Establishment of an Automatic Real-Time Monitoring System for Irrigation Water Quality Management
Abstract
1. Introduction
2. Materials and Methods
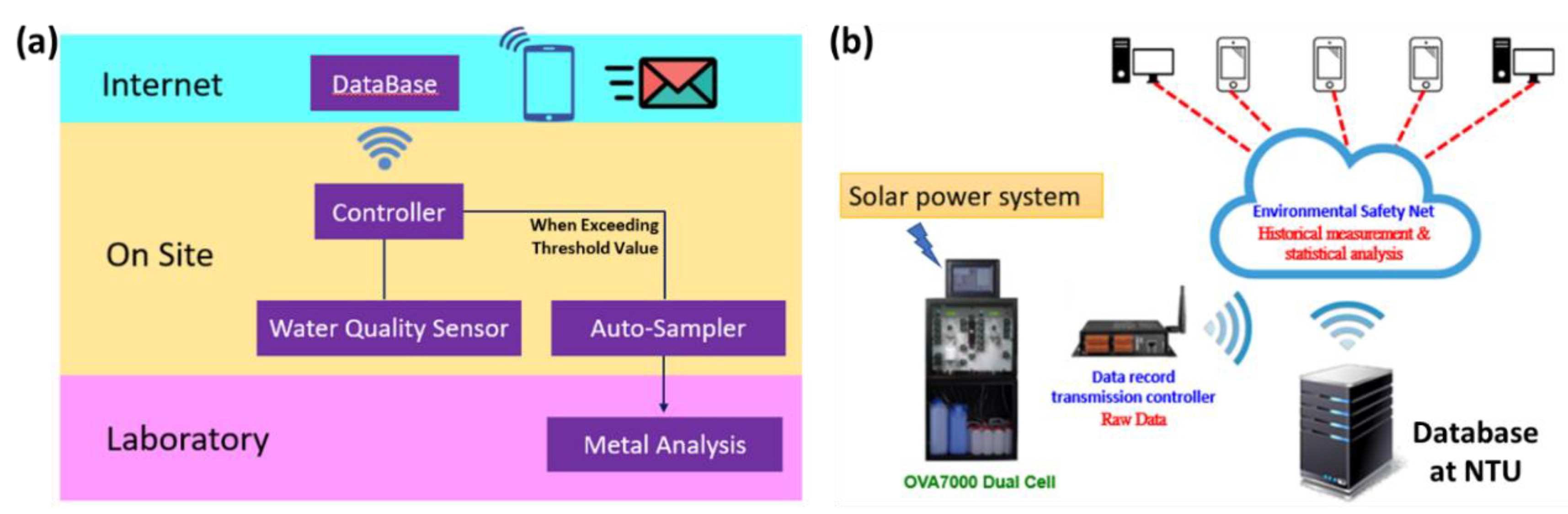
2.1. Establishment of Automatic Real-Time Monitoring System
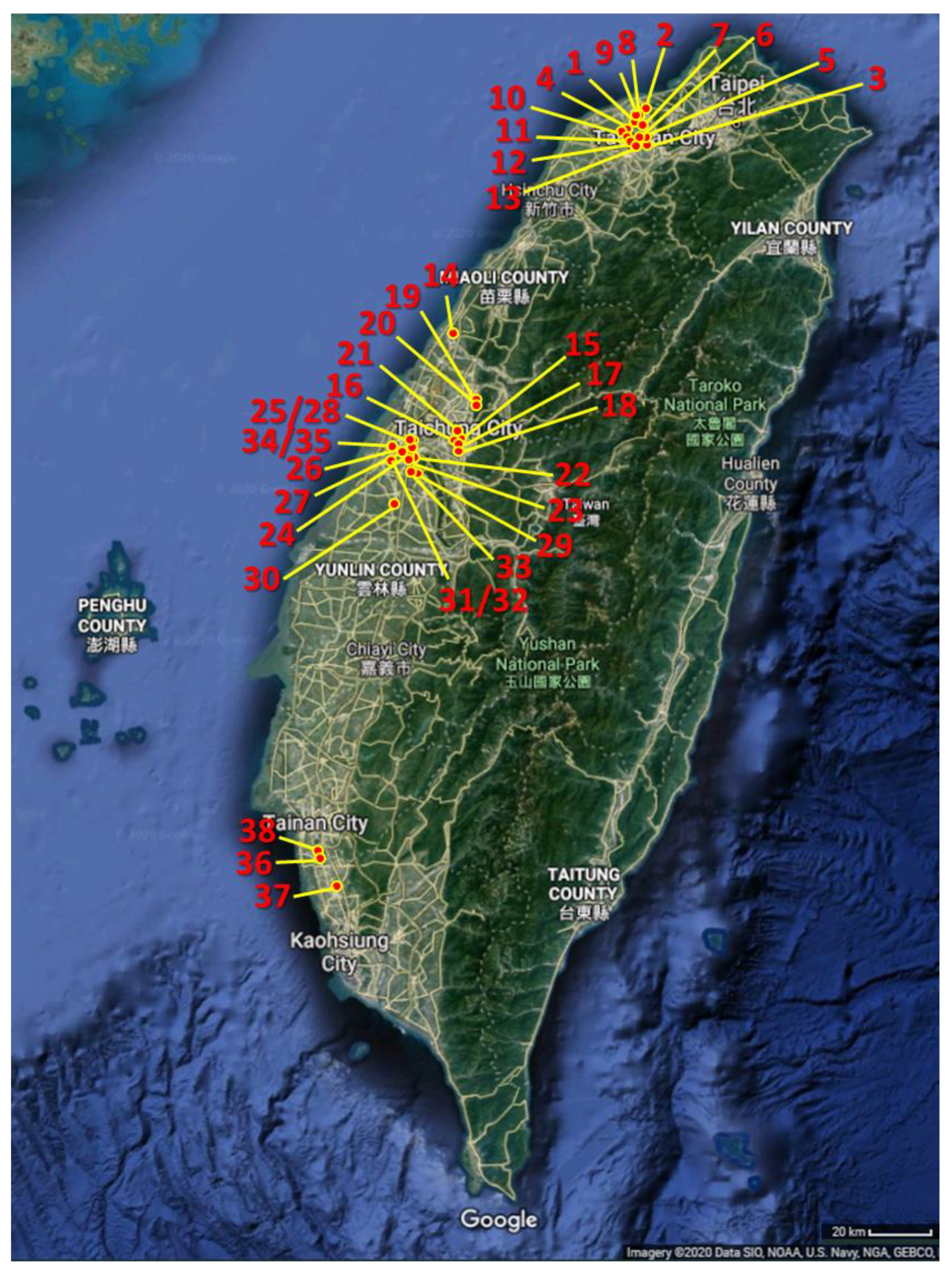
2.2. Locations of Basic Water Quality Stations
2.3. Data Acquisition, Collection, and Correction
3. Results and Discussion
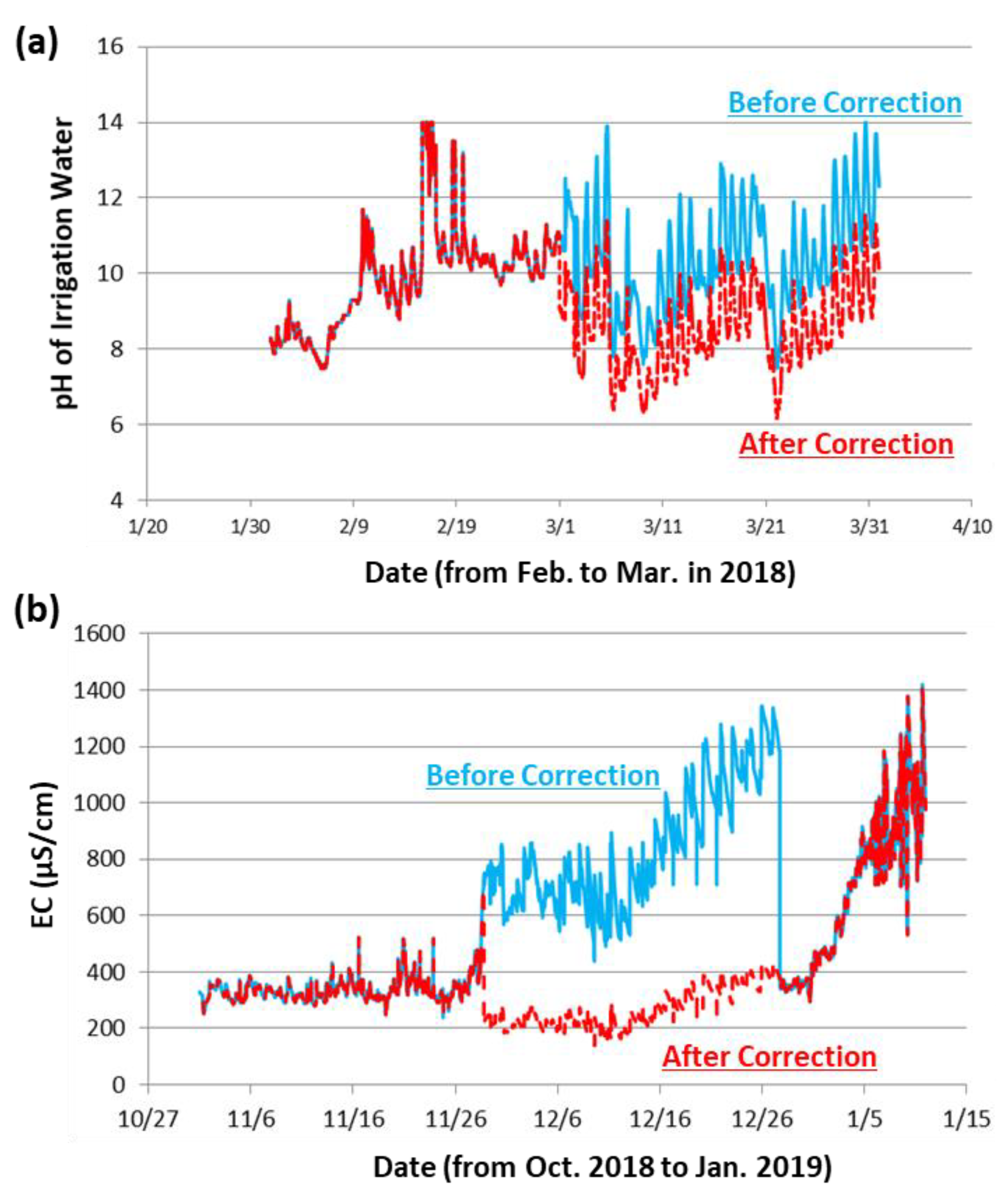
3.1. Data Correction for Basic Water Quality Parameters: pH and EC
