Factors Associated with Survey Non-Response in a Cross-Sectional Survey of Persons with an Axial Spondyloarthritis or Osteoarthritis Claims Diagnosis
Abstract
1. Introduction
- -
- Characterize respondents and non-respondents of the two surveys with respect to relevant characteristics available from the claims data (age, sex, health care utilization).
- -
- Compare differences in the response patterns among the two diagnoses and discuss the results in comparison to the diabetes sample in order to assess the generalizability of the results to other chronic diseases.
2. Materials and Methods
2.1. Sample Selection and Survey Procedure
- An introduction to the project with explanation of the data protection measures and an invitation to participate in the research project.
- A form to declare consent to participate.
- The study questionnaire (6 pages for the axSpA sample, 7 pages for the OA sample).
- A stamped envelope to send the consent form to the health insurance.
- A stamped envelope to send the questionnaire to the German rheumatism research center.
2.2. Claims Data Available for Analysis
2.3. Statistical Analysis
3. Results
3.1. Response by Age and Sex
3.2. Differences between Responders and Non-Responders
3.3. Analysis of the Response Time
4. Discussion
4.1. Implications
4.2. Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoffmann, W.; Terschuren, C.; Holle, R.; Kamtsiuris, P.; Bergmann, M.; Kroke, A.; Sauer, S.; Stang, A.; Latza, U. The problem of response in epidemiologic studies in Germany (Part II). Gesundheitswesen 2004, 66, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Eisner, N.L.; Murray, A.L.; Eisner, M.; Ribeaud, D. A practical guide to the analysis of non-response and attrition in longitudinal research using a real data example. Int. J. Behav. Dev. 2018, 43, 24–34. [Google Scholar] [CrossRef]
