Disparities in Risks of Malaria Associated with Climatic Variability among Women, Children and Elderly in the Chittagong Hill Tracts of Bangladesh
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Study Population
2.2. Ethical Approval
2.3. Data Sources and Study Variables
2.4. Statistical Analysis
3. Results
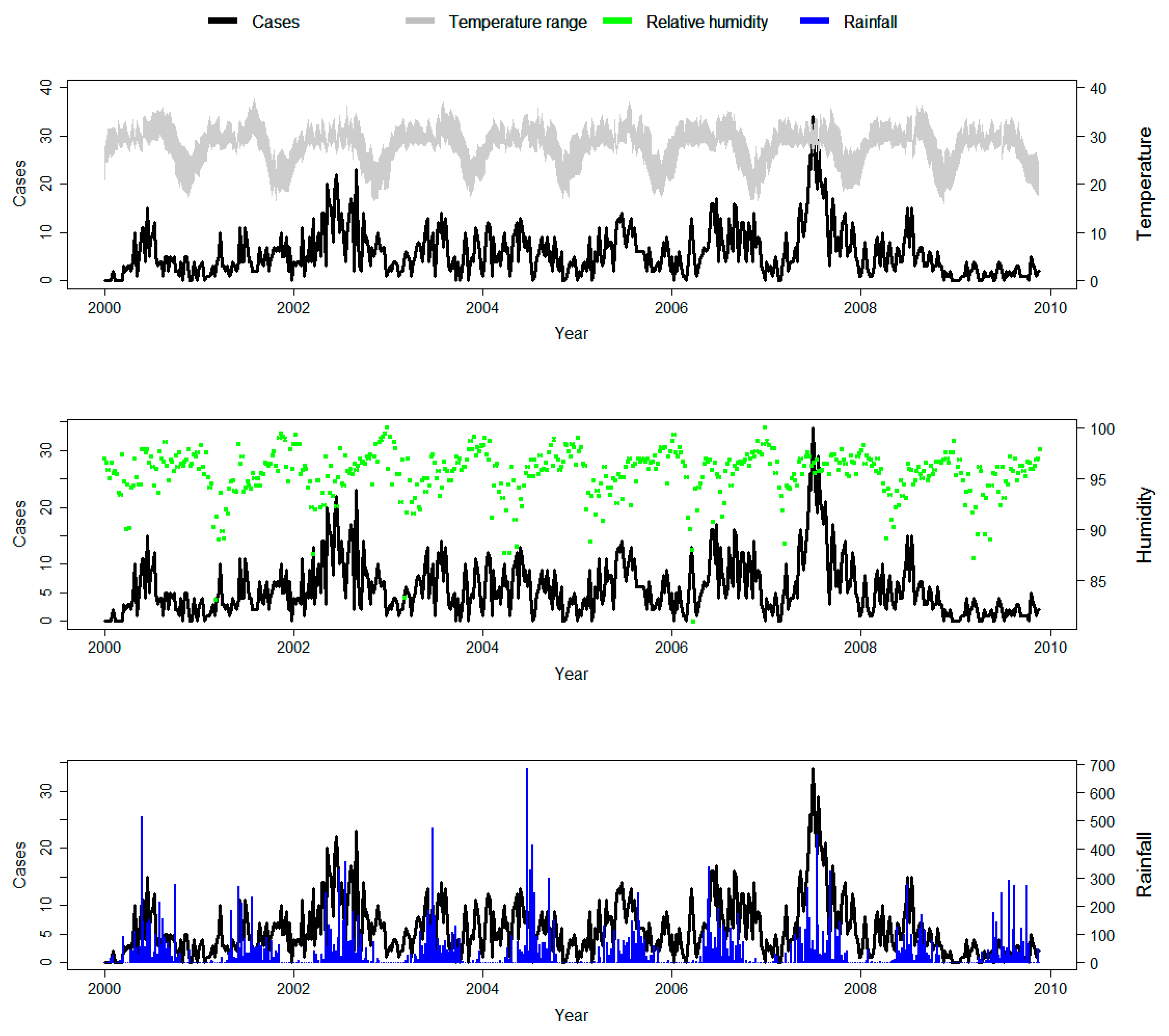
3.1. Descriptive Analysis
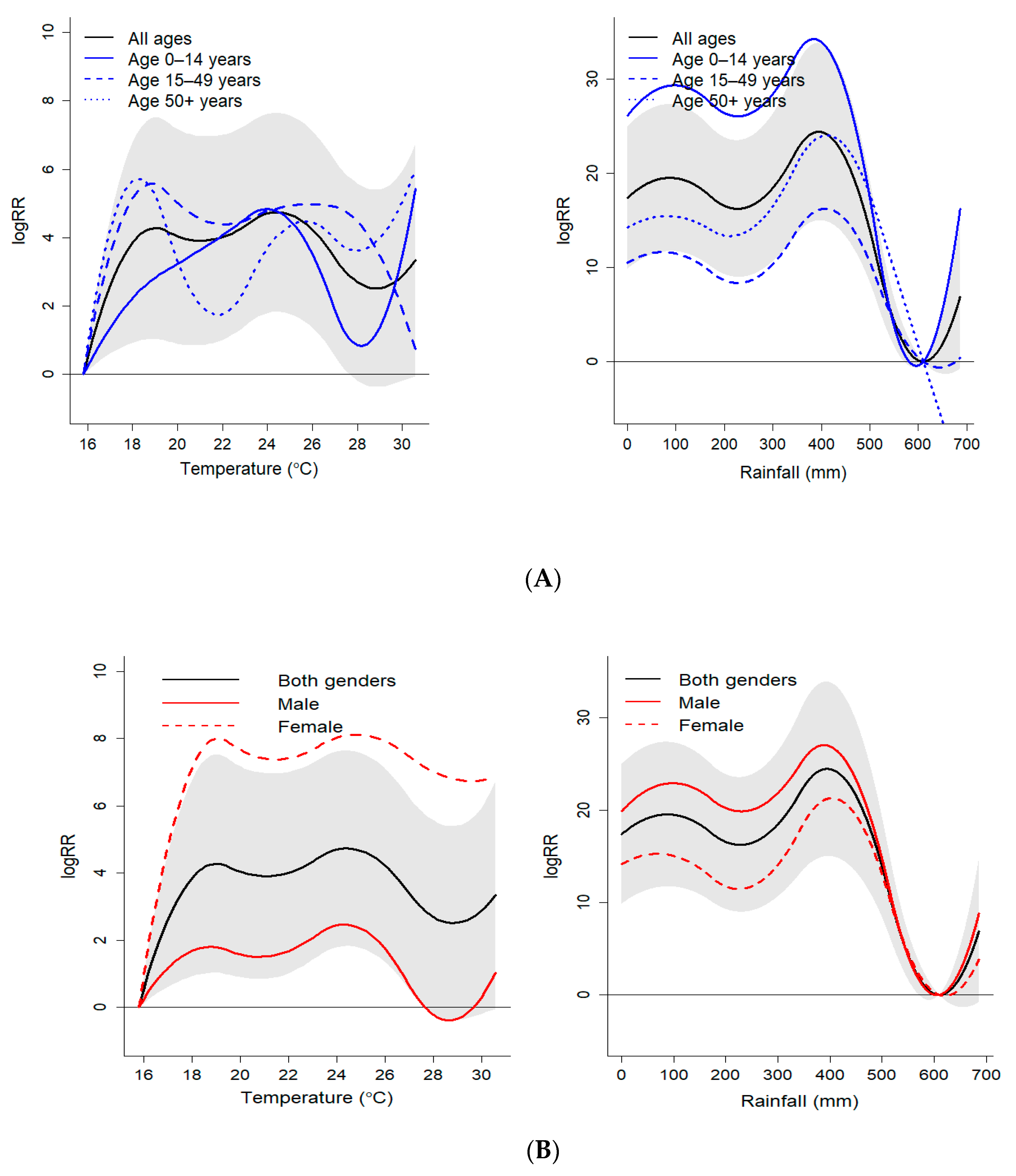
3.2. Association between Weather Variables and Malaria
3.3. Sub-Group Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cox, F.E. History of the discovery of the malaria parasites and their vectors. Parasites Vectors 2010, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Haque, U.; Overgaard, H.J.; Clements, A.C.A.; Norris, D.E.; Islam, N.; Karim, J.; Roy, S.; Haque, W.; Kabir, M.; Smith, D.L.; et al. Malaria burden and control in Bangladesh and prospects for elimination: An epidemiological and economic assessment. Lancet Glob. Health 2014, 2, e98–e105. [Google Scholar] [CrossRef]
- Noé, A.; Zaman, S.I.; Rahman, M.; Saha, A.K.; Aktaruzzaman, M.M.; Maude, R.J. Mapping the stability of malaria hotspots in Bangladesh from 2013 to 2016. Malar. J. 2018, 17, 259. [Google Scholar] [CrossRef] [PubMed]
- Haque, U.; Hashizume, M.; Glass, G.E.; Dewan, A.M.; Overgaard, H.J.; Yamamoto, T. The Role of Climate Variability in the Spread of Malaria in Bangladeshi Highlands. PLoS ONE 2010, 5, e14341. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A. Climate change and its impact on health in Bangladesh. Reg. Health Forum 2008, 12, 16–26. [Google Scholar]
- Baylis, M. Potential impact of climate change on emerging vector-borne and other infections in the UK. Environ. Health 2017, 16, 112. [Google Scholar] [CrossRef]
- Sutherst, R.W. Global Change and Human Vulnerability to Vector-Borne Diseases. Clin. Microbiol. Rev. 2004, 17, 136–173. [Google Scholar] [CrossRef]
- Githeko, A.K.; Lindsay, S.W.; Confalonieri, U.E.; Patz, J.A. Climate change and vector-borne diseases: A regional analysis. Bull. World Health Organ. 2000, 78, 1136–1147. [Google Scholar]
- Adegboye, O.; Leung, D.; Wang, Y. Analysis of spatial data with a nested correlation structure. J. R. Stat. Soc. Ser. C (Appl. Stat.) 2018, 67, 329–354. [Google Scholar] [CrossRef]
- Adegboye, O.; Al-Saghir, M.; Leung, D. Joint spatial time-series epidemiological analysis of malaria and cutaneous leishmaniasis infection. Epidemiol. Infect. 2017, 145, 685–700. [Google Scholar] [CrossRef]
