Clinical Profile, Treatment and Predictors during the First COVID-19 Wave: A Population-Based Registry Analysis from Castile and Leon Hospitals
Abstract
1. Introduction
2. Results
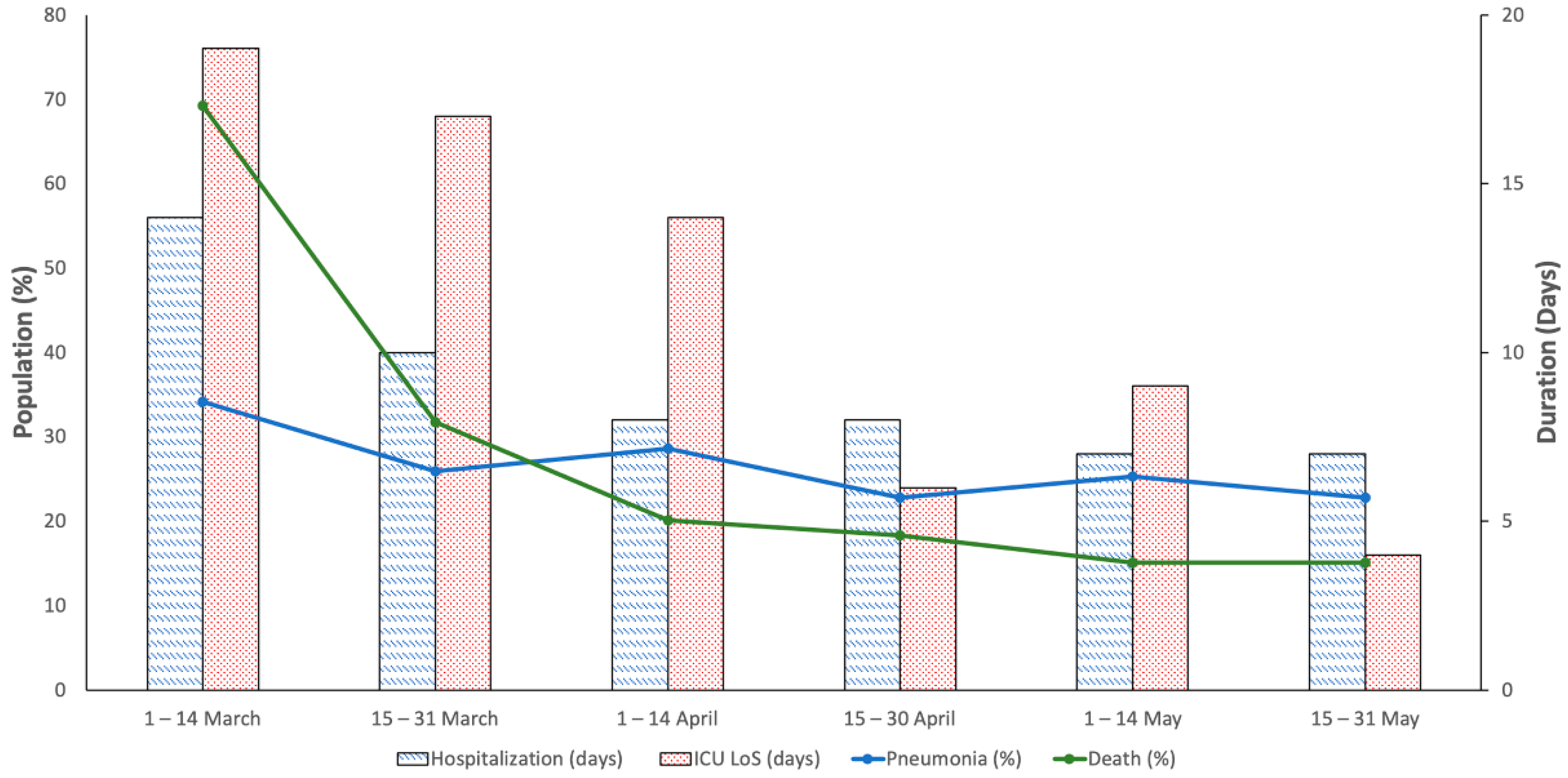
2.1. Clinical Findings
2.2. Pharmacological Treatment
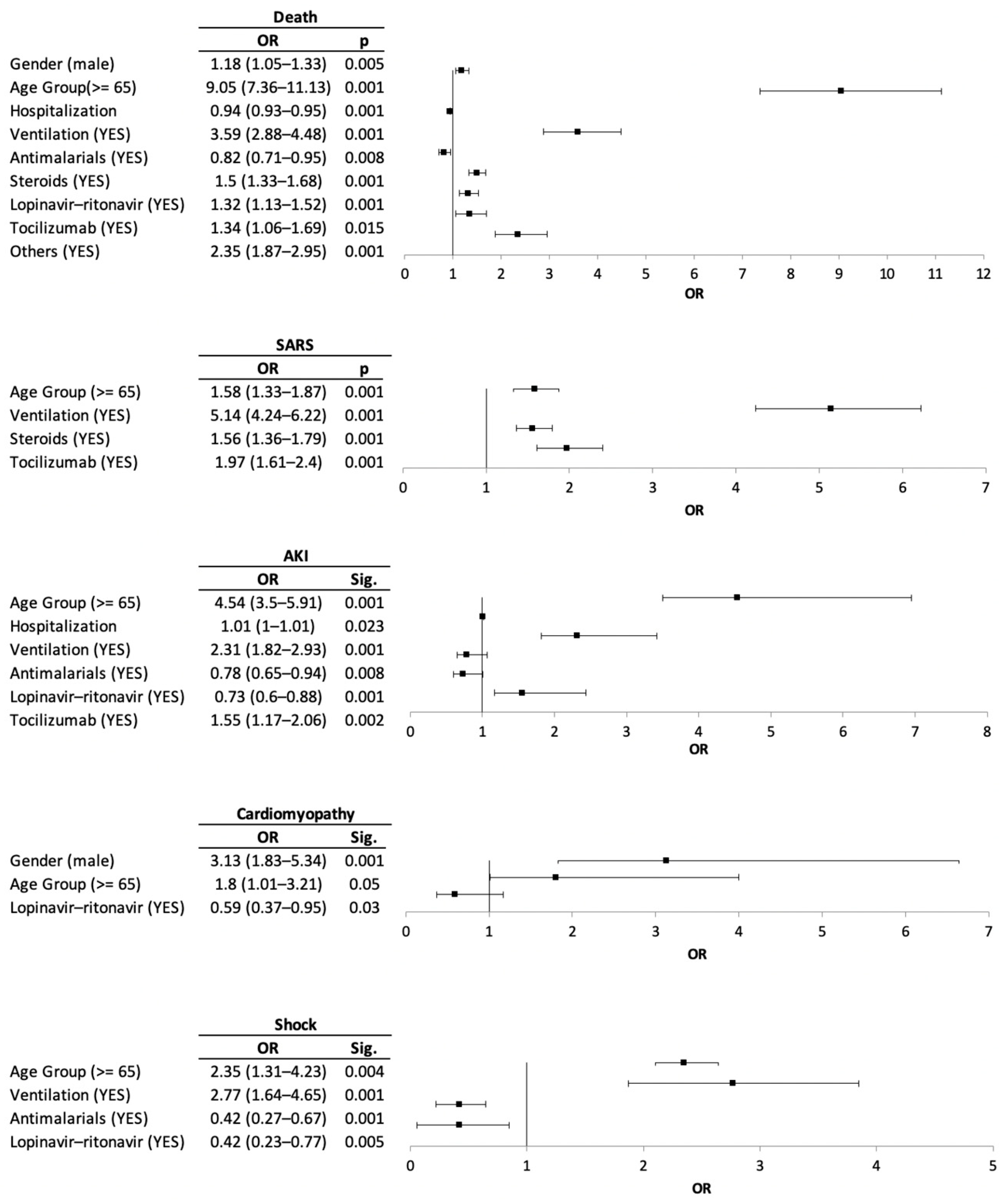
2.3. Risk Factor for Clinical Outcomes and Medication Prescribed
3. Discussion
4. Materials and Methods
4.1. Real-World Study Details
4.2. Variables
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, M.; Zhang, X.; Qu, J. Coronavirus disease 2019 (COVID-19): A clinical update. Front. Med. 2020, 14, 126–135. [Google Scholar] [CrossRef]
- Qian, S.-Z.; Hong, W.-D.; Mao, L.; Lin, C.; Fang, Z.; Pan, J.-Y. Clinical Characteristics and Outcomes of Severe and Critical Patients With 2019 Novel Coronavirus Disease (COVID-19) in Wenzhou: A Retrospective Study. Front. Med. (Lausanne) 2020, 7, 552002. [Google Scholar] [CrossRef] [PubMed]
- Del Sole, F.; Farcomeni, A.; Loffredo, L.; Carnevale, R.; Menichelli, D.; Vicario, T.; Pignatelli, P.; Pastori, D. Features of severe COVID-19: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2020, 50, e13378. [Google Scholar] [CrossRef] [PubMed]
- Pascarella, G.; Strumia, A.; Piliego, C.; Bruno, F.; Del Buono, R.; Costa, F.; Scarlata, S.; Agrò, F.E. COVID-19 diagnosis and management: A comprehensive review. J. Intern. Med. 2020, 288, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Director-General’s Remarks at the Media Briefing on 2019-nCoV on 11 February 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-mediabriefing-on-2019-ncov-on-11-february-2020 (accessed on 16 November 2020).
- World Health Organization (WHO). Director-General’s Opening Remarks at the Media Briefing on COVID-19: 11 March 2020. 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-themedia-briefing-on-covid-19---11-march-2020 (accessed on 16 November 2020).