- Gustavson, K.; von Soest, T.; Karevold, E.; Roysamb, E. Attrition and generalizability in longitudinal studies: Findings from a 15-year population-based study and a Monte Carlo simulation study. BMC Public Health 2012, 12, 918. [Google Scholar] [CrossRef] [PubMed]
- Raisanen, P.; Hedman, L.; Andersson, M.; Stridsman, C.; Lindberg, A.; Lundback, B.; Ronmark, E.; Backman, H. Non-response did not affect prevalence estimates of asthma and respiratory symptoms—Results from a postal questionnaire survey of the general population. Respir. Med. 2020, 173, 106017. [Google Scholar] [CrossRef]
- Neubauer, S.; Kreis, K.; Klora, M.; Zeidler, J. Access, use, and challenges of claims data analyses in Germany. Eur. J. Health Econ. 2017, 18, 533–536. [Google Scholar] [CrossRef]
- Kreis, K.; Neubauer, S.; Klora, M.; Lange, A.; Zeidler, J. Status and perspectives of claims data analyses in Germany—A systematic review. Health Policy 2016, 120, 213–226. [Google Scholar] [CrossRef]
- Kruger, K.; von Hinuber, U.; Meier, F.; Tian, H.; Bohm, K.; Jugl, S.M.; Borchert, K.; Meise, D.; Konig, C.; Braun, S. Ankylosing spondylitis causes high burden to patients and the healthcare system: Results from a German claims database analysis. Rheumatol. Int. 2018, 38, 2121–2131. [Google Scholar] [CrossRef]
- Redeker, I.; Callhoff, J.; Hoffmann, F.; Saam, J.; Haibel, H.; Sieper, J.; Zink, A.; Poddubnyy, D. Cost of illness in axial spondylarthritis for patients with and without tumor necrosis factor inhibitor treatment: Results of a routine data analysis. Z. Rheumatol. 2020, 79, 85–94. [Google Scholar] [CrossRef]
- Callhoff, J.; Albrecht, K.; Hoffmann, F.; Poddubnyy, D.; Gunther, K.P.; Zink, A. Reality of care for musculoskeletal diseases at the population level: Results of the PROCLAIR collaborative project. Z. Rheumatol. 2019, 78, 73–79. [Google Scholar] [CrossRef]