- Haque, U.; Ahmed, S.M.; Hossain, S.; Huda, M.; Hossain, A.; Alam, M.S.; Mondal, D.; Khan, W.A.; Khalequzzaman, M.; Haque, R. Malaria prevalence in endemic districts of Bangladesh. PLoS ONE 2009, 4, e6737. [Google Scholar] [CrossRef]
- McMichael, A.J.; Campbell-Lendrum, D.H.; Corvalán, C.F.; Ebi, K.L.; Githeko, A.; Scheraga, J.D.; Woodward, A. Climate Change and Human Health: Risks and Responses; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Islam, A.T.; Shen, S.; Yang, S.; Hu, Z.; Chu, R. Assessing recent impacts of climate change on design water requirement of Boro rice season in Bangladesh. Theor. Appl. Climatol. 2019, 138, 97–113. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ahmad, S.; Mahmud, A.S.; Hassan-uz-Zaman, M.; Nahian, M.A.; Ahmed, A.; Nahar, Q.; Streatfield, P.K. Health consequences of climate change in Bangladesh: An overview of the evidence, knowledge gaps and challenges. Wiley Interdiscip. Rev. Clim. Chang. 2019, 10, e601. [Google Scholar] [CrossRef]
- Rahman, M.M.; Bodrud-Doza, M.; Shammi, M.; Islam, A.R.M.T.; Khan, A.S.M. COVID-19 pandemic, dengue epidemic, and climate change vulnerability in Bangladesh: Scenario assessment for strategic management and policy implications. Environ. Res. 2020, 192, 110303. [Google Scholar] [CrossRef] [PubMed]
- Martens, W.J.; Niessen, L.W.; Rotmans, J.; Jetten, T.H.; McMichael, A.J. Potential impact of global climate change on malaria risk. Environ. Health Perspect. 1995, 103, 458–464. [Google Scholar] [CrossRef]
- Kilian, A.; Langi, P.; Talisuna, A.; Kabagambe, G. Rainfall pattern, El Niño and malaria in Uganda. Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 22–23. [Google Scholar] [CrossRef]
- Briët, O.J.; Vounatsou, P.; Gunawardena, D.M.; Galappaththy, G.N.; Amerasinghe, P.H. Temporal correlation between malaria and rainfall in Sri Lanka. Malar. J. 2008, 7, 77. [Google Scholar] [CrossRef]
- Huang, F.; Zhou, S.; Zhang, S.; Wang, H.; Tang, L. Temporal correlation analysis between malaria and meteorological factors in Motuo County, Tibet. Malar. J. 2011, 10, 54. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sharma, C.; Dhiman, R.; Mitra, A. Climate change and malaria in India. Curr. Sci. 2006, 90, 369–375. [Google Scholar]
- Armstrong, B. Models for the relationship between ambient temperature and daily mortality. Epidemiology 2006, 17, 624–631. [Google Scholar] [CrossRef]
