L | S | T | L | S | T | L | S | T | L | S | T | ||
| A | Stimulant | 989 | 1 | 990 | 941 | 4 | 945 | 0 | 0 | 0 | - | - | - | 0 | 21 | 22 | 1 | 65 | 66 | 0 | 0 | 0 |
| NSAIDs | 1613 | 11 | 1624 | 1697 | 17 | 1713 | 35 | 0 | 35 | - | - | - | 120 | 1041 | 1161 | 102 | 2883 | 2985 | 2 | 16 | 18 | |
| Antibiotics | 118 | 2 | 121 | 94 | 2 | 96 | 31 | 0 | 31 | - | - | - | 22 | 610 | 632 | 61 | 990 | 1051 | 1 | 27 | 28 | |
| Others | 140 | 1 | 141 | 138 | 1 | 138 | 127 | 0 | 127 | - | - | - | 154 | 257 | 411 | 106 | 602 | 709 | 2 | 9 | 11 | |
| Total | 2860 | 16 | 2876 | 2869 | 24 | 2893 | 193 | 0 | 193 | - | - | - | 297 | 1929 | 2226 | 270 | 4541 | 4811 | 5 | 53 | 58 | |
| B | Stimulant | 1196 | 1 | 1197 | 1120 | 1 | 1121 | 1 | 0 | 1 | 1 | 2 | 3 | 0 | 9 | 9 | 0 | 7 | 7 | 0 | 0 | 0 |
| NSAIDs | 4096 | 13 | 4109 | 4547 | 18 | 4564 | 191 | 7 | 198 | 218 | 12 | 231 | 301 | 411 | 712 | 81 | 292 | 373 | 3 | 12 | 15 | |
| Antibiotics | 298 | 6 | 303 | 321 | 4 | 325 | 113 | 10 | 123 | 109 | 2 | 111 | 200 | 368 | 568 | 15 | 413 | 427 | 1 | 16 | 17 | |
| Others | 939 | 2 | 941 | 804 | 3 | 807 | 458 | 5 | 463 | 443 | 2 | 444 | 328 | 104 | 432 | 112 | 283 | 395 | 4 | 11 | 16 | |
| Total | 6529 | 21 | 6550 | 6791 | 26 | 6817 | 763 | 23 | 785 | 771 | 18 | 789 | 828 | 892 | 1720 | 208 | 995 | 1203 | 8 | 39 | 48 | |
| C | Stimulant | 832 | 1 | 833 | 740 | 1 | 741 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 4 | 0 | 111 | 111 | 0 | 0 | 0 |
| NSAIDs | 1597 | 7 | 1604 | 1762 | 6 | 1768 | 85 | 2 | 87 | 78 | 3 | 81 | 89 | 294 | 383 | 308 | 2818 | 3126 | 2 | 11 | 13 | |
| Antibiotics | 105 | 2 | 107 | 136 | 1 | 137 | 94 | 1 | 95 | 89 | 1 | 90 | 75 | 328 | 403 | 110 | 1417 | 1527 | 0 | 6 | 6 | |
| Others | 181 | 0 | 182 | 188 | 0 | 188 | 130 | 0 | 131 | 125 | 0 | 125 | 89 | 80 | 169 | 363 | 879 | 1243 | 2 | 4 | 6 | |
| Total | 2716 | 10 | 2725 | 2826 | 8 | 2834 | 309 | 4 | 313 | 292 | 5 | 297 | 253 | 706 | 959 | 782 | 5225 | 6006 | 4 | 22 | 25 | |
| D | Stimulant | 2242 | 11 | 2253 | 2159 | 5 | 2164 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 15 | 15 | 0 | 29 | 29 | 0 | 0 | 0 |
| NSAIDs | 2315 | 15 | 2329 | 2914 | 11 | 2925 | 55 | 2 | 57 | 60 | 3 | 63 | 93 | 204 | 297 | 75 | 423 | 498 | 1 | 6 | 7 | |
| Antibiotics | 156 | 3 | 160 | 143 | 2 | 144 | 73 | 8 | 82 | 58 | 4 | 62 | 58 | 110 | 169 | 19 | 392 | 411 | 0 | 6 | 6 | |
| Others | 210 | 1 | 211 | 208 | 0 | 209 | 111 | 0 | 112 | 85 | 0 | 85 | 136 | 57 | 194 | 96 | 245 | 341 | 1 | 4 | 5 | |
| Total | 4923 | 30 | 4953 | 5424 | 18 | 5443 | 240 | 11 | 251 | 204 | 8 | 212 | 289 | 386 | 675 | 190 | 1089 | 1279 | 3 | 16 | 19 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Kim, C.; Hong, Y.; Lee, W.; Chung, H.; Jeong, D.-H.; Kim, H. Distribution and Removal of Pharmaceuticals in Liquid and Solid Phases in the Unit Processes of Sewage Treatment Plants. Int. J. Environ. Res. Public Health 2020, 17, 687. https://doi.org/10.3390/ijerph17030687
Park J, Kim C, Hong Y, Lee W, Chung H, Jeong D-H, Kim H. Distribution and Removal of Pharmaceuticals in Liquid and Solid Phases in the Unit Processes of Sewage Treatment Plants. International Journal of Environmental Research and Public Health. 2020; 17(3):687. https://doi.org/10.3390/ijerph17030687
Chicago/Turabian StylePark, Junwon, Changsoo Kim, Youngmin Hong, Wonseok Lee, Hyenmi Chung, Dong-Hwan Jeong, and Hyunook Kim. 2020. "Distribution and Removal of Pharmaceuticals in Liquid and Solid Phases in the Unit Processes of Sewage Treatment Plants" International Journal of Environmental Research and Public Health 17, no. 3: 687. https://doi.org/10.3390/ijerph17030687
APA StylePark, J., Kim, C., Hong, Y., Lee, W., Chung, H., Jeong, D.-H., & Kim, H. (2020). Distribution and Removal of Pharmaceuticals in Liquid and Solid Phases in the Unit Processes of Sewage Treatment Plants. International Journal of Environmental Research and Public Health, 17(3), 687. https://doi.org/10.3390/ijerph17030687