- Chandran, U.; Reps, J.; Stang, P.E.; Ryan, P.B. Inferring disease severity in rheumatoid arthritis using predictive modeling in administrative claims databases. PLoS ONE 2019, 14, e0226255. [Google Scholar] [CrossRef]
- Garris, C.; Jhingran, P.; Bass, D.; Engel-Nitz, N.M.; Riedel, A.; Dennis, G. Healthcare utilization and cost of systemic lupus erythematosus in a US managed care health plan. J. Med. Econ. 2013, 16, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Speyer, C.B.; Li, D.; Guan, H.; Yoshida, K.; Stevens, E.; Jorge, A.M.; Everett, B.M.; Feldman, C.H.; Costenbader, K.H. Comparison of an administrative algorithm for SLE disease severity to clinical SLE Disease Activity Index scores. Rheumatol. Int. 2020, 40, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Linnenkamp, U.; Gontscharuk, V.; Brune, M.; Chernyak, N.; Kvitkina, T.; Arend, W.; Fiege, A.; Schmitz-Losem, I.; Kruse, J.; Evers, S.; et al. Using statutory health insurance data to evaluate non-response in a cross-sectional study on depression among patients with diabetes in Germany. Int. J. Epidemiol. 2020, 49, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Sieper, J.; Braun, J.; Dougados, M.; Baeten, D. Axial spondyloarthritis. Nat. Rev. Dis. Primers 2015, 1, 15013. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef]
- Roddy, E.; Thomas, M.J.; Marshall, M.; Rathod, T.; Myers, H.; Menz, H.B.; Thomas, E.; Peat, G. The population prevalence of symptomatic radiographic foot osteoarthritis in community-dwelling older adults: Cross-sectional findings from the clinical assessment study of the foot. Ann. Rheum. Dis. 2015, 74, 156–163. [Google Scholar] [CrossRef]