- Ali, A. Climate change impacts and adaptation assessment in Bangladesh. Clim. Res. 1999, 12, 109–116. [Google Scholar] [CrossRef]
- McMichael, A.J. Global climate change: Will it affect vector-borne infectious diseases? Intern. Med. J. 2003, 33, 554–555. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H. Climate change and vector-borne diseases of public health significance. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef] [PubMed]
- Adekunle, A.I.; Adegboye, O.A.; Rahman, K.M. Flooding in Townsville, North Queensland, Australia, in February 2019 and Its Effects on Mosquito-Borne Diseases. Int. J. Environ. Res. Public Health 2019, 16, 1393. [Google Scholar] [CrossRef] [PubMed]
- Caminade, C.; McIntyre, K.M.; Jones, A.E. Impact of recent and future climate change on vector-borne diseases. Ann. N. Y. Acad. Sci. 2019, 1436, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Mordecai, E.A.; Ryan, S.J.; Caldwell, J.M.; Shah, M.M.; LaBeaud, A.D. Climate change could shift disease burden from malaria to arboviruses in Africa. Lancet Planet. Health 2020, 4, e416–e423. [Google Scholar] [CrossRef]
- Caminade, C.; Kovats, S.; Rocklov, J.; Tompkins, A.M.; Morse, A.P.; Colón-González, F.J.; Stenlund, H.; Martens, P.; Lloyd, S.J. Impact of climate change on global malaria distribution. Proc. Natl. Acad. Sci. USA 2014, 111, 3286–3291. [Google Scholar] [CrossRef]
- Ryan, S.J.; Lippi, C.A.; Zermoglio, F. Shifting transmission risk for malaria in Africa with climate change: A framework for planning and intervention. Malar. J. 2020, 19, 1–14. [Google Scholar] [CrossRef]
- Ohm, J.R.; Baldini, F.; Barreaux, P.; Lefevre, T.; Lynch, P.A.; Suh, E.; Whitehead, S.A.; Thomas, M.B. Rethinking the extrinsic incubation period of malaria parasites. Parasites Vectors 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Gasparrini, A.; Armstrong, B.; Kenward, M.G. Distributed lag non-linear models. Stat. Med. 2010, 29, 2224–2234. [Google Scholar] [CrossRef]
- Dominici, F.; Samet, J.M.; Zeger, S.L. Combining evidence on air pollution and daily mortality from the 20 largest US cities: A hierarchical modelling strategy. J. R. Stat. Soc. Ser. A (Stat. Soc.) 2000, 163, 263–302. [Google Scholar] [CrossRef]
