Development and Molecular Investigation into the Effects of Carbamazepine Exposure in the Zebrafish (Danio rerio)
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Culture Conditions
2.2. Exposure Proposal
2.2.1. Exposure Experiment 1
2.2.2. Exposure Experiment 2
2.3. Gene Expression Analysis
2.4. Measurement of AChE Activity, GABA, and Glu Concentrations
2.5. Statistical Analysis
3. Results
3.1. Carbamazepine Quantification
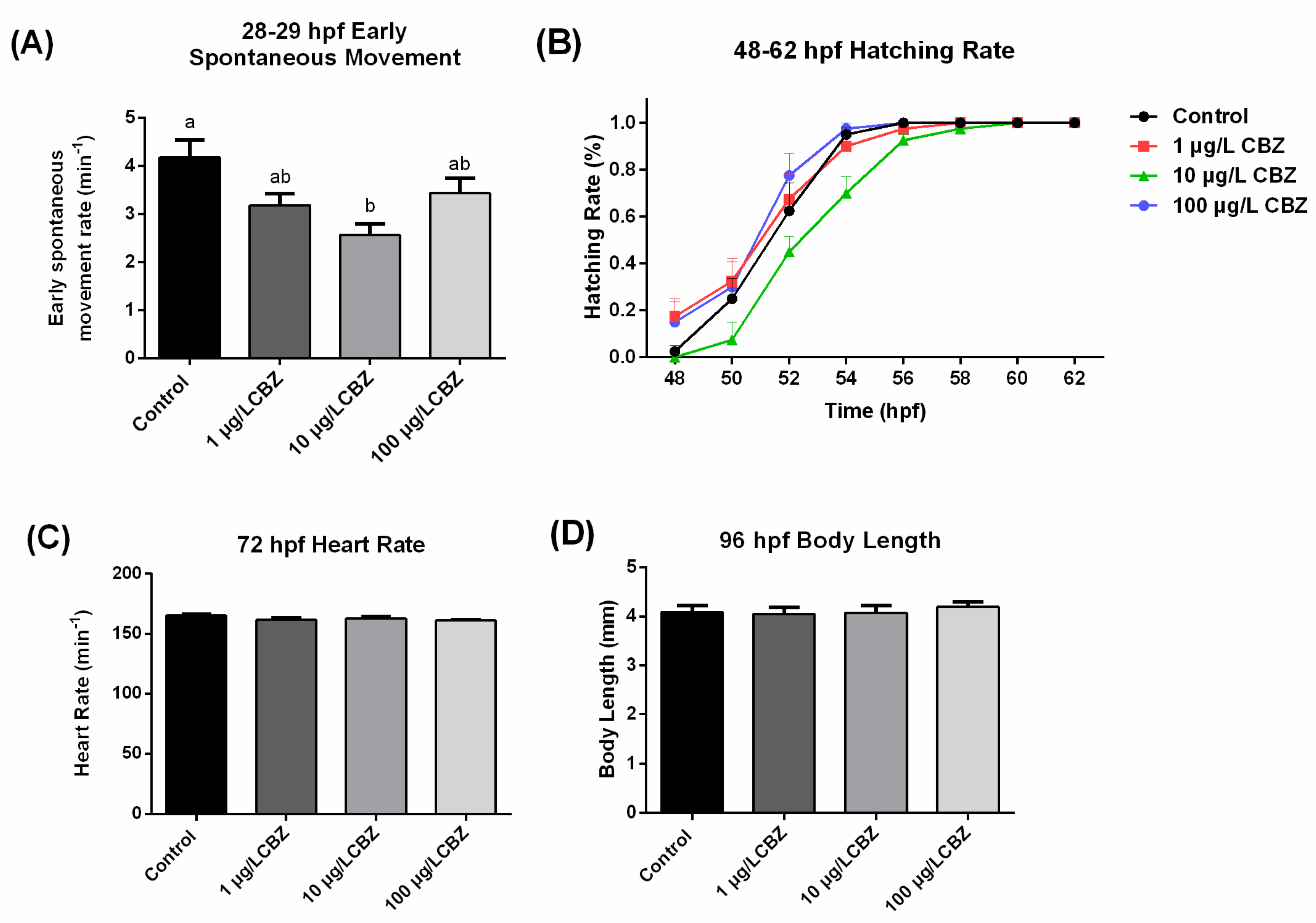
3.2. Early Spontaneous Movement and Development
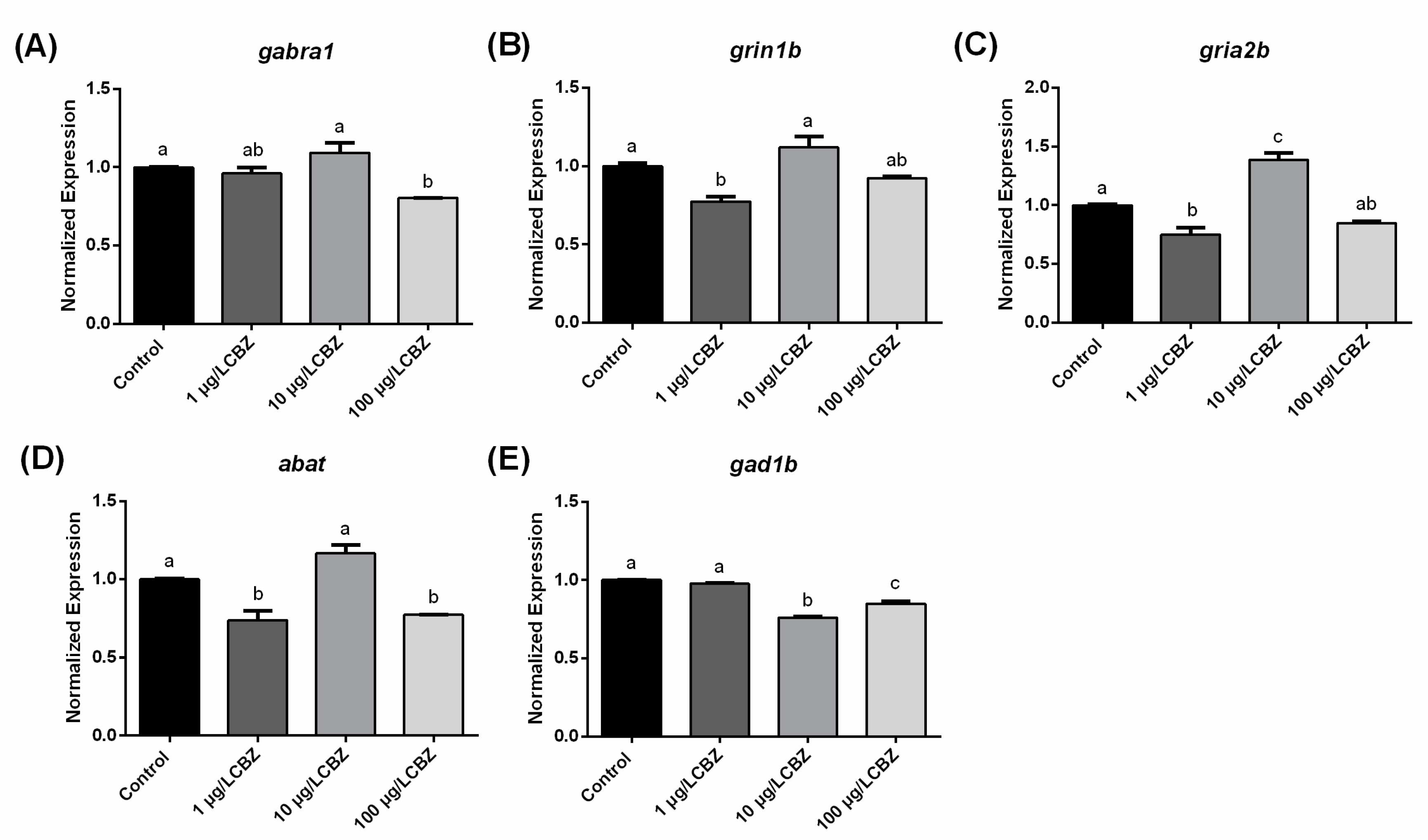
3.3. Gene Expression
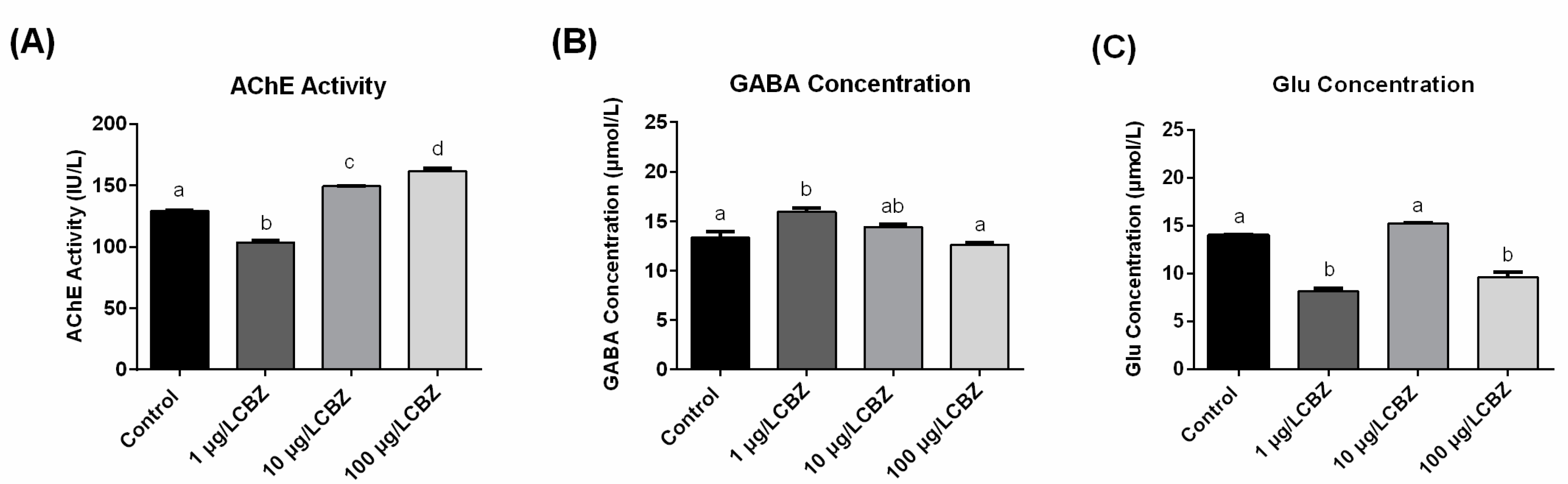
3.4. AChE Activity, GABA and Glu Concentrations Measurement
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect 1999, 107 (Suppl 6), 907–938. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Niu, X.; Yao, C.; Yang, W.; Lu, G. Distribution, Removal, and Risk Assessment of Pharmaceuticals and Their Metabolites in Five Sewage Plants. Int. J. Environ. Res. Public Health 2019, 16, 4729. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Jin, X.; Feng, C.; Wang, Z.; Wu, F.; Johnson, A.C.; Xiao, H.; Hollert, H.; Giesy, J.P. Ecological risk assessment of fifty pharmaceuticals and personal care products (PPCPs) in Chinese surface waters: A proposed multiple-level system. Environ. Int. 2020, 136, 105454. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Wong, M.H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59C, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Jin, X.; Yan, Z.; Luo, Y.; Feng, C.; Fu, Z.; Tang, Z.; Wu, F.; Giesy, J.P. Occurrence and multiple-level ecological risk assessment of pharmaceuticals and personal care products (PPCPs) in two shallow lakes of China. Environ. Sci. Eur. 2020, 32. [Google Scholar] [CrossRef]
- Chen, H.; Gu, X.; Zeng, Q.; Mao, Z.; Liang, X.; Martyniuk, C.J. Carbamazepine disrupts molting hormone signaling and inhibits molting and growth of Eriocheir sinensis at environmentally relevant concentrations. Aquat. Toxicol. 2019, 208, 138–145. [Google Scholar] [CrossRef]