- Peat, G.; Thomas, E.; Handy, J.; Wood, L.; Dziedzic, K.; Myers, H.; Wilkie, R.; Duncan, R.; Hay, E.; Hill, J.; et al. The Knee Clinical Assessment Study—CAS(K). A prospective study of knee pain and knee osteoarthritis in the general population: Baseline recruitment and retention at 18 months. BMC Musculoskelet. Disord. 2006, 7, 30. [Google Scholar] [CrossRef][Green Version]
- Callhoff, J.; Albrecht, K.; Redeker, I.; Lange, T.; Goronzy, J.; Gunther, K.P.; Zink, A.; Schmitt, J.; Saam, J.; Postler, A. Disease Burden of Patients with Osteoarthritis: Results of a Cross-Sectional Survey Linked to Claims Data. Arthritis Care Res. 2020, 72, 193–200. [Google Scholar] [CrossRef]
- Redeker, I.; Hoffmann, F.; Callhoff, J.; Haibel, H.; Sieper, J.; Zink, A.; Poddubnyy, D. Determinants of psychological well-being in axial spondyloarthritis: An analysis based on linked claims and patient-reported survey data. Ann. Rheum. Dis. 2018, 77, 1017–1024. [Google Scholar] [CrossRef]
- Elixhauser, A.; Steiner, C.; Harris, D.R.; Coffey, R.M. Comorbidity measures for use with administrative data. Med. Care 1998, 36, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.A.R.; Ekholm, O.; Davidsen, M.; Christensen, A.I. The Danish health and morbidity surveys: Study design and participant characteristics. BMC Med. Res. Methodol. 2019, 19, 91. [Google Scholar] [CrossRef]
- Li, A.; Cronin, S.; Bai, Y.Q.; Walker, K.; Ammi, M.; Hogg, W.; Wong, S.T.; Wodchis, W.P. Assessing the representativeness of physician and patient respondents to a primary care survey using administrative data. BMC Fam. Pract. 2018, 19, 77. [Google Scholar] [CrossRef] [PubMed]
- Shortreed, S.M.; Von Korff, M.; Thielke, S.; LeResche, L.; Saunders, K.; Rosenberg, D.; Turner, J.A. Electronic Health Records to Evaluate and Account for Non-response Bias: A Survey of Patients Using Chronic Opioid Therapy. Obs. Stud. 2016, 2, 24–38. [Google Scholar] [PubMed]