- Adegboye, M.A.; Olumoh, J.; Saffary, T.; Elfaki, F.; Adegboye, O.A. Effects of time-lagged meteorological variables on attributable risk of leishmaniasis in central region of Afghanistan. Sci. Total Environ. 2019, 685, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Kuddus, M.A.; McBryde, E.S.; Adegboye, O.A. Delay effect and burden of weather-related tuberculosis cases in Rajshahi province, Bangladesh, 2007–2012. Sci. Rep. 2019, 9, 12720. [Google Scholar] [CrossRef] [PubMed]
- Adegboye, O.A.; McBryde, E.S.; Eisen, D.P. Epidemiological analysis of association between lagged meteorological variables and pneumonia in wet-dry tropical North Australia, 2006–2016. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 448–458. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, F.; Feng, Z.; Li, X.; Zhou, X.-H. The temporal lagged association between meteorological factors and malaria in 30 counties in south-west China: A multilevel distributed lag non-linear analysis. Malar. J. 2014, 13, 57. [Google Scholar] [CrossRef]
- Guo, C.; Yang, L.; Ou, C.-Q.; Li, L.; Zhuang, Y.; Yang, J.; Zhou, Y.-X.; Qian, J.; Chen, P.-Y.; Liu, Q.-Y. Malaria incidence from 2005–2013 and its associations with meteorological factors in Guangdong, China. Malar. J. 2015, 14, 116. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Park, J.-W.; Cheong, H.-K. Estimated effect of climatic variables on the transmission of Plasmodium vivax malaria in the Republic of Korea. Environ. Health Perspect. 2012, 120, 1314–1319. [Google Scholar] [CrossRef]
- Sewe, M.O.; Ahlm, C.; Rocklöv, J. Remotely sensed environmental conditions and malaria mortality in three malaria endemic regions in western Kenya. PLoS ONE 2016, 11, e0154204. [Google Scholar] [CrossRef]
- Wu, Y.; Qiao, Z.; Wang, N.; Yu, H.; Feng, Z.; Li, X.; Zhao, X. Describing interaction effect between lagged rainfalls on malaria: An epidemiological study in south–west China. Malar. J. 2017, 16, 53. [Google Scholar] [CrossRef]
- Gasparrini, A. Modeling exposure–lag–response associations with distributed lag non-linear models. Stat. Med. 2014, 33, 881–899. [Google Scholar] [CrossRef]
- Gasparrini, A. Modelling lagged associations in environmental time series data: A simulation study. Epidemiology 2016, 27, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Ravishankaran, S.; Justin, N.J.A.; Asokan, A.; Kalsingh, T.M.J.; Mathai, M.T.; Valecha, N.; Montgomery, J.; Thomas, M.B.; Eapen, A. Microclimate variables of the ambient environment deliver the actual estimates of the extrinsic incubation period of Plasmodium vivax and Plasmodium falciparum: A study from a malaria-endemic urban setting, Chennai in India. Malar. J. 2018, 17, 201. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, X.-Q. A malaria transmission model with temperature-dependent incubation period. Bull. Math. Biol. 2017, 79, 1155–1182. [Google Scholar] [CrossRef] [PubMed]