3.2. Trend and Variance of Water Quality Parameters for Different Types of Monitoring Stations: Exemplified by Electrical Conductance
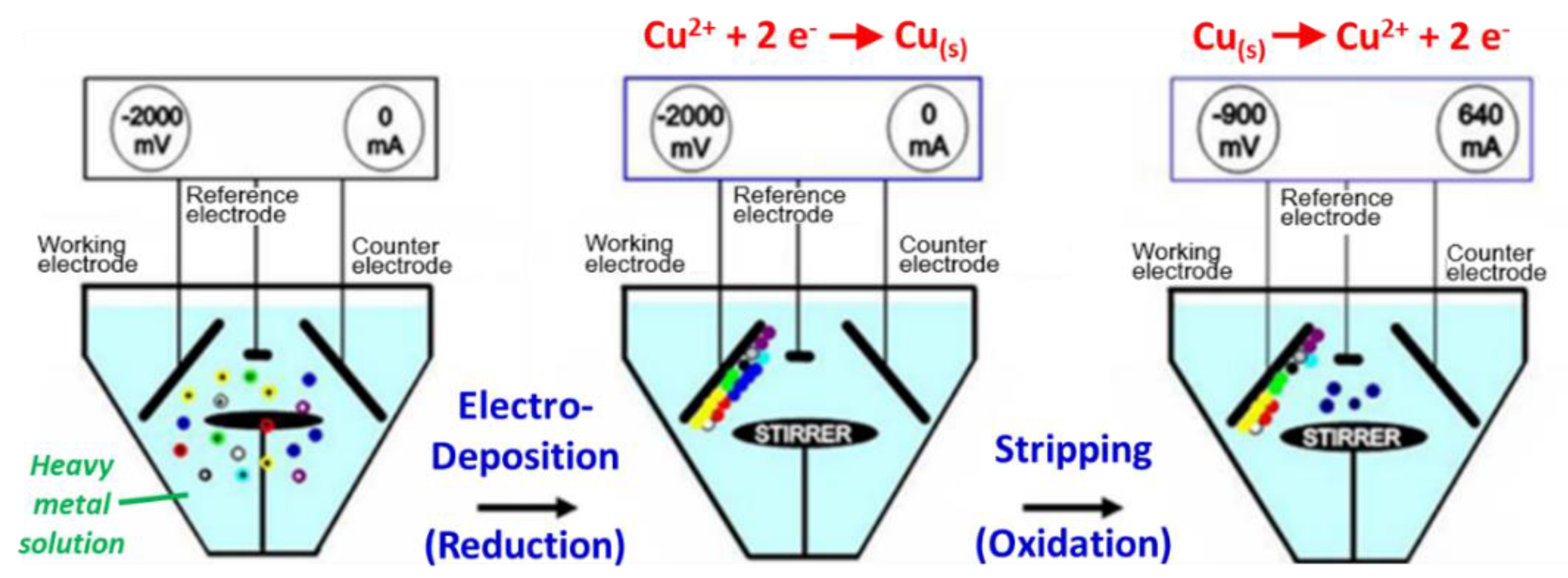
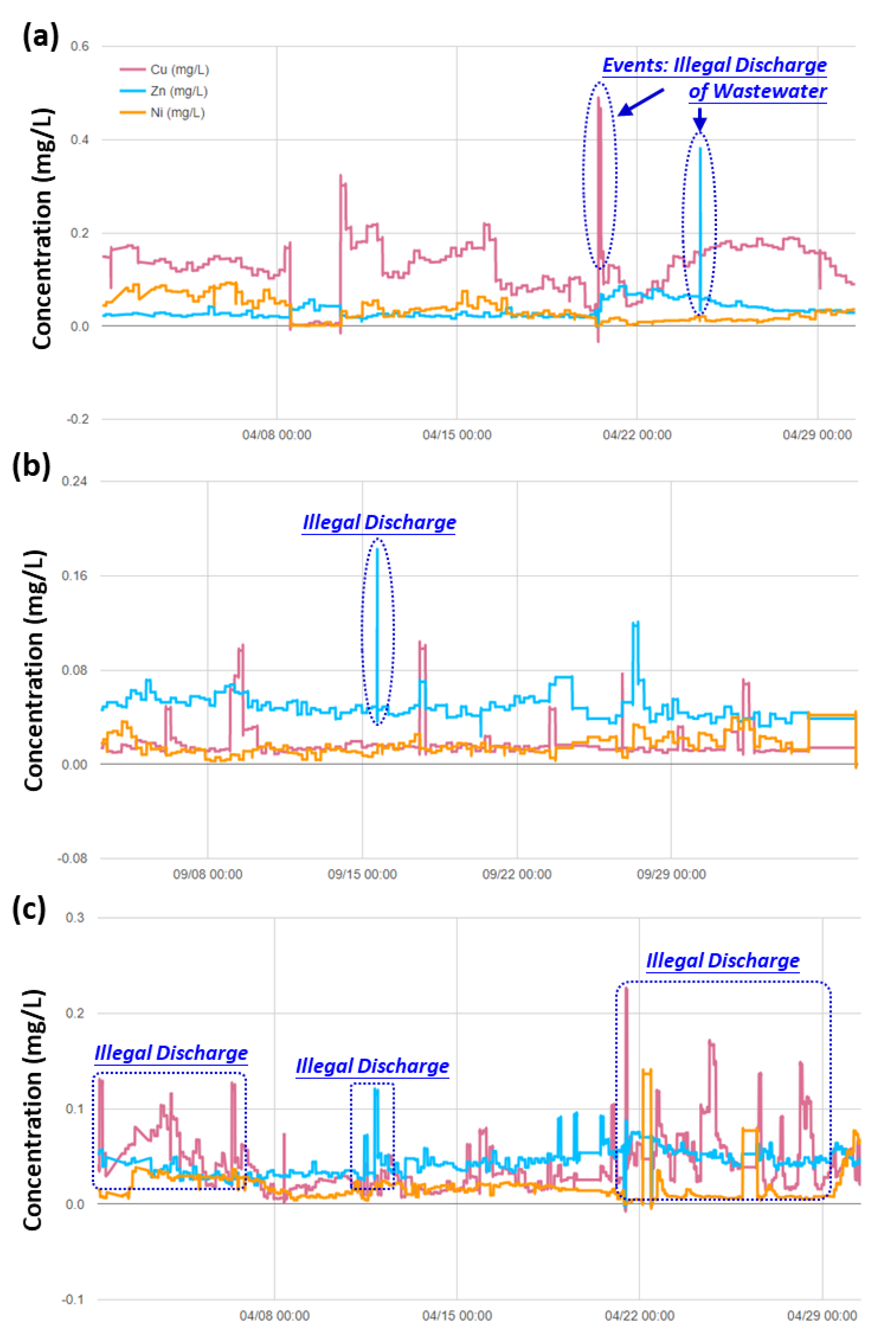
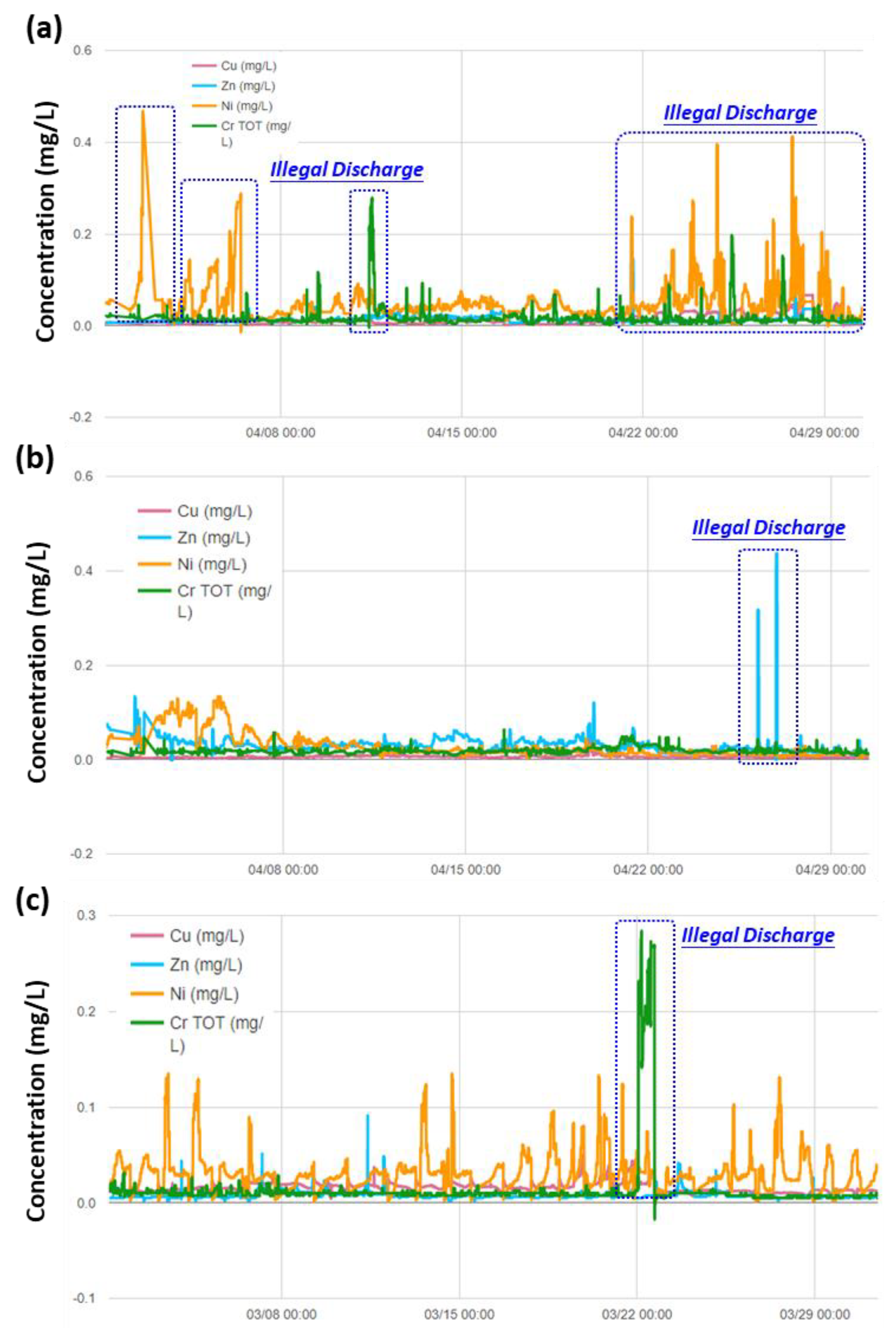
3.3. Real-time Automatic Monitoring for Heavy Metals
3.4. Implications to Ensuring the Safety of Irrigation Water
- (1)
- Integration with Automatic Sampling for Establishing Information Exchange Platform.
- (2)