- Vercambre, M.N.; Gilbert, F. Respondents in an epidemiologic survey had fewer psychotropic prescriptions than nonrespondents: An insight into health-related selection bias using routine health insurance data. J. Clin. Epidemiol. 2012, 65, 1181–1189. [Google Scholar] [CrossRef]
- Kamtsiuris, P.; Lange, M.; Hoffmann, R.; Rosario, A.S.; Dahm, S.; Kuhnert, R.; Kurth, B.M. The first wave of the German Health Interview and Examination Survey for Adults (DEGS1): Sample design, response, weighting and representativeness. Bundesgesundheitsblatt-Gesundh.-Gesundh. 2013, 56, 620–630. [Google Scholar] [CrossRef]
- Mindell, J.S.; Giampaoli, S.; Goesswald, A.; Kamtsiuris, P.; Mann, C.; Mannisto, S.; Morgan, K.; Shelton, N.J.; Verschuren, W.M.; Tolonen, H.; et al. Sample selection, recruitment and participation rates in health examination surveys in Europe—experience from seven national surveys. BMC Med. Res. Methodol. 2015, 15, 78. [Google Scholar] [CrossRef]
- Lutter, J.I.; Lukas, M.; Schwarzkopf, L.; Jorres, R.A.; Studnicka, M.; Kahnert, K.; Karrasch, S.; Bewig, B.; Vogelmeier, C.F.; Holle, R. Utilization and determinants of use of non-pharmacological interventions in COPD: Results of the COSYCONET cohort. Respir. Med. 2020, 171, 106087. [Google Scholar] [CrossRef]
- Applewhite, A.; Stancampiano, F.F.; Harris, D.M.; Manaois, A.; Dimuna, J.; Glenn, J.; Heckman, M.G.; Brushaber, D.E.; Sher, T.; Valery, J.R. A Retrospective Analysis of Gender-Based Difference in Adherence to Influenza Vaccination during the 2018–2019 Season. J. Prim. Care Community Health 2020, 11, 2150132720958532. [Google Scholar] [CrossRef]
- Polk, A.; Rasmussen, J.V.; Brorson, S.; Olsen, B.S. Reliability of patient-reported functional outcome in a joint replacement registry. A comparison of primary responders and non-responders in the Danish Shoulder Arthroplasty Registry. Acta Orthop. 2013, 84, 12–17. [Google Scholar] [CrossRef]
