Using Rutilus rutilus (L.) and Perca fluviatilis (L.) as Bioindicators of the Environmental Condition and Human Health: Lake Łańskie, Poland
Abstract
1. Introduction
- Differences between mercury content, hepatosomatic index (HSI) and gonadosomatic index (GSI) in two freshwater fish species examined.
- Differences between mercury content in organs (muscles, liver, gills and gonads) of the same species.
- The impact of biometric parameters (body weight and total length) and Fulton’s condition factor (FCF) on the content of mercury in selected organs of fish.
- The health risk posed by mercury using estimated daily intake (EDI), tolerable weekly intake (EWI) and target hazard quotient (THQ).
- Metal pollution index (MPI) and bioconcentration factor (BCF) based on the mercury content of the four organs.
- Differences between the fatty acids profile and the lipid quality indices (atherogenicity index (AI), thrombogenicity index (TI), flesh-lipid quality index (FLQ), hypercholesterolemic fatty acids (OFA) and hypocholesterolemic fatty acids (DFA)) in muscles of perch (Perca fluviatilis L.) and roach (Rutilus rutilus L.).
2. Materials and Methods
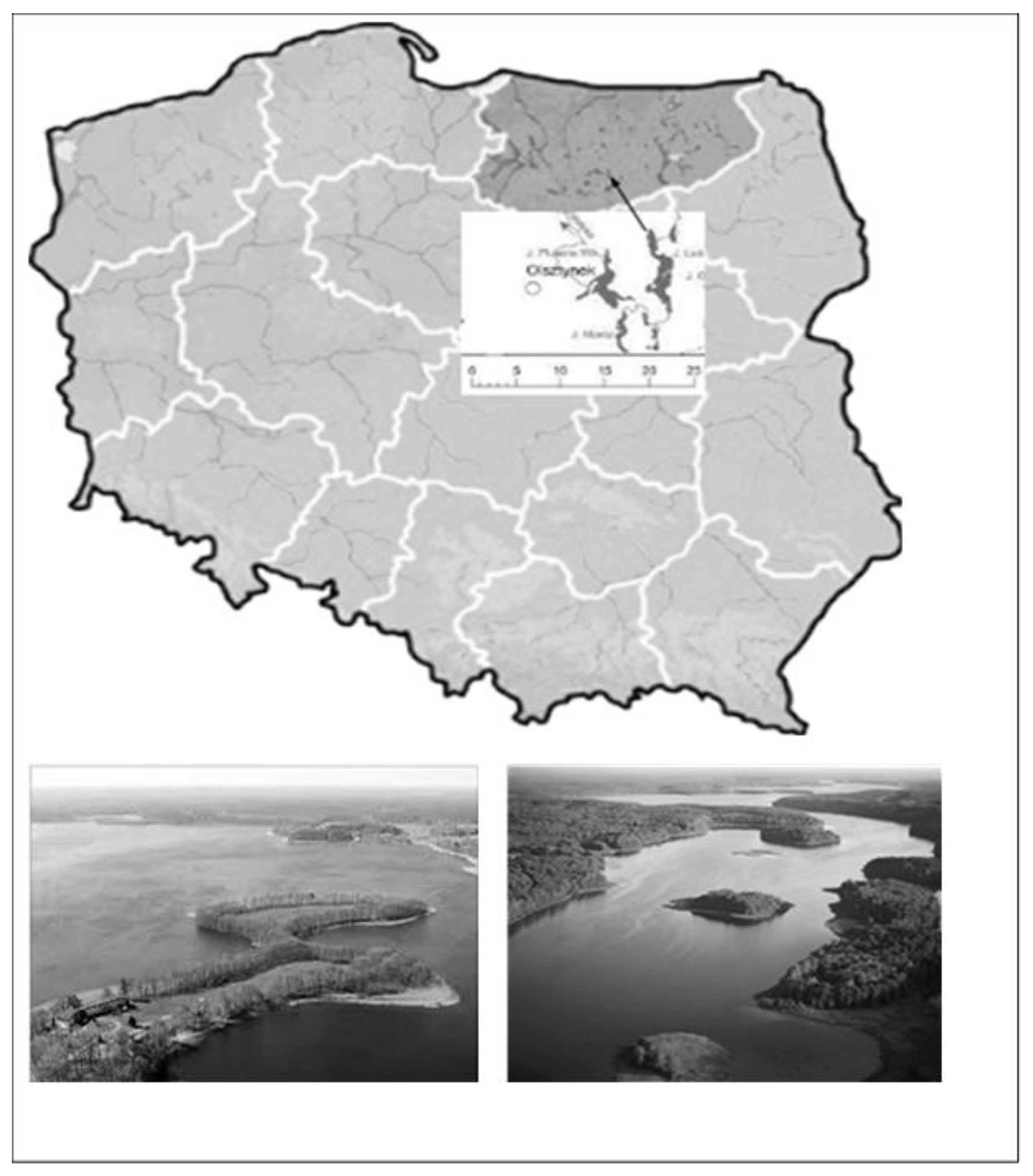
2.1. Sampling and Sample Preparation
2.2. Element Analysis
2.2.1. Mercury
2.2.2. Fat and Fatty Acid Analysis
2.2.3. Indicators of Environmental Conditions and Fish Quality (FCF, HSI, GSI, MPI and BCF)
2.2.4. The Lipid Quality Indices
2.2.5. Human Health Risk Assessment
2.3. Statistical Analysis
3. Results
3.1. Mercury and Tools for Monitoring Fish and Environmental Conditions (BCF, MPI, FCF, HSI and GSI)
3.2. Human Health Risk Assessment
3.3. Fatty Acids
3.4. Lipid Quality Indexes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Starmach, K.; Wróbel, S.; Pasternak, K. Hydrobiologia. Limnologia; PWN: Warsaw, Poland, 1976. [Google Scholar]
- Sadowska, U. The importance of bioindication in water ecotoxicology. SEeB 2012, 10, 33–52. [Google Scholar] [CrossRef]
- Sauliutė, G.; Stankevičiūtė, M.; Svecevičius, G.; Baršiene, J.; Valskienė, R. Assessment of heavy metals bioconcentration factor (BCF) and genotoxicity response induced by metal mixture in Salmo salar tissues. In Proceedings of the Environmental Engineering 10th International Conference, Vilnius, Lithuania, 27–28 April 2017. [Google Scholar] [CrossRef]
- Barron, M.G. Bioaccumulation and bioconcentration in aquatic organisms. In Handbook of Ecotoxicology; Hoffman, D.J., Rattner, B.A., Burton, G.A., Jr., Cairns, J., Jr., Eds.; CRC Press Company: Boca Raton, FL, USA, 2003; pp. 877–892. [Google Scholar]
- Yarsan, E.; Yipel, M. The Important Terms of Marine Pollution “Biomarkers and Biomonitoring, Bioaccumulation, Bioconcentration, Biomagnification”. J. Mol. Biomark. Diagn. 2013, 1, 1–4. [Google Scholar] [CrossRef]
- Mackay, D.; Celsie, A.K.D.; Powell, D.E.; Parnis, J.M. Bioconcentration, bioaccumulation, biomagnification and trophic magnification: A modelling perspective. Environ. Sci. Process. Impacts 2018, 20, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Zeitoun, M.M.; Mehana, E.E. Impact of water pollution with heavy metals on fish health: Overview and updates. Glob. Vet. 2014, 12, 219–231. [Google Scholar] [CrossRef]