- Estimating Fluxes of Heavy Metals to Paddy Fields.
- (3)
- Combining with Green Technologies for Nonpoint Source Pollution Control.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- USEPA. Online Water Quality Monitoring Resources. Available online: https://www.epa.gov/waterqualitysurveillance/online-water-quality-monitoring-resources (accessed on 7 October 2019).
- Ruiqi Technology. Continuous Automatic Monitoring and Analysis of Irrigation Water Quality Commissioned Professional Service Plan (2014–2016); Farmland Water Conservation Association: Taipei, Taiwan, 2017. [Google Scholar]
- Tamkang University. Irrigation Water Quality Monitoring Survey and Technical Counseling Plan (2015–2017); Agricultural Council of the Executive Yuan: Taipei, Taiwan, 2018.
- Council of Agriculture. Agricultural area. In Statistic Information Database; Council of Agriculture, Executive Yuan, Eds.; Executive Yuan: Taipei, Taiwan, 2018. [Google Scholar]
- Rock, C.M.; Brassill, N.; Dery, J.L.; Carr, D.; McLain, J.E.; Bright, K.R.; Gerba, C.P. Review of water quality criteria for water reuse and risk-based implications for irrigated produce under the FDA Food Safety Modernization Act, produce safety rule. Environ. Res. 2019, 172, 616–629. [Google Scholar] [CrossRef] [PubMed]
- National Taiwan University. Established Automatic Monitoring Network and Monitoring Technology for Irrigation Water Quality (2016–2018); Agricultural Committee of the Executive Yuan: Taipei, Taiwan, 2019.