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Dong, X.; Cao, Y.-Y.; Yuan, Y.-D.; Yang, Y.-B.; Yan, Y.-Q.; Akdis, C.A.; Gao, Y.-D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef]
- Cummings, M.J.; Baldwin, M.R.; Abrams, D.; Jacobson, S.D.; Meyer, B.J.; Balough, E.M.; Aaron, J.G.; Claassen, J.; Rabbani, L.E.; Hastie, J.; et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 2020, 395, 1763–1770. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef]
- Bonanad, C.; García-Blas, S.; Tarazona-Santabalbina, F.; Sanchis, J.; Bertomeu-González, V.; Fácila, L.; Ariza, A.; Núñez, J.; Cordero, A. The Effect of Age on Mortality in Patients with COVID-19: A Meta-Analysis With 611,583 Subjects. J. Am. Med. Dir. Assoc. 2020, 21, 915–918. [Google Scholar] [CrossRef]
- Giorgi Rossi, P.; Marino, M.; Formisano, D.; Venturelli, F.; Vicentini, M.; Grilli, R.; Emilia, R. COVID-19 Working Group Characteristics and outcomes of a cohort of COVID-19 patients in the Province of Reggio Emilia, Italy. PLoS ONE 2020, 15, e0238281. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-Y.; Xu, X.-X.; Yin, H.-S.; Hu, Q.-M.; Xiong, T.; Tang, Y.-Y.; Yang, A.-Y.; Yu, B.-P.; Huang, Z.-P. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: A retrospective study. BMC Infect. Dis. 2020, 20, 311. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. Remdesivir: First Approval. Drugs 2020, 80, 1355–1363. [Google Scholar] [CrossRef]
- Spanish Ministry of Health Clinical Management of COVID-19. Available online: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/Protocolo_manejo_clinico_ah_COVID-19.pdf (accessed on 16 November 2020).
- Spanish Agency for Medicine and Health Products Available Treatments for the Management of Respiratory Infection by SARS-CoV-2. Available online: https://www.aemps.gob.es/laAEMPS/docs/medicamentos-disponibles-SARS-CoV-2-22-5-2020.pdf?x57200 (accessed on 16 November 2020).
- Jamshaid, H.; Zahid, F.; Din, I.U.; Zeb, A.; Choi, H.G.; Khan, G.M.; Din, F.U. Diagnostic and Treatment Strategies for COVID-19. AAPS PharmSciTech 2020, 21, 222. [Google Scholar] [CrossRef]
- Wu, R.; Wang, L.; Kuo, H.-C.D.; Shannar, A.; Peter, R.; Chou, P.J.; Li, S.; Hudlikar, R.; Liu, X.; Liu, Z.; et al. An Update on Current Therapeutic Drugs Treating COVID-19. Curr. Pharmacol. Rep. 2020, 1–15. [Google Scholar] [CrossRef]
- Guo, Y.-R.; Cao, Q.-D.; Hong, Z.-S.; Tan, Y.-Y.; Chen, S.-D.; Jin, H.-J.; Tan, K.-S.; Wang, D.-Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak-an update on the status. Mil. Med. Res. 2020, 7, 11. [Google Scholar] [CrossRef]
- Siemieniuk, R.A.; Bartoszko, J.J.; Ge, L.; Zeraatkar, D.; Izcovich, A.; Kum, E.; Pardo-Hernandez, H.; Rochwerg, B.; Lamontagne, F.; Han, M.A.; et al. Drug treatments for covid-19: Living systematic review and network meta-analysis. BMJ 2020, 370, m2980. [Google Scholar] [CrossRef]
- Scavone, C.; Brusco, S.; Bertini, M.; Sportiello, L.; Rafaniello, C.; Zoccoli, A.; Berrino, L.; Racagni, G.; Rossi, F.; Capuano, A. Current pharmacological treatments for COVID-19: What’s next? Br. J. Pharmacol. 2020, 177, 4813–4824. [Google Scholar] [CrossRef]
- Sanders, J.M.; Monogue, M.L.; Jodlowski, T.Z.; Cutrell, J.B. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 323, 1824–1836. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Magro, G. COVID-19: Review on latest available drugs and therapies against SARS-CoV-2. Coagulation and inflammation cross-talking. Virus Res. 2020, 286, 198070. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, M.; Yin, L.; Wang, K.; Zhou, Y.; Zhou, M.; Lu, Y. COVID-19 treatment: Close to a cure? A rapid review of pharmacotherapies for the novel coronavirus (SARS-CoV-2). Int. J. Antimicrob. Agents 2020, 56, 106080. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, Y.; Zhang, F.; Wang, Q.; Li, T.; Liu, Z.; Wang, J.; Qin, Y.; Zhang, X.; Yan, X.; et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin. Immunol. 2020, 214, 108393. [Google Scholar] [CrossRef]