- Chen, H.; Gu, X.; Zeng, Q.; Mao, Z.; Martyniuk, C.J. Characterization of the GABAergic system in Asian clam Corbicula fluminea: Phylogenetic analysis, tissue distribution, and response to the aquatic contaminant carbamazepine. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 239, 108896. [Google Scholar] [CrossRef]
- Zhang, Y.; Geißen, S.-U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef]
- Xie, Z.; Lu, G.; Liu, J.; Yan, Z.; Ma, B.; Zhang, Z.; Chen, W. Occurrence, bioaccumulation, and trophic magnification of pharmaceutically active compounds in Taihu Lake, China. Chemosphere 2015, 138, 140–147. [Google Scholar] [CrossRef]
- Ferrer, I.; Thurman, E.M. Analysis of 100 pharmaceuticals and their degradates in water samples by liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2012, 1259, 148–157. [Google Scholar] [CrossRef]
- Miao, X.-S.; Metcalfe, C.D. Determination of carbamazepine and its metabolites in aqueous samples using liquid chromatography−Electrospray tandem mass spectrometry. Anal. Chem. 2003, 75, 3731–3738. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, G.; Xie, Z.; Zhang, Z.; Li, S.; Yan, Z. Occurrence, bioaccumulation and risk assessment of lipophilic pharmaceutically active compounds in the downstream rivers of sewage treatment plants. Sci. Total Environ. 2015, 511, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zha, J.; Liang, X.; Li, J.; Wang, Z. Effects of the human antiepileptic drug carbamazepine on the behavior, biomarkers, and heat shock proteins in the Asian clam Corbicula fluminea. Aquat. Toxicol. 2014, 155, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gu, X.; Zeng, Q.; Mao, Z. Acute and Chronic Toxicity of Carbamazepine on the Release of Chitobiase, Molting, and Reproduction in Daphnia similis. Int. J. Environ. Res. Public Health 2019, 16, 209. [Google Scholar] [CrossRef]
- Fraz, S.; Lee, A.H.; Wilson, J.Y. Gemfibrozil and carbamazepine decrease steroid production in zebrafish testes (Danio rerio). Aquat. Toxicol. 2018, 198, 1–9. [Google Scholar] [CrossRef]