- Huang, J.S.; Lin, S.C.; Lowemark, L.; Liou, S.Y.H.; Chang, Q.; Chang, T.K.; Wei, K.Y.; Croudace, I.W. Rapid assessment of heavy metal pollution using ion-exchange resin sachets and micro-XRF core-scanning. Sci. Rep. 2019, 9, 6601. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.-K.; Chiang, L.-C.; Lin, S.-C.; Chang, T.-K.; Hsu, W.-C. Application of Time-Lapse Ion Exchange Resin Sachets (TIERS) for Detecting Illegal Effluent Discharge in Mixed Industrial and Agricultural Areas, Taiwan. Sustainability 2019, 11, 3129. [Google Scholar] [CrossRef]
- Chowdury, M.S.U.; Emran, T.B.; Ghosh, S.; Pathak, A.; Alam, M.M.; Absar, N.; Andersson, K.; Hossain, M.S. IoT Based Real-time River Water Quality Monitoring System. Procedia Comput. Sci. 2019, 155, 161–168. [Google Scholar] [CrossRef]
- Meng, D.; Zhao, N.; Wang, Y.; Ma, M.; Fang, L.; Gu, Y.; Jia, Y.; Liu, J. On-line/on-site analysis of heavy metals in water and soils by laser induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2017, 137, 39–45. [Google Scholar] [CrossRef]
- Borrill, A.J.; Reily, N.E.; Macpherson, J.V. Addressing the practicalities of anodic stripping voltammetry for heavy metal detection: A tutorial review. Analyst 2019, 144, 6834–6849. [Google Scholar] [CrossRef] [PubMed]
- PAR. Fundamentals of Stripping Voltammetry. OVA7000 On-line Metal. Monitor; Princeton Applied Research: Oak Ridge, TN, USA, 2019; Volume Application Note S-6. [Google Scholar]
- Council of Agriculture. Irrigation Water Quality Standard; Executive Yuan: Taipei, Taiwan, 2003; Volume 0920031524.
- Wagner, R.J.; Boulger, R.W.; Oblinger, C.J.; Smith, B.A. Guidelines and Standard Procedures for Continuous Water-Quality Monitors: Station Operation, Record Computation, and Data Reporting; U.S. Geological Survey: Reston, VA, USA, 2006.
- Yi, K.; Fan, W.; Chen, J.; Jiang, S.; Huang, S.; Peng, L.; Zeng, Q.; Luo, S. Annual input and output fluxes of heavy metals to paddy fields in four types of contaminated areas in Hunan Province, China. Sci. Total Environ. 2018, 634, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, H. Accumulation of Heavy Metals in Roadside Soil in Urban Area and the Related Impacting Factors. Int. J. Environ. Res. Public Health 2018, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, H.; Alvenäs, G.; Nilsson, S.I.; Hultman, B.; Öborn, I. Cadmium, copper and zinc leaching and surface run-off losses at the Öjebyn farm in Northern Sweden—Temporal and spatial variation. Agric. Ecosyst. Environ. 2006, 113, 120–138. [Google Scholar] [CrossRef]
- Srinivas, R.; Singh, A.P.; Dhadse, K.; Garg, C. An evidence based integrated watershed modelling system to assess the impact of non-point source pollution in the riverine ecosystem. J. Clean. Prod. 2019. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, T.; Li, J.; Li, Z.; Xu, G.; Yang, R. Reducing Nitrogen and Phosphorus Losses from Different Crop Types in the Water Source Area of the Danjiang River, China. Int. J. Environ. Res. Public Health 2019, 16, 3442. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.-Y.; Lin, Y.J.; Snyder, S.W.; Ma, H.-W.; Chiang, P.-C. Development of Low-Carbon-Driven Bio-product Technology Using Lignocellulosic Substrates from Agriculture: Challenges and Perspectives. Curr. Sustain. Renew. Energy Rep. 2015, 2, 145–154. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.; Pei, M.; Qiu, L.; Qiang, H.; Yao, Y. Performance of Anaerobic Digestion of Chicken Manure Under Gradually Elevated Organic Loading Rates. Int. J. Environ. Res. Public Health 2019, 16, 2239. [Google Scholar] [CrossRef]


| Item | Water Quality Parameter | |||