- Nittari, G.; Pallotta, G.; Amenta, F.; Tayebati, S.K. Current pharmacological treatments for SARS-COV-2: A narrative review. Eur. J. Pharmacol. 2020, 882, 173328. [Google Scholar] [CrossRef]
- Barlow, A.; Landolf, K.M.; Barlow, B.; Yeung, S.Y.A.; Heavner, J.J.; Claassen, C.W.; Heavner, M.S. Review of Emerging Pharmacotherapy for the Treatment of Coronavirus Disease 2019. Pharmacotherapy 2020, 40, 416–437. [Google Scholar] [CrossRef]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell COVID-19 Research Consortium; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Berenguer, J.; Ryan, P.; Rodríguez-Baño, J.; Jarrín, I.; Carratalà, J.; Pachón, J.; Yllescas, M.; Arriba, J.R.; COVID-19@Spain Study Group; Fundación SEIMC-GESIDA; et al. Characteristics and predictors of death among 4035 consecutively hospitalized patients with COVID-19 in Spain. Clin. Microbiol. Infect. 2020, 26, 1525–1536. [Google Scholar] [CrossRef]
- Casas-Rojo, J.M.; Antón-Santos, J.M.; Millán-Núñez-Cortés, J.; Lumbreras-Bermejo, C.; Ramos-Rincón, J.M.; Roy-Vallejo, E.; Artero-Mora, A.; Arnalich-Fernández, F.; García-Bruñén, J.M.; Vargas-Núñez, J.A.; et al. Clinical characteristics of patients hospitalized with COVID-19 in Spain: Results from the SEMI-COVID-19 Registry. Rev. Clin. Esp. 2020, 220, 480–494. [Google Scholar] [CrossRef]
- Onder, G.; Rezza, G.; Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA 2020, 323, 1775–1776. [Google Scholar] [CrossRef] [PubMed]
- Goyal, P.; Choi, J.J.; Pinheiro, L.C.; Schenck, E.J.; Chen, R.; Jabri, A.; Satlin, M.J.; Campion, T.R.; Nahid, M.; Ringel, J.B.; et al. Clinical Characteristics of Covid-19 in New York City. N. Engl. J. Med. 2020, 382, 2372–2374. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Deng, X.; Li, Y.; Sun, X.; Chen, Q.; Xie, M.; Liu, S.; Qu, H.; Liu, S.; Wang, L.; et al. Clinical characteristics and drug therapies in patients with the common-type coronavirus disease 2019 in Hunan, China. Int. J. Clin. Pharm. 2020, 42, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Hung, I.F.-N.; Lung, K.-C.; Tso, E.Y.-K.; Liu, R.; Chung, T.W.-H.; Chu, M.-Y.; Ng, Y.-Y.; Lo, J.; Chan, J.; Tam, A.R.; et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial. Lancet 2020, 395, 1695–1704. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Kebriaei, R.; Dresser, L.D. Remdesivir: Review of Pharmacology, Pre-clinical Data, and Emerging Clinical Experience for COVID-19. Pharmacotherapy 2020, 40, 659–671. [Google Scholar] [CrossRef]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.-X.; et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef]
- Goldman, J.D.; Lye, D.C.B.; Hui, D.S.; Marks, K.M.; Bruno, R.; Montejano, R.; Spinner, C.D.; Galli, M.; Ahn, M.-Y.; Nahass, R.G.; et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Cortegiani, A.; Ingoglia, G.; Ippolito, M.; Giarratano, A.; Einav, S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care 2020, 57, 279–283. [Google Scholar] [CrossRef]
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C.; et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020, 71, 732–739. [Google Scholar] [CrossRef]
- Zhou, D.; Dai, S.-M.; Tong, Q. COVID-19: A recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J. Antimicrob. Chemother. 2020, 75, 1667–1670. [Google Scholar] [CrossRef]