- da Silva Santos, N.; Oliveira, R.; Lisboa, C.A.; Mona, E.P.J.; Sousa-Moura, D.; Camargo, N.S.; Perillo, V.; Oliveira, M.; Grisolia, C.K.; Domingues, I. Chronic effects of carbamazepine on zebrafish: Behavioral, reproductive and biochemical endpoints. Ecotoxicol. Environ. Saf. 2018, 164, 297–304. [Google Scholar] [CrossRef]
- Van den Brandhof, E.J.; Montforts, M. Fish embryo toxicity of carbamazepine, diclofenac and metoprolol. Ecotoxicol. Environ. Saf. 2010, 73, 1862–1866. [Google Scholar] [CrossRef]
- Qiang, L.; Cheng, J.; Yi, J.; Rotchell, J.M.; Zhu, X.; Zhou, J. Environmental concentration of carbamazepine accelerates fish embryonic development and disturbs larvae behavior. Ecotoxicology 2016, 25, 1426–1437. [Google Scholar] [CrossRef]
- Yan, S.; Wang, M.; Liang, X.; Martyniuk, C.J.; Zha, J.; Wang, Z. Environmentally relevant concentrations of carbamazepine induce liver histopathological changes and a gender-specific response in hepatic proteome of Chinese rare minnows (Gobiocypris rarus). Environ. Pollut. 2018, 243, 480–491. [Google Scholar] [CrossRef]
- Yan, S.; Wang, M.; Zha, J.; Zhu, L.; Li, W.; Luo, Q.; Sun, J.; Wang, Z. Environmentally Relevant Concentrations of Carbamazepine Caused Endocrine-Disrupting Effects on Nontarget Organisms, Chinese Rare Minnows (Gobiocypris rarus). Environ. Sci. Technol. 2018, 52, 886–894. [Google Scholar] [CrossRef]
- Galus, M.; Kirischian, N.; Higgins, S.; Purdy, J.; Chow, J.; Rangaranjan, S.; Li, H.; Metcalfe, C.; Wilson, J.Y. Chronic, low concentration exposure to pharmaceuticals impacts multiple organ systems in zebrafish. Aquat. Toxicol. 2013, 132–133, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Christen, V.; Hickmann, S.; Rechenberg, B.; Fent, K. Highly active human pharmaceuticals in aquatic systems: A concept for their identification based on their mode of action. Aquat. Toxicol. 2010, 96, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Ambrósio, A.F.; Soares-da-Silva, P.; Carvalho, C.M.; Carvalho, A.P. Mechanisms of action of carbamazepine and its derivatives, oxcarbazepine, BIA 2-093, and BIA 2-024. Neurochem. Res. 2002, 27, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Potschka, H. Pharmacological treatment strategies: Mechanisms of antiepileptic drugs. Epileptology 2013. [Google Scholar] [CrossRef]