- Juto, H.; Nilsson, M.G.; Moller, M.; Wennergren, D.; Morberg, P. Evaluating non-responders of a survey in the Swedish fracture register: No indication of different functional result. BMC Musculoskelet. Disord. 2017, 18, 278. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P.J.; Roberts, I.; Clarke, M.J.; Diguiseppi, C.; Wentz, R.; Kwan, I.; Cooper, R.; Felix, L.M.; Pratap, S. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst. Rev. 2009, 3, MR000008. [Google Scholar] [CrossRef] [PubMed]
- Kelfve, S.; Kivi, M.; Johansson, B.; Lindwall, M. Going web or staying paper? The use of web-surveys among older people. BMC Med. Res. Methodol. 2020, 20, 252. [Google Scholar] [CrossRef] [PubMed]
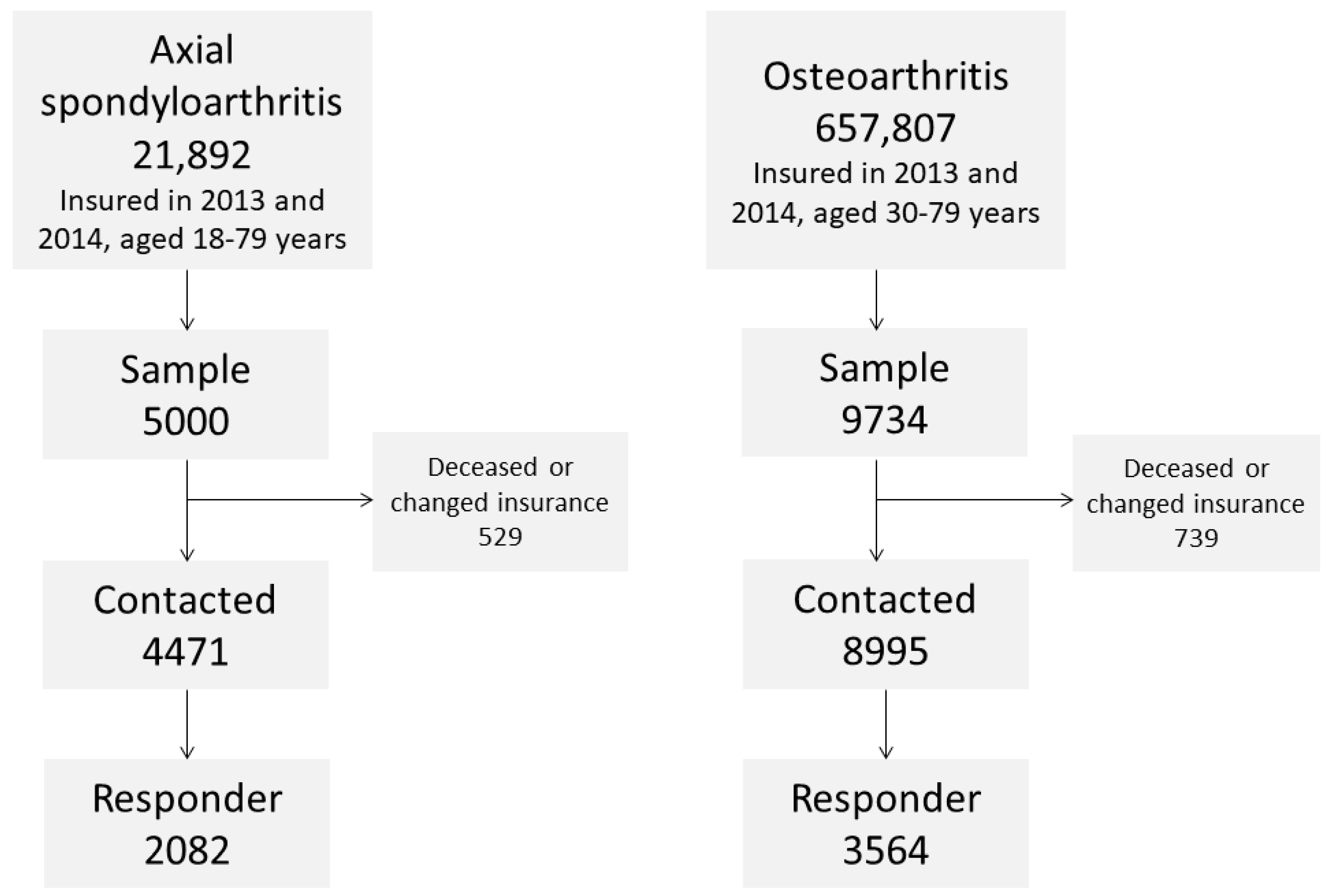
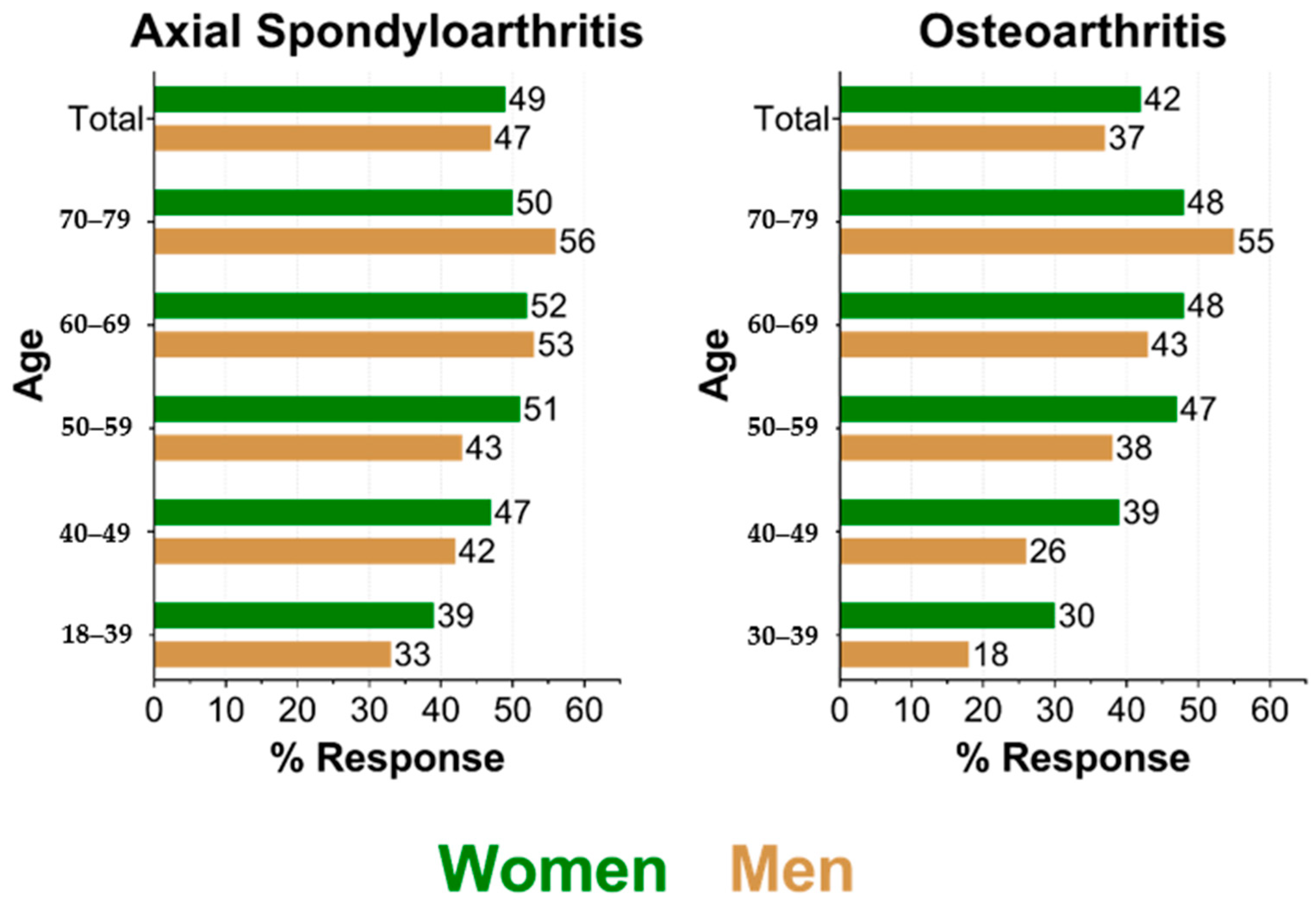
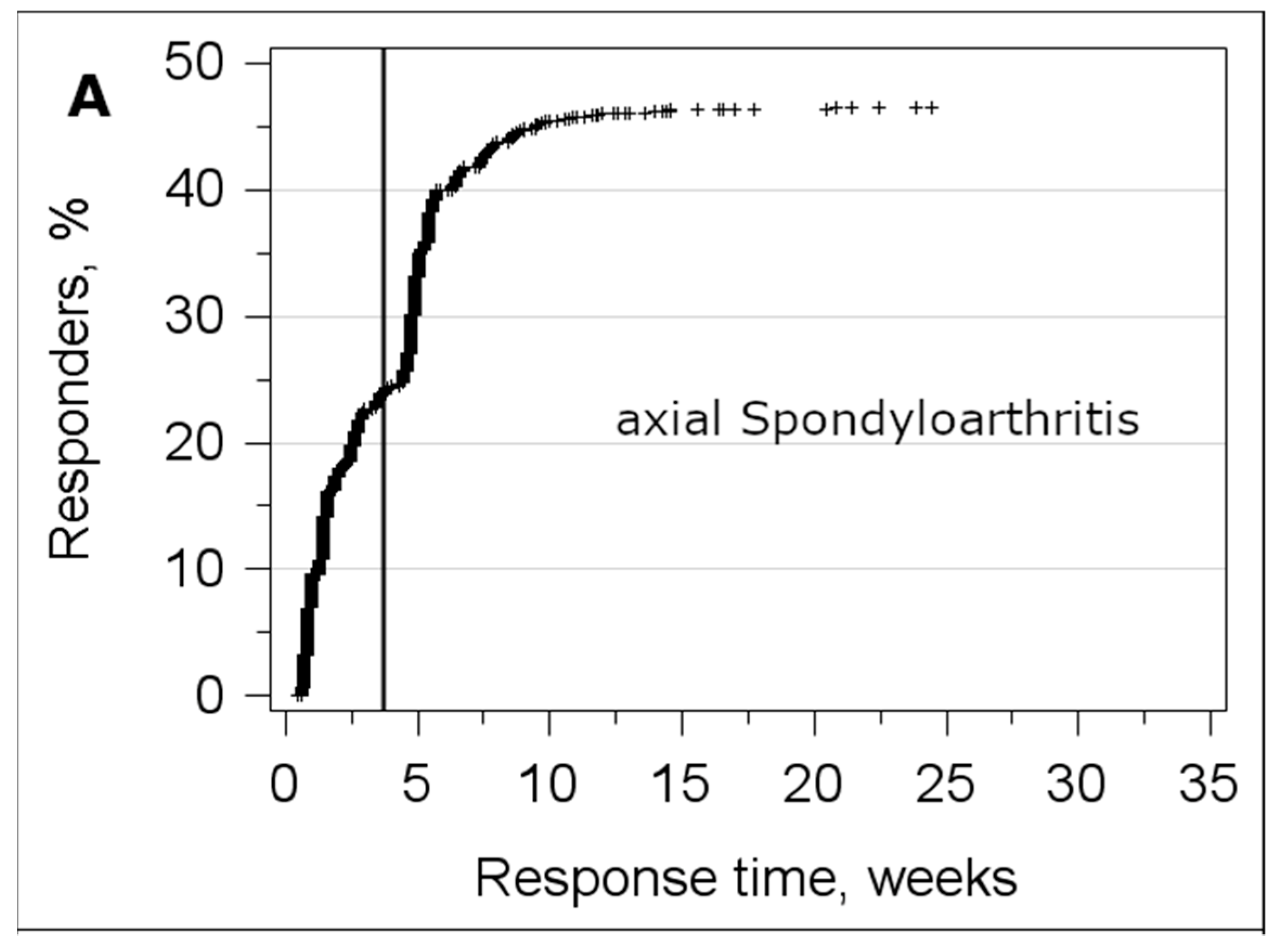
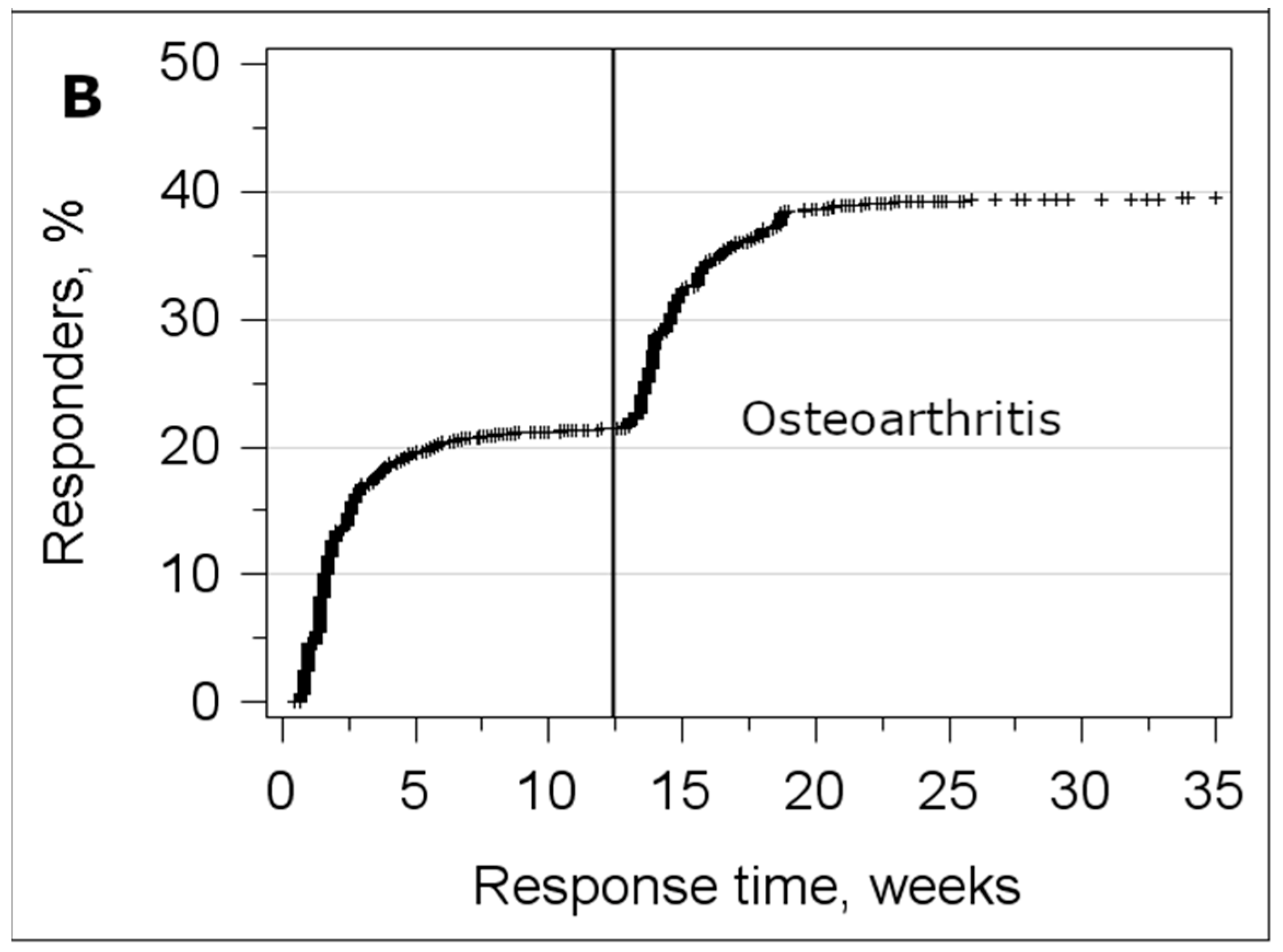
| Sample | Axial Spondyloarthritis | Osteoarthritis | ||
|---|---|---|---|---|
| Response status | Responder n = 2082 | Non-Responder n = 2389 | Responder n = 3564 | Non-Responder n = 5420 |
| Age (years), mean | 58.3 | 56.7 | 67.2 | 67.5 |
| Women, % | 49.9 | 48.2 | 70.5 | 69.0 |
| NSAIDs 1, % | 57.9 | 50.1 | 48.0 | 39.6 |
| Biologic disease-modifying anti-rheumatic drug, % | 13.9 | 9.0 | 0.9 | 0.5 |
| Opioids, % | 16.5 | 14.8 | 15.2 | 14.3 |
| Seeing a Rheumatologist, % | 32.7 | 21.1 | 7.1 | 3.9 |
| Seeing an Orthopedist, % | 39.6 | 31.3 | 56.8 | 43.7 |
| Elixhauser Score (0–31), Median | 2.6 | 2.5 | 2.8 | 2.6 |
| Number of prescribed medication, Median | 6.0 | 5.0 | 6.4 | 5.8 |
| Influenza vaccination, % | 32.5 | 22.3 | 39.2 | 29.0 |
| Hospitalized, % | 27.1 | 25.1 | 30.9 | 28.1 |