- Boyd, M.F.; Kitchen, S. The Duration of the Intrinsic Incubation Period in Falciparum Malaria in Relation to Certain Factors Affecting the Parasites1. Am. J. Trop. Med. Hyg. 1937, 1, 845–848. [Google Scholar] [CrossRef]
- Gasparrini, A. Distributed Lag Linear and Non-Linear Models in R: The Package dlnm. J. Stat. Softw. 2011, 43, 1–20. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Paaijmans, K.P.; Read, A.F.; Thomas, M.B. Understanding the link between malaria risk and climate. Proc. Natl. Acad. Sci. USA 2009, 106, 13844–13849. [Google Scholar] [CrossRef]
- Blanford, J.I.; Blanford, S.; Crane, R.G.; Mann, M.E.; Paaijmans, K.P.; Schreiber, K.V.; Thomas, M.B. Implications of temperature variation for malaria parasite development across Africa. Sci. Rep. 2013, 3, 1300. [Google Scholar] [CrossRef]
- Orish, V.; Afutu, L.; Ayodele, O.; Likaj, L.; Marinkovic, A.; Sanyaolu, A. A 4-Day Incubation Period of Plasmodium falciparum Infection in a Nonimmune Patient in Ghana: A Case Report. Open Forum Infect. Dis. 2019, 6, ofy169. [Google Scholar] [CrossRef]
- Parham, P.E.; Michael, E. Modeling the effects of weather and climate change on malaria transmission. Environ. Health Perspect. 2009, 118, 620–626. [Google Scholar] [CrossRef]
- Arab, A.; Jackson, M.C.; Kongoli, C. Modelling the effects of weather and climate on malaria distributions in West Africa. Malar. J. 2014, 13, 126. [Google Scholar] [CrossRef]
- Kipruto, E.K.; Ochieng, A.O.; Anyona, D.N.; Mbalanya, M.; Mutua, E.N.; Onguru, D.; Nyamongo, I.K.; Estambale, B.B. Effect of climatic variability on malaria trends in Baringo County, Kenya. Malar. J. 2017, 16, 220. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, F.; Feng, Z.; Li, X.; Zhou, X.-H. Characterizing the effect of temperature fluctuation on the incidence of malaria: An epidemiological study in south-west China using the varying coefficient distributed lag non-linear model. Malar. J. 2014, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- Faulde, M.K.; Hoffmann, R.; Fazilat, K.M.; Hoerauf, A. Malaria reemergence in northern Afghanistan. Emerg. Infect. Dis. 2007, 13, 1402. [Google Scholar] [CrossRef] [PubMed]