- Schoch, P.; Richards, J.; Häring, P.; Takacs, B.; Stähli, C.; Staehelin, T.; Haefely, W.; Möhler, H. Co-localization of GABAA receptors and benzodiazepine receptors in the brain shown by monoclonal antibodies. Nature 1985, 314, 168. [Google Scholar] [CrossRef] [PubMed]
- Charych, E.I.; Liu, F.; Moss, S.J.; Brandon, N.J. GABAA receptors and their associated proteins: Implications in the etiology and treatment of schizophrenia and related disorders. Neuropharmacology 2009, 57, 481–495. [Google Scholar] [CrossRef]
- Dionisio, L.; De Rosa, M.J.; Bouzat, C.; del Carmen Esandi, M. An intrinsic GABAergic system in human lymphocytes. Neuropharmacology 2011, 60, 513–519. [Google Scholar] [CrossRef]
- Yan, W.; Li, L.; Li, G.; Zhao, S. Microcystin-LR induces changes in the GABA neurotransmitter system of zebrafish. Aquat. Toxicol. 2017, 188, 170–176. [Google Scholar] [CrossRef]
- Zheng, S.; Liu, C.; Huang, Y.; Bao, M.; Huang, Y.; Wu, K. Effects of 2,2′,4,4′-tetrabromodiphenyl ether on neurobehavior and memory change and bcl-2, c-fos, grin1b and lingo1b gene expression in male zebrafish (Danio rerio). Toxicol. Appl. Pharmacol. 2017, 333, 10–16. [Google Scholar] [CrossRef]
- Soghomonian, J.-J.; Martin, D.L. Two isoforms of glutamate decarboxylase: Why? Trends Pharmacol. Sci. 1998, 19, 500–505. [Google Scholar] [CrossRef]
- Atama, C.I.; Nnaji, E.C.; Christian Ezeoyili, I.; Udeani, F.O.; Onovo, C.J.; Ike Ossai, N.; Oscar Aguzie, I.; Nwani, C.D. Neuromodulatory and oxidative stress evaluations in African catfish Clarias gariepinus exposed to antipsychotic drug chlorpromazine. Drug. Chem. Toxicol. 2020. [Google Scholar] [CrossRef]