|---|---|---|---|---|
| pH | Temp. | EC | Heavy Metal | |
| Range of measurement | 0‒14 | −5 °C~50 °C | 0‒100 mS/cm | 0.5‒10.0 µg/L |
| Accuracy | ±0.2 | ±0.1 °C | ±1% full scale | 0.01 µg/L |
| Resolution | 0.01 | 0.01 °C | 1 mV | −2 V~+2 V (Sensitivity: 1 nA) |
| Frequency of Data | 1 min | 1 min | 1 min | <30 min |
| Volume of Feed Water | In-situ | In-situ | In-situ | 10 mL |
| Maintenance | Once a month | Once a month | Once a month | Once a month |
| ID | Irrigation Associations | Station Location | Associated Watershed | Monitoring Items a | Threshold for pH (-) | Threshold for EC (µS/cm) |
|---|---|---|---|---|---|---|
| 1 | Taoyuan | No. 25-3 River Dam | Sanquaichuo Branch | pH, EC, Tm | 6 and 9 | 750 |
| 2 | Shuchuo Intake | Shuchuo Drainage | pH, EC, Tm | 6 and 9 | 750 | |
| 3 | Erzhixian | Taoyuan Daquan | pH, EC, Tm, Water Level | 6 and 9 | 750 | |
| 4 | No 6-14 Intake | Laojie Stream | pH, EC, Tm, Water Level | 6 and 9 | 750 | |
| 5 | Qiedong Upstream | Qiedong Stream | pH, EC, Tm, Water Level | 6 and 9 | 750 | |
| 6 | Xiazhongfu Drainage | Xiazhongfu Drainage | pH, EC, Tm, Water Level | 6 and 9 | 750 | |
| 7 | No. 24 Puxin Stream | Puxin Stream | pH, EC, Tm, Water Level | 6 and 9 | 1200 | |
| 8 | No. 25-7 Puxin Stream | Puxin Stream | pH, EC, Tm, Water Level | 6 and 9 | 1200 | |
| 9 | No. 25 River Dam | Sanquaichuo Branch | pH, EC, Tm, Water Level | 6 and 9 | 1500 | |
| 10 | No. 6-5 Intake | Qia Stream | pH, EC, Tm, Water Level | 6 and 9 | 750 | |
| 11 | No. 34 Intake | Xinjie Stream | pH, EC, Tm, Water Level | 6 and 9 | 750 | |
| 12 | Xinjie Upstream | Xinjie Stream | pH, EC, Tm, Cd, Pb, Cu, Ni, Zn | 6 and 9 | 750 | |
| 13 | Xinzhuang No. 3 Bridge | Sanquaichuo Branch | pH, EC, Tm, Cd, Pb, Cu, Ni, Zn | 6 and 9 | 4000 | |
| 14 | Taichung | No. 35 Yuanlizhen Shangan | Xinfugou | pH, EC, Tm | 6 and 9 | 750 |
| 15 | Datuliaozhenyigei Intake | Dali Stream | pH, EC, Tm, Water Level | 6 and 9 | 750 | |
| 16 | Datuliaozhenyigei Downstream | Dali Stream | pH, EC, Tm, Water Level | 6 and 9 | 750 | |
| 17 | Zhancuoyuanzhen Zhongxing Drainage | Zhongxing Drainage | pH, EC, Tm, Water Level | 6 and 9 | 750 | |
| 18 | Zhancuoyuanzhen | Toubiankeng Stream | pH, EC, Tm, Water Level | 6 and 9 | 750 | |
| 19 | Babaozhen Niuchou Branch | Dajia Stream | pH, EC, Tm, Water Level | 6 and 9 | 750 | |
| 20 | Babaozhen Shangpi Branch | Dajia Stream | pH, EC, Tm, Water Level | 6 and 9 | 750 | |
| 21 | No. 2, 3 Zhancuoyuanzhen | Zhongxing Drainage | pH, EC, Tm, Cd, Pb, Cu, Ni, Zn, Cr | 6 and 9 | 750 | |
| 22 | Changhua | Dongxi Erzhen Jiali Branch | Wu Stream | pH, EC, Tm, Water Level | 6 and 9 | 750 |
| 23 | Dongxi Erzhen Gongcuo Branch | Wu Stream | pH, EC, Tm, Water Level | 6 and 9 | 750 | |
| 24 | Dongxi Shanzhen Tieshan Branch | Wu Stream | pH, EC, Tm, Water Level | 6 and 9 | 750 | |
| 25 | Si-Liu Guzhen | Wu Stream | pH, EC, Tm, Water Level | 6 and 9 | 750 | |
| 26 | Xinpijiuzhen Intake | Fanyagou | pH, EC, Tm, Water Level | 6 and 9 | 750 | |
| 27 | Xinzhen Intake | Yangzicuo Stream | pH, EC, Tm, Water Level | 6 and 9 | 750 | |
| 28 | Dongxi Shanzhen Midstream | Wu Stream | pH, EC, Tm, Water Level | 6 and 9 | 750 | |
| 29 | Jintunzhen Intake | Shigou Drainage | pH, EC, Tm, Water Level | 6 and 9 | 1500 | |
| 30 | Zhangshui Road | jiu zhuo shui Stream | pH, EC, Tm, Water Level | 6 and 9 | 750 | |
| 31 | Andong Erpai | Andong Erpai | pH, EC, Tm, Water Level | 6 and 9 | 1500 | |
| 32 | Gongcuo Branch | Wu Stream | pH, EC, Tm, Water Level | 6 and 9 | 1200 | |
| 33 | Sanjiachun Drainage | Sanjiachun Drainage | pH, EC, Tm, Water Level | 6 and 9 | 1200 | |