- Hasyim, H.; Nursafingi, A.; Haque, U.; Montag, D.; Groneberg, D.A.; Dhimal, M.; Kuch, U.; Müller, R. Spatial modelling of malaria cases associated with environmental factors in South Sumatra, Indonesia. Malar. J. 2018, 17, 87. [Google Scholar] [CrossRef] [PubMed]
- Charlwood, J.D.; Graves, P.; Alpers, M. The ecology of the Anopheles punctulatus group of mosquitoes from Papua New Guinea: A review of recent work. PNG Med. J. 1986, 29, 19–26. [Google Scholar]
- Bates, I.; Fenton, C.; Gruber, J.; Lalloo, D.; Lara, A.M.; Squire, S.B.; Theobald, S.; Thomson, R.; Tolhurst, R. Vulnerability to malaria, tuberculosis, and HIV/AIDS infection and disease. Part 1: Determinants operating at individual and household level. Lancet Infect. Dis. 2004, 4, 267–277. [Google Scholar] [CrossRef]
- Steketee, R.W.; Nahlen, B.L.; Parise, M.E.; Menendez, C. The burden of malaria in pregnancy in malaria-endemic areas. Am. J. Trop. Med. Hyg. 2001, 64, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Duffy, P.; Fried, M. Malaria in the pregnant woman. In Malaria: Drugs, Disease and Post-Genomic Biology; Springer: Heidelberg, Germany, 2005; pp. 169–200. [Google Scholar]
- Galagan, S.R.; Prue, C.S.; Khyang, J.; Khan, W.A.; Ahmed, S.; Ram, M.; Alam, M.S.; Haq, M.Z.; Akter, J.; Streatfield, P.K. The practice of jhum cultivation and its relationship to Plasmodium falciparum infection in the Chittagong Hill Districts of Bangladesh. Am. J. Trop. Med. Hyg. 2014, 91, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Akanbi, O.; Badaki, J.; Adeniran, O.; Olotu, O. Effect of blood group and demographic characteristics on malaria infection, oxidative stress and haemoglobin levels in South Western Nigeria. Afr. J. Microbiol. Res. 2010, 4, 877–880. [Google Scholar]
- Vlassoff, C.; Manderson, L. Incorporating gender in the anthropology of infectious diseases. Trop. Med. Int. Health 1998, 3, 1011–1019. [Google Scholar] [CrossRef]
- Müller, I.; Smith, T.; Mellor, S.; Rare, L.; Genton, B. The effect of distance from home on attendance at a small rural health centre in Papua New Guinea. Int. J. Epidemiol. 1998, 27, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Dysoley, L.; Kaneko, A.; Eto, H.; Mita, T.; Socheat, D.; Börkman, A.; Kobayakawa, T. Changing patterns of forest malaria among the mobile adult male population in Chumkiri District, Cambodia. Acta Trop. 2008, 106, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Yé, Y.; Louis, V.R.; Simboro, S.; Sauerborn, R. Effect of