- Jia, D.; Li, X.; Du, S.; Xu, N.; Zhang, W.; Yang, R.; Zhang, Y.; He, Y.; Zhang, Y. Single and combined effects of carbamazepine and copper on nervous and antioxidant systems of zebrafish (Danio rerio). Environ. Toxicol. 2020, 35, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Rico, E.P.; de Oliveira, D.L.; Rosemberg, D.B.; Mussulini, B.H.; Bonan, C.D.; Dias, R.D.; Wofchuk, S.; Souza, D.O.; Bogo, M.R. Expression and functional analysis of Na(+)-dependent glutamate transporters from zebrafish brain. Brain Res. Bull 2010, 81, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choi, K.; Jung, J.; Park, S.; Kim, P.-G.; Park, J. Aquatic toxicity of acetaminophen, carbamazepine, cimetidine, diltiazem and six major sulfonamides, and their potential ecological risks in Korea. Environ. Int. 2007, 33, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Hampel, M.; Bron, J.E.; Taggart, J.B.; Leaver, M.J. The antidepressant drug carbamazepine induces differential transcriptome expression in the brain of Atlantic salmon, Salmo salar. Aquat. Toxicol. 2014, 151, 114–123. [Google Scholar] [CrossRef]
- Zindler, F.; Beedgen, F.; Brandt, D.; Steiner, M.; Stengel, D.; Baumann, L.; Braunbeck, T. Analysis of tail coiling activity of zebrafish (Danio rerio) embryos allows for the differentiation of neurotoxicants with different modes of action. Ecotox Environ. Safe 2019, 186, 109754. [Google Scholar] [CrossRef]
- Schetinger, M.R.; Porto, N.M.; Moretto, M.B.; Morsch, V.M.; da Rocha, J.B.; Vieira, V.; Moro, F.; Neis, R.T.; Bittencourt, S.; Bonacorso, H.G.; et al. New benzodiazepines alter acetylcholinesterase and ATPDase activities. Neurochem. Res. 2000, 25, 949–955. [Google Scholar] [CrossRef]
- Tõugu, V.; Kesvatera, T. Role of ionic interactions in cholinesterase catalysis. Biochim. Biophys. Acta BBA Protein Struct. Mol. Enzymol. 1996, 1298, 12–30. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, X.; Mu, L.; Hou, L.; Yang, B.; Zhao, J.; Schlenk, D.; Dong, W.; Xie, L.; Zhang, Q. Effects of acute and chronic exposures of fluoxetine on the Chinese fish, topmouth gudgeon Pseudorasbora parva. Ecotoxicol. Environ. Saf. 2018, 160, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Prange, O.; Wong, T.P.; Gerrow, K.; Wang, Y.T.; El-Husseini, A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc. Natl. Acad. Sci. USA 2004, 101, 13915. [Google Scholar] [CrossRef] [PubMed]