| Level of care present, % | 3.8 | 4.4 | 3.1 | 5.9 |
| Physical therapy, % | 50.4 | 38.7 | 48.6 | 40.1 |
| Persons with diagnosis in all quarters of survey year, % | 71.9 | 59.9 | 58.3 | 46.5 |
| -Sample | - | - | Axial Spondyloarthritis | Osteoarthritis | ||
|---|---|---|---|---|---|---|
| Parameter | Effect | Reference | Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value |
| Age | 40–49 | 18–39 | 1.40 (1.06; 1.85) | 0.02 | - | - |
| 50–59 | 1.73 (1.31; 2.28) | <0.001 | - | - | ||
| 60–69 | 1.84 (1.38; 2.45) | <0.001 | - | - | ||
| 70–79 | 1.79 (1.33; 2.40) | <0.001 | - | - | ||
| 40–49 | 30–39 | - | - | 1.32 (1.05; 1.66) | 0.02 | |
| 50–59 | - | - | 1.67 (1.33; 2.09) | <0.001 | ||
| 60–69 | - | - | 1.69 (1.35; 2.12) | <0.001 | ||
| 70–79 | - | - | 1.70 (1.34; 2.14) | <0.001 | ||
| Sex | male | female | 0.75 (0.56; 1.00) | 0.047 | 0.51 (0.38; 0.67) | <0.001 |
| Age * Sex | 18–39, male | 18–39, female | Reference | - | - | - |
| 30–39, male | 30–39, female | - | - | Reference | - | |
| 40–49, male | 40–49, female | 1.06 (0.71; 1.57) | 0.78 | 1.30 (0.90; 1.87) | 0.16 | |
| 50–59, male | 50–59, female | 1.03 (0.69; 1.52) | 0.89 | 1.45 (1.02; 2.05) | 0.04 | |
| 60–69, male | 60–69, female | 1.47 (0.99; 2.18) | 0.06 | 1.67 (1.19; 2.35) | 0.003 | |
| 70–79, male | 70–79, female | 1.83 (1.23; 2.73) | 0.003 | 2.56 (1.83; 3.63) | <0.001 | |
| Elixhauser Comorbidity Index | per comorbidity | 0.95 (0.92; 0.98) | <0.001 | 1.00 (0.98; 1.04) | 0.78 | |
| Opioids | Yes | No | - | - | 1.00 (0.84; 1.21) | 0.96 |
| Biologic disease-modifying anti-rheumatic drug | Yes | No | 1.28 (1.02; 1.59) | 0.03 | - | - |
| In Rheumatologic treatment | Yes | No | 1.71 (1.46; 2.01) | <0.001 | - | - |
| Orthopedist treating | Yes | No | - | - | 1.56 (1.37; 1.78) | <0.001 |
| Influenza Vaccination | Yes | No | 1.32 (1.13; 1.55) | <0.001 | 1.40 (1.22; 1.61) | <0.001 |