- Million, M.; Lagier, J.-C.; Gautret, P.; Colson, P.; Fournier, P.-E.; Amrane, S.; Hocquart, M.; Mailhe, M.; Esteves-Vieira, V.; Doudier, B.; et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille, France. Travel Med. Infect. Dis. 2020, 35, 101738. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, J.; Zhang, Z.; Jiang, S.; Han, S.; Yan, D.; Zhuang, R.; Hu, B.; Zhang, Z. Efficacy of hydroxychloroquine in patients with COVID-19: Results of a randomized clinical trial. MedRxiv 2020. [Google Scholar] [CrossRef]
- Horby, P.; Mafham, M.; Linsell, L.; Bell, J.L.; Staplin, N.; Emberson, J.R.; Wiselka, M.; Ustianowski, A.; Elmahi, E.; Prudon, B.; et al. Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19: Preliminary results from a multi-centre, randomized, controlled trial. MedRxiv 2020. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, J.; Zhou, Y.; Zhao, X.; Zhao, Q.; Liu, J. The effect of corticosteroid treatment on patients with coronavirus infection: A systematic review and meta-analysis. J. Infect. 2020, 81, e13–e20. [Google Scholar] [CrossRef]
- The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group Association between Administration of Systemic Corticosteroids and Mortality among Critically Ill Patients with COVID-19: A Meta-Analysis. JAMA 2020, 324, 1330–1341. [CrossRef] [PubMed]
- Zhang, S.; Li, L.; Shen, A.; Chen, Y.; Qi, Z. Rational Use of Tocilizumab in the Treatment of Novel Coronavirus Pneumonia. Clin. Drug. Investig. 2020, 40, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Rossi, B.; Nguyen, L.S.; Zimmermann, P.; Boucenna, F.; Dubret, L.; Baucher, L.; Guillot, H.; Bouldouyre, M.-A.; Allenbach, Y.; Salem, J.-E.; et al. Effect of Tocilizumab in Hospitalized Patients with Severe COVID-19 Pneumonia: A Case-Control Cohort Study. Pharmaceuticals 2020, 13, 317. [Google Scholar] [CrossRef] [PubMed]
- Schijns, V.; Lavelle, E.C. Prevention and treatment of COVID-19 disease by controlled modulation of innate immunity. Eur. J. Immunol. 2020, 50, 932–938. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e19. [Google Scholar] [CrossRef]
- Sherman, R.E.; Anderson, S.A.; Dal Pan, G.J.; Gray, G.W.; Gross, T.; Hunter, N.L.; LaVange, L.; Marinac-Dabic, D.; Marks, P.W.; Robb, M.A.; et al. Real-World Evidence-What Is It and What Can It Tell Us? N. Engl. J. Med. 2016, 375, 2293–2297. [Google Scholar] [CrossRef]
- Benchimol, E.I.; Smeeth, L.; Guttmann, A.; Harron, K.; Moher, D.; Petersen, I.; Sørensen, H.T.; von Elm, E.; Langan, S.M. RECORD Working Committee the REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015, 12, e1001885. [Google Scholar] [CrossRef]
| TOTAL | MALE | FEMALE | p | |
|---|---|---|---|---|
| n | 7307 | 4169 | 3138 | |
| Age (median and IQR) | 76 (63–86) | 75 (63–84) | 79 (64–88) | 0.001 |
| Age < 65 (95% CI) | 27.23 (26.21–28.25) | 27.87 (26.51–29.23) | 26.39 (24.84–27.93) | 0.158 |
| Age >= 65 (95% CI) | 72.77 (71.75–73.79) | 72.13 (70.77–73.49) | 73.61 (72.07–75.16) | 0.158 |
| Treatment | ||||
| Oxygenation and ventilation (95% CI) | ||||
| Oxygenation only | 2.52 (2.16–2.88) | 2.85 (2.35–3.36) | 2.07 (1.57–2.57) | 0.034 |
| NIPPV | 1.63 (1.34–1.92) | 2.16 (1.72–2.6) | 0.92 (0.59–1.26) | 0.001 |
| IMV | 3.5 (3.08–3.93) | 4.73 (4.08–5.37) | 1.88 (1.4–2.36) | 0.001 |
| Drugs (95% CI) | ||||
| Antibiotics | 90.83 (90.17–91.49) | 90.19 (89.29–91.09) | 91.68 (90.72–92.65) | 0.029 |
| Antimalarial | 69.74 (68.69–70.79) | 71.7 (70.33–73.06) | 67.14 (65.5–68.79) | 0.001 |
| Steroids | 44.37 (43.23–45.51) | 47.83 (46.31–49.35) | 39.77 (38.06–41.48) | 0.001 |
| Antivirals | 42.63 (41.52–43.93) | 45.42 (43.5–46.93) | 38.95 (37.22–40.66) | 0.001 |
| Tocilizumab | 9.37 (8.71–10.04) | 12.14 (11.15–13.13) | 5.7 (4.89–6.52) | 0.001 |