- Ravenelle, R.; Neugebauer, N.M.; Niedzielak, T.; Donaldson, S.T. Sex differences in diazepam effects and parvalbumin-positive GABA neurons in trait anxiety Long Evans rats. Behav. Brain Res. 2014, 270, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Snigirov, S.; Sylantyev, S. GABAA receptors activate fish feeding behaviour via two distinct functional pathways. J. Exp. Biol. 2018, 221. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Qiu, X.; Chen, C.; Chen, K.; Li, M.; Xu, H.; Wu, X.; Shimasaki, Y.; Oshima, Y. Short-term and persistent impacts of sublethal exposure to diazepam on behavioral traits and brain GABA levels in juvenile zebrafish (Danio rerio). Sci. Total Environ. 2020, 740, 140392. [Google Scholar] [CrossRef] [PubMed]
- Orger, M.B.; Smear, M.C.; Anstis, S.M.; Baier, H. Perception of Fourier and non-Fourier motion by larval zebrafish. Nat. Neurosci. 2000, 3, 1128–1133. [Google Scholar] [CrossRef]
- Wang, H.; Bedford, F.K.; Brandon, N.J.; Moss, S.J.; Olsen, R.W. GABAA-receptor-associated protein links GABAA receptors and the cytoskeleton. Nature 1999, 397, 69–72. [Google Scholar] [CrossRef]
- Martin, D.L.; Rimvall, K. Regulation of γ-Aminobutyric Acid Synthesis in the Brain. J. Neurochem. 1993, 60, 395–407. [Google Scholar] [CrossRef]
- Leclercq, K.; Afrikanova, T.; Langlois, M.; De Prins, A.; Buenafe, O.E.; Rospo, C.C.; Van Eeckhaut, A.; de Witte, P.A.M.; Crawford, A.D.; Smolders, I.; et al. Cross-species pharmacological characterization of the allylglycine seizure model in mice and larval zebrafish. Epilepsy Behav. 2015, 45, 53–63. [Google Scholar] [CrossRef]
- Sands, T.T.; Balestri, M.; Bellini, G.; Mulkey, S.B.; Danhaive, O.; Bakken, E.H.; Taglialatela, M.; Oldham, M.S.; Vigevano, F.; Holmes, G.L.; et al. Rapid and safe response to low-dose carbamazepine in neonatal epilepsy. Epilepsia 2016, 57, 2019–2030. [Google Scholar] [CrossRef]
| Symbol | Gene Name | Primer (5′-3′) | NCBI (National Center for Biotechnology Information) Accession Number |
|---|---|---|---|