meteorological factors on clinical malaria risk among children: An assessment using village-based meteorological stations and community-based parasitological survey. BMC Public Health 2007, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- Haque, U.; Hashizume, M.; Sunahara, T.; Hossain, S.; Ahmed, S.M.; Haque, R.; Yamamoto, T.; Glass, G.E. Progress and challenges to control malaria in a remote area of Chittagong hill tracts, Bangladesh. Malar. J. 2010, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Odongo-Aginya, E.; Ssegwanyi, G.; Kategere, P.; Vuzi, P. Relationship between malaria infection intensity and rainfall pattern in Entebbe peninsula, Uganda. Afr. Health Sci. 2005, 5, 238–245. [Google Scholar]
- Gasparrini, A.; Scheipl, F.; Armstrong, B.; Kenward, M. A penalized framework for distributed lag non-linear models. Biometrics 2017, 73, 938–948. [Google Scholar] [CrossRef]
- Shah, M.M.; Krystosik, A.R.; Ndenga, B.A.; Mutuku, F.M.; Caldwell, J.M.; Otuka, V.; Chebii, P.K.; Maina, P.W.; Jembe, Z.; Ronga, C. Malaria smear positivity among Kenyan children peaks at intermediate temperatures as predicted by ecological models. Parasites Vectors 2019, 12, 1–9. [Google Scholar] [CrossRef]
- Peña-García, V.H.; Triana-Chávez, O.; Arboleda-Sánchez, S. Estimating effects of temperature on dengue transmission in colombian cities. Ann. Glob. Health 2017, 83, 509–518. [Google Scholar] [CrossRef]

| Variables | Mean | SD | Min | Percentile | Max | Cor. (p-Value) * | ||
|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 75 | ||||||
| Malaria Cases | 5.7 | 4.9 | 0 | 2 | 4 | 8 | 34 | |
| Mean Temperature (°C) | 25.2 | 3.7 | 15.8 | 22.0 | 26.8 | 28.0 | 30.7 | 0.32 (<0.001) |
| Minimum Temperature (°C) | 21.9 | 4.2 | 11.2 | 18.2 | 24.1 | 25.3 | 27.8 | 0.34 (<0.001) |
| Maximum Temperature (°C) | 31.1 | 2.8 | 23.1 | 29.4 | 31.7 | 33.1 | 37.8 | 0.21 (<0.001) |
| Rainfall (mm) | 47.8 | 79.5 | 0.0 | 0.0 | 16.0 | 61.0 | 686.0 | 0.16 (<0.001) |
| Relative Humidity (%) | 95.5 | 2.4 | 81.0 | 94.4 | 96.0 | 97.0 | 100.0 | −0.02 (0.704) |
| Characteristics | Overall N = 2995 | Age Group, n (%) | Gender, n (%) | |||
|---|---|---|---|---|---|---|
| 0–14 Years | 15–49 Years | 50 + Years | Male | Female | ||
| 1381(45.1) | 1424(47.6) | 190(6.3) | 1740(58.1) | 1255(41.9) | ||
| Temperature | ||||||
| 2.5th (17.3 °C) | 3.1(0.7, 5.5) | 1.7(−1.5, 4.8) | 4.2(1.4, 6.9) | 4.9(−1.1, 10.6) | 1.4(−1.3, 4.0) | 5.6(2.6, 8.7) |