| Others anti SIRS * | 7.34 (6.74–7.93) | 9.35 (8.47–10.24) | 4.65 (3.92–5.39) | 0.001 |
| Outcomes | ||||
| Hospitalization days (median and IQR) | 9 (5–15) | 9 (5–15) | 8 (5–14) | 0.001 |
| ICU LoS (median and IQR) | 15 (7–30) | 15 (7–32) | 15 (8–24) | 0.359 |
| SARS (95% CI) | 14.03 (13.23–14.82) | 15.59 (14.49–16.69) | 11.95 (10.82–13.09) | 0.001 |
| AKI (95% CI) | 10.87 (10.15–11.58) | 11.3 (10.34–12.26) | 10.29 (9.23–11.36) | 0.172 |
| Cardiomyopathy (95% CI) | 1.15 (0.91–1.39) | 1.61 (1.23–1.99) | 0.54 (0.28–0.8) | 0.001 |
| Shock (95% CI) | 1.51 (1.23–1.78) | 1.66 (1.27–2.04) | 1.31 (0.91–1.7) | 0.226 |
| Bacterial superinfection (95% CI) | 3.59 (3.16–4.01) | 3.89 (3.3–4.47) | 3.19 (2.57–3.8) | 0.112 |
| Fungal superinfection (95% CI) | 2.23 (1.89–2.57) | 2.11 (1.67–2.55) | 2.39 (1.86–2.92) | 0.424 |
| DIC (95% CI) | 0.18 (0.08–0.27) | 0.29 (0.13–0.45) | 0.03 (0.01–0.05) | 0.001 |
| Death (95% CI) | 24.43 (23.44–25.41) | 26.12 (24.79–27.45) | 22.18 (20.73–23.63) | 0.001 |
| Medicines | 1–14 March | 15–31 March | 1–14 April | 15–30 April | 1–14 May | 15–31 May |
|---|---|---|---|---|---|---|
| n = 117 | n = 2819 | n = 2155 | n = 1250 | n = 588 | n = 378 | |
| Antibiotics | 79.49 (72.17–86.8) | 91.63 (90.61–92.65) | 90.95 (89.74–92.16) | 91.6 (90.06–93.14) | 88.1 (85.48–90.71) | 89.42 (86.32–92.52) |
| Ceftriaxone | 47.01 (37.96–56.05) | 71.83 (70.17–73.49) | 68.45 (66.48–70.41) | 66.96 (64.35–69.57) | 69.73 (66.01–73.44) | 68.78 (64.11–73.45) |
| Azithromycin | 25.64 (17.73–33.55) | 70.95 (69.27–72.62) | 73.74 (71.88–75.59) | 67.28 (64.68–69.88) | 52.21 (48.17–56.25) | 52.12 (47.08–57.15) |
| Levofloxacin | 44.44 (35.44–53.45) | 16.42 (15.06–17.79) | 10.95 (9.63–12.27) | 15.52 (13.51–17.53) | 17.52 (14.44–20.59) | 17.72 (13.88–21.57) |
| Cefditoren | 0.85 (0.1–1.69) | 3.12 (2.48–3.76) | 2.55 (1.89–3.22) | 2.4 (1.55–3.25) | 2.04 (0.9–3.18) | 1.32 (0.17–2.47) |
| Teicoplanin | 2.56 (0.3–5.49) | 1.21 (0.8–1.61) | 1.62 (1.09–2.16) | 1.36 (0.72–2) | 1.19 (0.31–2.07) | 1.59 (0.33–2.85) |
| Clarithromycin | 0.85 (0.1–1.69) | 0.32 (0.11–0.53) | 0.19 (0–0.37) | 0.48 (0.1–0.86) | 0.34 (0.13–0.55) | 1.32 (0.17–2.47) |
| Cefotaxime | 1.71 (0.03–2.65) | 0.14 (0–0.28) | 0.23 (0.03–0.44) | 0.4 (0.05–0.75) | 0.51 (0.07–0.95) | 0.26 (0.05–0.58) |
| Moxifloxacin | 0 (0–0) | 0.18 (0.02–0.33) | 0.42 (0.15–0.69) | 0.24 (0.03–0.45) | 0.51 (0.07–0.95) | 0.26 (0.25–0.58) |
| Ceftaroline | 0 (0–0) | 0.11 (0.01–0.21) | 0 (0–0) | 0.08 (0.02–0.14) | 0 (0–0) | 0 (0–0) |
| Antimalarials | 42.74 (33.77–51.7) | 84.04 (82.68–85.39) | 76.29 (74.49–78.08) | 60 (57.28–62.72) | 32.14 (28.37–35.92) | 24.87 (20.51–29.23) |
| Hydroxycloroquine | 36.75 (28.02–45.49) | 76.48 (74.92–78.05) | 71.18 (69.27–73.1) | 58.96 (56.23–61.69) | 31.63 (27.87–35.39) | 23.81 (19.52–28.1) |
| Cloroquine | 6.84 (2.26–11.41) | 10.85 (9.71–12) | 5.15 (4.22–6.08) | 1.12 (0.54–1.7) | 0.51 (0.07–0.95) | 1.59 (0.33–2.85) |
| Steroids | 59.83 (50.95–68.71) | 44.45 (42.61–46.28) | 43.67 (41.57–45.76) | 44.72 (41.96–47.48) | 42.69 (38.69–46.68) | 44.44 (39.44–49.45) |
| Methylprednisolone | 56.41 (47.43–65.4) | 42.21 (40.39–44.04) | 40.23 (38.16–42.3) | 41.52 (38.79–44.25) | 37.59 (33.67–41.5) | 39.42 (34.49–44.34) |
| Prednisone | 11.11 (5.42–16.81) | 8.09 (7.08–9.09) | 10.12 (8.84–11.39) | 9.44 (7.82–11.06) | 11.56 (8.98–14.15) | 10.32 (7.25–13.38) |
| Antivirals | 38.46 (29.65–47.28) | 65.02 (63.01–66.43) | 42.51 (40.25–44.32) | 20.24 (18.01–22.47) | 8.5 (6.25–10.76) | 6.61 (4.11–9.12) |
| Lopinavir-Ritonavir | 38.46 (29.65–47.28) | 64.88 (63.12–66.64) | 42.37 (40.28–44.45) | 20.24 (18.01–22.47) | 8.5 (6.25–10.76) | 6.61 (4.11–9.12) |