| Level of care present | Yes | No | 0.79 (0.56; 1.11) | 0.17 | 0.43 (0.30; 0.62) | <0.001 |
| Physical therapy | Yes | No | 1.42 (1.25; 1.62) | <0.001 | 1.18 (1.03; 1.35) | 0.01 |
| Number of quarters with diagnosis in survey year | per quarter | 1.18 (1.12; 1.25) | <0.001 | 1.04 (1.00; 1.09) | 0.07 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Callhoff, J.; Jacobs, H.; Albrecht, K.; Saam, J.; Zink, A.; Hoffmann, F. Factors Associated with Survey Non-Response in a Cross-Sectional Survey of Persons with an Axial Spondyloarthritis or Osteoarthritis Claims Diagnosis. Int. J. Environ. Res. Public Health 2020, 17, 9186. https://doi.org/10.3390/ijerph17249186
Callhoff J, Jacobs H, Albrecht K, Saam J, Zink A, Hoffmann F. Factors Associated with Survey Non-Response in a Cross-Sectional Survey of Persons with an Axial Spondyloarthritis or Osteoarthritis Claims Diagnosis. International Journal of Environmental Research and Public Health. 2020; 17(24):9186. https://doi.org/10.3390/ijerph17249186
Chicago/Turabian StyleCallhoff, Johanna, Hannes Jacobs, Katinka Albrecht, Joachim Saam, Angela Zink, and Falk Hoffmann. 2020. "Factors Associated with Survey Non-Response in a Cross-Sectional Survey of Persons with an Axial Spondyloarthritis or Osteoarthritis Claims Diagnosis" International Journal of Environmental Research and Public Health 17, no. 24: 9186. https://doi.org/10.3390/ijerph17249186
APA StyleCallhoff, J., Jacobs, H., Albrecht, K., Saam, J., Zink, A., & Hoffmann, F. (2020). Factors Associated with Survey Non-Response in a Cross-Sectional Survey of Persons with an Axial Spondyloarthritis or Osteoarthritis Claims Diagnosis. International Journal of Environmental Research and Public Health, 17(24), 9186. https://doi.org/10.3390/ijerph17249186