| 10th (18.9 °C) | 4.2(1.1, 7.5) | 2.8(−1.5, 7.2) | 5.6(1.8, 9.4) | 5.3(−2.8, 13.6) | 1.8(−1.9, 5.4) | 7.9(3.7, 12.8) |
| 90th (28.7 °C) | 2.5(−0.4, 5.4) | 1.0(−2.8, 4.9) | 3.9(0.5, 7.2) | 4.4(−3.6, 11.2) | −0.4(−3.6, 2.8) | 6.8(3.1, 10.6) |
| 97.5th (29.5 °C) | 2.6(−0.3, 5.6) | 2.2(−1.7, 6.1) | 2.7(−0.7, 6.2) | 4.8(−2.5, 12.0) | −0.1(−3.4, 3.5) | 6.7(2.9, 10.5) |
| Rainfall | ||||||
| 10th (0 mm) | 17.3(9.8, 25.0) | 26.2(15.5, 36.8) | 10.5(1.8, 19.1) | 14.2(−4.8, 33.2) | 19.9(11.4, 28.3) | 14.1(4.4, 23.1) |
| 90th (130 mm) | 19.1(11.4, 26.7) | 29.0(18.3, 39.8) | 10.6(2.1, 19.8) | 15.0(4.3, 34.3) | 22.6(13.9, 31.2) | 14.4(4.5, 24.2) |
| 97.5th (274 mm) | 17.2(9.6, 24.3) | 17.2(16.5, 37.8) | 9.2(0.6, 17.8) | 14.9(−3.9, 33.7) | 20.7(12.3, 29.1) | 12.6(2.9, 22.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emeto, T.I.; Adegboye, O.A.; Rumi, R.A.; Khan, M.-U.I.; Adegboye, M.; Khan, W.A.; Rahman, M.; Streatfield, P.K.; Rahman, K.M. Disparities in Risks of Malaria Associated with Climatic Variability among Women, Children and Elderly in the Chittagong Hill Tracts of Bangladesh. Int. J. Environ. Res. Public Health 2020, 17, 9469. https://doi.org/10.3390/ijerph17249469
Emeto TI, Adegboye OA, Rumi RA, Khan M-UI, Adegboye M, Khan WA, Rahman M, Streatfield PK, Rahman KM. Disparities in Risks of Malaria Associated with Climatic Variability among Women, Children and Elderly in the Chittagong Hill Tracts of Bangladesh. International Journal of Environmental Research and Public Health. 2020; 17(24):9469. https://doi.org/10.3390/ijerph17249469
Chicago/Turabian StyleEmeto, Theophilus I., Oyelola A. Adegboye, Reza A. Rumi, Mahboob-Ul I. Khan, Majeed Adegboye, Wasif A. Khan, Mahmudur Rahman, Peter K. Streatfield, and Kazi M. Rahman. 2020. "Disparities in Risks of Malaria Associated with Climatic Variability among Women, Children and Elderly in the Chittagong Hill Tracts of Bangladesh" International Journal of Environmental Research and Public Health 17, no. 24: 9469. https://doi.org/10.3390/ijerph17249469
APA StyleEmeto, T. I., Adegboye, O. A., Rumi, R. A., Khan, M.-U. I., Adegboye, M., Khan, W. A., Rahman, M., Streatfield, P. K., & Rahman, K. M. (2020). Disparities in Risks of Malaria Associated with Climatic Variability among Women, Children and Elderly in the Chittagong Hill Tracts of Bangladesh. International Journal of Environmental Research and Public Health, 17(24), 9469. https://doi.org/10.3390/ijerph17249469