| Remdesevir | 0 (0–0) | 0.14 (0–0.28) | 0.14 (0.02–0.26) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Tocilizumab | 7.69 (2.86–12.52) | 13.2 (11.95–14.45) | 10.58 (9.28–11.88) | 4.8 (3.61–5.99) | 1.87 (0.78–2.97) | 1.32 (0.17–2.47) |
| Other Anti-SIRS | 15.38 (8.85–21.92) | 13.2 (11.95–14.45) | 5.06 (4.13–5.98) | 2.16 (1.35–2.97) | 1.02 (0.21–1.83) | 1.06 (0.03–2.09) |
| Interferon Beta | 15.38 (8.85–21.92) | 12.2 (10.99–13.41) | 2.18 (1.56–2.8) | 0 (0–0) | 0 (0–0) | 0.79 (0.1–1.67) |
| Anakinra | 0 (0–0) | 0.89 (0.54–1.23) | 2.51 (1.85–3.17) | 1.68 (0.97–2.39) | 0.17 (0.06–0.35) | 0.53 (0.2–1.22) |
| Baricitinib | 0 (0–0) | 0.11 (0.01–0.21) | 0.42 (0.15–0.69) | 0.64 (0.2–1.08) | 0.85 (0.11–1.59) | 0 (0–0) |
| Siltuximab | 0 (0–0) | 0.18 (0.02–0.33) | 0.23 (0.03–0.44) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Ruxolitinib | 0 (0–0) | 0.11 (0.01–0.21) | 0.19 (0–0.37) | 0.08 (0.02–0.14) | 0 (0–0) | 0 (0–0) |
| Medicines | TOTAL (95 CI) | No Death (95 CI) | Death (95 CI) | p |
|---|---|---|---|---|
| n = 7307 | n = 5522 | n = 1785 | ||
| Antibiotics | 90.83 (90.17–91.49) | 91.22 (90.47–91.96) | 89.64 (88.22–91.05) | 0.044 |
| Ceftriaxone | 69.28 (68.22–70.33) | 69.21 (68–70.43) | 69.47 (67.33–71.6) | 0.84 |
| Azithromycin | 67.93 (66.86–69) | 69.68 (68.47–70.9) | 62.52 (60.28–64.77) | 0.001 |
| Levofloxacin | 15.26 (14.43–16.08) | 13.94 (13.03–14.86) | 19.33 (17.5–21.16) | 0.001 |
| Cefditoren | 2.61 (2.25–2.98) | 2.95 (2.51–3.4) | 1.57 (0.99–2.15) | 0.001 |
| Teicoplanin | 1.4 (1.13–1.66) | 1.18 (0.89–1.46) | 2.07 (1.41–2.73) | 0.005 |
| Clarithromycin | 0.37 (0.23–0.51) | 0.31 (0.16–0.45) | 0.56 (0.21–0.91) | 0.127 |
| Moxifloxacin | 0.29 (0.16–0.41) | 0.29 (0.15–0.43) | 0.28 (0.03–0.53) | 0.947 |
| Cefotaxime | 0.27 (0.15–0.39) | 0.33 (0.18–0.48) | 0.11 (0.04–0.19) | 0.133 |
| Ceftaroline | 0.05 (0–0.11) | 0.07 (0–0.14) | 0 (0–0) | |
| Antimalarials | 69.74 (68.69–70.79) | 71.01 (69.81–72.2) | 65.83 (63.63–68.03) | 0.001 |
| Hydroxycloroquine | 64.95 (63.86–66.05) | 66.37 (65.12–67.62) | 60.56 (58.29–62.83) | 0.001 |
| Cloroquine | 6.13 (5.58–6.68) | 5.94 (5.32–6.56) | 6.72 (5.56–7.88) | 0.231 |
| Steroids | 44.37 (43.23–45.51) | 42.12 (40.82–43.42) | 51.32 (49–53.64) | 0.001 |
| Methylprednisolone | 41.22 (40.09–42.35) | 38.59 (37.31–39.88) | 49.36 (47.04–51.68) | 0.001 |
| Prednisone | 9.36 (8.69–10.03) | 10.45 (9.64–11.26) | 5.99 (4.89–7.1) | 0.001 |
| Antivirals | 42.64 (41.52–43.93) | 42.99 (41.61–44.36) | 41.91 (39.46–44.35) | 0.35 |
| Lopinavir-Ritonavir | 42.63 (41.5–43.76) | 42.92 (41.61–44.22) | 41.74 (39.45–44.02) | 0.38 |
| Remdesevir | 0.1 (0.02–0.17) | 0.07 (0–0.14) | 0.17 (0.01–0.33) | 0.256 |
| Tocilizumab | 9.37 (8.71–10.04) | 9.69 (8.91–10.47) | 8.4 (7.12–9.69) | 0.105 |
| Other Anti-SIRS | 7.34 (6.74–7.93) | 6.25 (5.61–6.89) | 10.7 (9.27–12.13) | 0.001 |
| Interfereon Beta | 5.64 (5.11–6.17) | 4.85 (4.29–5.42) | 8.07 (6.8–9.33) | 0.001 |
| Anakinra | 1.41 (1.14–1.68) | 1.16 (0.88–1.44) | 2.18 (1.51–2.86) | 0.001 |
| Baricitinib | 0.34 (0.21–0.48) | 0.33 (0.18–0.48) | 0.39 (0.1–0.68) | 0.677 |
| Siltuximab | 0.14 (0.05–0.22) | 0.07 (0–0.14) | 0.34 (0.07–0.6) | 0.009 |
| Ruxolitinib | 0.11 (0.03–0.19) | 0.07 (0–0.14) | 0.22 (0–0.44) | 0.092 |
| Remdesevir | 0.1 (0.02–0.17) | 0.07 (0–0.14) | 0.17 (0.01–0.33) | 0.256 |
| Drugs | No Pneumonia (95% CI) | Pneumonia (95% CI) | p |
|---|---|---|---|
| n = 5399 | n = 1908 | ||
| Antibiotics | 90.31 (89.52–91.1) | 92.3 (91.1–93.49) | 0.001 |
| Ceftriaxone | 69.05 (67.82–70.28) | 69.92 (67.86–71.97) | 0.481 |
| Azithromycin | 66.64 (65.38–67.9) | 71.59 (69.57–73.62) | 0.001 |
| Levofloxacin | 15.1 (14.14–16.05) | 15.72 (14.09–17.36) | 0.512 |
| Cefditoren | 2.43 (2.02–2.84) | 3.14 (2.36–3.93) | 0.091 |