| 34 | Fanyagou Branch Intake | Fanyagou | pH, EC, Tm, Water Level | 6 and 9 | 750 | |
| 35 | Xinzhen Midstream | yang zi cuo Stream | pH, EC, Tm, Cd, Pb, Cu, Ni, Zn, Cr | 6 and 9 | 750 | |
| 36 | Kaohsiung | Hunei Erren Downstream | Erren River | pH, EC, Tm Water Level | 6 and 9 | 1200 |
| 37 | Weisui Pumping Station | Wujiawei | pH, EC, Tm, Water Level | 6 and 9 | 3500 | |
| 38 | Hankou | District Road | pH, EC, Tm, Cd, Pb, Cu, Ni, Zn, Cr | 6 and 9 | 750 |
| Item | Guideline | Description |
|---|---|---|
| 1 | Head distance of external autosampler | The head should be 5 m, and the horizontal distance should be 30 m. |
| 2 | Avoid use in harsh environments | High humidity and high gas flow rate would easily cause corrosion and failure of the motherboard’s signal collection board. |
| 3 | The extent of water quality would affect the maintenance frequency | If the water sample in the field is viscous, it would increase the frequency of temporary cleaning and polishing of analytical electrodes. |
| 4 | Sunlight must be sufficient to provide solar energy | If there is a shelter above the solar panel, use the AC power instead. |
| 5 | Avoid using in the vibrating environment | Do not place the equipment in a vibrating environment. Vibration would malfunction the equipment’s circuit board of precision. |
| 6 | Keep it in a horizontal position, in a customized sun and heat removal device | If the position is not maintained horizontally, the sample will be tilted and the stirring will be uneven, which will cause deviation in the analysis value. It is recommended to use a customized protection box (with double-layer insulation, top ventilation and heat dissipation), being fixed to the horizontal concrete foundation seat. Prevent dumping and theft! |
| 7 | Maintenance by professional and experienced engineers | Careless maintenance will increase the frequency and costs of repairs, or even damage the instrument. |
| Type | Heavy Metal Monitoring Station (This Study) | Water Quality Environment Monitoring Station | Manual Sampling (Laboratory Test) |
|---|---|---|---|
| Measure | Heavy metals | pH, EC, etc. | Heavy metals, pH, EC, etc. |
| Power supply | AC 90‒260 V (Solar energy) | AC 110/240 V | - |
| Types of water body sample | Rivers, lakes, groundwater | Rivers, lakes, groundwater | Rivers, lakes, groundwater |
| Accuracy of measure | High | High | Highest |
| Price of equipment (US $) | 7900 | 11,700 | Depending on the test item |
| Labor costs | Low | Low | High |
| Maintenance | Once a month | Once a month | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syu, W.-J.; Chang, T.-K.; Pan, S.-Y. Establishment of an Automatic Real-Time Monitoring System for Irrigation Water Quality Management. Int. J. Environ. Res. Public Health 2020, 17, 737. https://doi.org/10.3390/ijerph17030737
Syu W-J, Chang T-K, Pan S-Y. Establishment of an Automatic Real-Time Monitoring System for Irrigation Water Quality Management. International Journal of Environmental Research and Public Health. 2020; 17(3):737. https://doi.org/10.3390/ijerph17030737
Chicago/Turabian StyleSyu, Wei-Jhan, Tsun-Kuo Chang, and Shu-Yuan Pan. 2020. "Establishment of an Automatic Real-Time Monitoring System for Irrigation Water Quality Management" International Journal of Environmental Research and Public Health 17, no. 3: 737. https://doi.org/10.3390/ijerph17030737
APA StyleSyu, W.-J., Chang, T.-K., & Pan, S.-Y. (2020). Establishment of an Automatic Real-Time Monitoring System for Irrigation Water Quality Management. International Journal of Environmental Research and Public Health, 17(3), 737. https://doi.org/10.3390/ijerph17030737