| β-actin | Beta-actin | F: CGAGCAGGAGATGGGAACC R: CAACGGAAACGCTCATTGC | AF057040.1 |
| gabra1 | GABAA receptor, α1 | F: TCAGGCAGAGCTGGAAGGAT R: TGCCGTTGTGGAAGAACGT | NM_001077326 |
| grin1b | Glutamate receptor, ionotropic, N-methyl D-aspartate 1b | F: CATGAGAACGGCTTCATGG R: GCCAGCTGCATTTGCTTCC | NM_001144131 |
| gria2b | Glutamate receptor, ionotropic, AMPA 2b | F: ATGACAGTGACCGAGGAC R: CTTGAAAGAGTGAGCGATA | NM_131895 |
| abat | GABA transaminase | F: GCGTTCAGGCAAAGCTCT R: GCAGGACGGAAACGGAT | NM_201498 |
| gad1b | Glutamate decarboxylase 1b | F: AACTCAGGCGATTGTTGCAT R: TGAGGACATTTCCAGCCTTC | NM_194419 |
| Conditions | Control | 1 μg/L | 10 μg/L | 100 μg/L |
|---|---|---|---|---|
| Nominal concentration (μg/L) | 0 | 1.00 | 10.00 | 100.00 |
| Measured concentration (μg/L) | No detected | 0.90 ± 0.02 | 9.50 ± 0.22 | 92.80 ± 3.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Yang, H.; Zhao, Y.; Gu, X.; Martyniuk, C.J. Development and Molecular Investigation into the Effects of Carbamazepine Exposure in the Zebrafish (Danio rerio). Int. J. Environ. Res. Public Health 2020, 17, 8882. https://doi.org/10.3390/ijerph17238882
Chen H, Yang H, Zhao Y, Gu X, Martyniuk CJ. Development and Molecular Investigation into the Effects of Carbamazepine Exposure in the Zebrafish (Danio rerio). International Journal of Environmental Research and Public Health. 2020; 17(23):8882. https://doi.org/10.3390/ijerph17238882
Chicago/Turabian StyleChen, Huihui, Huiting Yang, Yanyan Zhao, Xiaohong Gu, and Christopher J. Martyniuk. 2020. "Development and Molecular Investigation into the Effects of Carbamazepine Exposure in the Zebrafish (Danio rerio)" International Journal of Environmental Research and Public Health 17, no. 23: 8882. https://doi.org/10.3390/ijerph17238882
APA StyleChen, H., Yang, H., Zhao, Y., Gu, X., & Martyniuk, C. J. (2020). Development and Molecular Investigation into the Effects of Carbamazepine Exposure in the Zebrafish (Danio rerio). International Journal of Environmental Research and Public Health, 17(23), 8882. https://doi.org/10.3390/ijerph17238882