| Teicoplanin | 1.28 (0.98–1.58) | 1.73 (1.14–2.31) | 0.148 |
| Clarithromycin | 0.37 (0.21–0.53) | 0.37 (0.1–0.64) | 0.982 |
| Moxifloxacin | 0.28 (0.14–0.42) | 0.31 (0.06–0.57) | 0.797 |
| Cefotaxime | 0.28 (0.14–0.42) | 0.26 (0.03–0.49) | 0.91 |
| Ceftaroline | 0.06 (0.01–0.10) | 0.05 (0.01–0.12) | 0.96 |
| Antimalarials | 68.4 (67.16–69.64) | 73.53 (71.55–75.51) | 0.001 |
| Hydroxycloroquine | 63.12 (61.84–64.41) | 70.13 (68.07–72.18) | 0.001 |
| Cloroquine | 6.82 (6.14–7.49) | 4.19 (3.29–5.09) | 0.001 |
| Steroids | 44.67 (43.35–46) | 43.5 (41.28–45.73) | 0.375 |
| Methylprednisolone | 41.71 (40.4–43.03) | 39.83 (37.64–42.03) | 0.152 |
| Prednisone | 8.8 (8.04–9.55) | 10.95 (9.55–12.36) | 0.005 |
| Antivirals | 41.08 (39.34–42.35) | 47.38 (44.92–49.43) | 0.001 |
| Lopinavir-Ritonavir | 41.01 (39.7–42.32) | 47.22 (44.98–49.46) | 0.001 |
| Remdesevir | 0.07 (0–0.15) | 0.16 (0.001–0.31) | 0.313 |
| Tocilizumab | 7.89 (7.17–8.61) | 13.57 (12.04–15.11) | 0.001 |
| Other Anti-SIRS | 7.08 (6.39–7.76) | 8.07 (6.85–9.29) | 0.152 |
| Interfereon Beta | 5.52 (4.91–6.13) | 5.97 (4.91–7.04) | 0.459 |
| Anakinra | 1.28 (0.98–1.58) | 1.78 (1.19–2.38) | 0.108 |
| Baricitinib | 0.31 (0.17–0.46) | 0.42 (0.13–0.71) | 0.502 |
| Ruxolitinib | 0.09 (0.01–0.17) | 0.16 (0.01–0.31) | 0.469 |
| Siltuximab | 0.09 (0.01–0.17) | 0.26 (0.03–0.49) | 0.085 |
| Medicines Group | ATC Code | Medicine | Medicines Group | ATC Code | Medicine |
|---|---|---|---|---|---|
| Antibiotics | J01DD01 | Cefotaxime | Anti-SIRS Drugs | L01XE18 | Ruxolitinib |
| J01DD04 | Ceftriaxone | L03AB05 | Interferon alpha 2b | ||
| J01DD16 | Cefditoren | L03AB08 | Interferon beta 1b | ||
| J01DI02 | Ceftaroline | L04AA37 | Baricitinib | ||
| J01FA09 | Clarithromycin | L04AC03 | Anakinra | ||
| J01FA10 | Azithromycin | L04AC07 | Tocilizumab | ||
| J01MA12 | Levofloxacin | L04AC11 | Siltuximab | ||
| J01MA14 | Moxifloxacin | L04AC14 | Sarilumab | ||
| J01XA02 | Teicoplanine | Antivirals | J05AR10 | Lopinavir/Ritonavir | |
| Antimalarials | P01BA01 | Chloroquine | J05AX95 * | Remdesivir | |
| P01BA02 | Hidroxychloroquine | Steroids | H02AB04 | Methylprednisolone | |
| H02AB07 | Prednisone |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Abejón, E.; Tamayo, E.; Martín-García, D.; Álvarez, F.J.; Herrera-Gómez, F. Clinical Profile, Treatment and Predictors during the First COVID-19 Wave: A Population-Based Registry Analysis from Castile and Leon Hospitals. Int. J. Environ. Res. Public Health 2020, 17, 9360. https://doi.org/10.3390/ijerph17249360
Gutiérrez-Abejón E, Tamayo E, Martín-García D, Álvarez FJ, Herrera-Gómez F. Clinical Profile, Treatment and Predictors during the First COVID-19 Wave: A Population-Based Registry Analysis from Castile and Leon Hospitals. International Journal of Environmental Research and Public Health. 2020; 17(24):9360. https://doi.org/10.3390/ijerph17249360
Chicago/Turabian StyleGutiérrez-Abejón, Eduardo, Eduardo Tamayo, Débora Martín-García, F. Javier Álvarez, and Francisco Herrera-Gómez. 2020. "Clinical Profile, Treatment and Predictors during the First COVID-19 Wave: A Population-Based Registry Analysis from Castile and Leon Hospitals" International Journal of Environmental Research and Public Health 17, no. 24: 9360. https://doi.org/10.3390/ijerph17249360
APA StyleGutiérrez-Abejón, E., Tamayo, E., Martín-García, D., Álvarez, F. J., & Herrera-Gómez, F. (2020). Clinical Profile, Treatment and Predictors during the First COVID-19 Wave: A Population-Based Registry Analysis from Castile and Leon Hospitals. International Journal of Environmental Research and Public Health, 17(24), 9360. https://doi.org/10.3390/ijerph17249360