- Lodenius, M. Accumulation and fluxes of mercury in terrestrial and aquatic food chains with special reference to Finland. Eur. J. Environ. Sci. 2012, 2, 77–83. [Google Scholar] [CrossRef][Green Version]
- World Health Organization (WHO). Elemental Mercury and Inorganic Mercury Compounds: Human Health Aspects. Concise International Chemical Assessment Document 50; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Szczerbowski, A.; Kucharczyk, D.; Mamcarz, A.; Łuczyński, M.J.; Targońska, K.; Kujawa, R. Artificial off-season spawning of Eurasian perch Perca fluviatilis L. Arch. Pol. Fish. 2009, 17, 95–98. [Google Scholar] [CrossRef]
- Kujawa, R.; Kucharczyk, D.; Mamcarz, A.; Żarski, D.; Targońska, K. Artificial spawning of common tench Tinca tinca (Linnaeus, 1758), obtained from wild and domestic stocks. Aquac. Int. 2011, 19, 513–521. [Google Scholar] [CrossRef]
- Kucharczyk, D.; Nowosad, J.; Kujawa, R.; Dietrich, G.; Biegaj, M.; Sikora, M.; Łuczyński, M.J. Comparison of spontaneous and hormone-induced reproduction of burbot (Lota lota L.) under hatchery conditions. Aquaculture 2018, 485, 25–29. [Google Scholar] [CrossRef]
- Kucharczyk, D.; Nowosad, J.; Kucharczyk, D.J.; Kupren, K.; Targońska, K.; Wyszomirska, E.; Kujawa, R. Out-of-season artificial reproduction of common dace (Leuciscus leuciscus L.) under controlled conditions. Anim. Reprod. Sci. 2019, 202, 21–25. [Google Scholar] [CrossRef]
- Kucharczyk, D.; Nowosad, J.; Wyszomirska, E.; Cejko, B.I.; Arciuch-Rutkowska, M.; Juchno, D.; Boroń, A. Comparison of artificial spawning effectiveness of hCG, CPH and GnRHa in combination with dopamine inhibitors in a wild strain of ide Leuciscus idus (L.) in hatchery conditions. Anim. Reprod. Sci. 2020, 221, 106543. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Nowosad, J.; Biegaj, M.; Kucharczyk, D.; Dębowski, M. The possibility of application of agglomerate elastomers (EPP) as media for biological bed in aquaculture. Aquac. Res. 2018, 49, 2988–2994. [Google Scholar] [CrossRef]
- Sikora, M.; Nowosad, J.; Kucharczyk, D. Comparison of Different Biofilter Media during Biological Bed Maturation Using Common Carp as a Biogen Donor. Appl. Sci. 2020, 10, 626. [Google Scholar] [CrossRef]
- Nowosad, J.; Kucharczyk, D. First report of the occurrence and different types of conjoined twins in common whitefish Coregonus maraena larvae originating from the Baltic Sea. Dis. Aquat. Org. 2019, 132, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Nowosad, J.; Kucharczyk, D.; Łuczyńska, J. Changes in mercury concentration in muscles, ovaries and eggs of European eel during maturation under controlled conditions. Ecotoxicol. Environ. Saf. 2018, 148, 857–861. [Google Scholar] [CrossRef]
- Nowosad, J.; Sieszputowska, J.; Kucharczyk, D.; Łuczyńska, J.; Sikora, M.; Kujawa, R. Dynamics of mercury content in adult sichel (Pelecus cultratus L.) tissues from the Baltic Sea before and during spawning. Mar. Environ. Res. 2019, 148, 75–80. [Google Scholar] [CrossRef]
- Helfrich, L.A.; Neves, R.J. Sustaining America’s aquatic biodiversity—Freshwater fish biodiversity and conservation. VCE Publ. 2009, 420–525, 1–6. [Google Scholar]
- Zdrojewicz, Z.; Adamek, M.; Machelski, A.; Wójcik, E. The influence of fatty acids (omega) contained in fish on the man organism. Med. Rodzinna. 2015, 3, 137–143. [Google Scholar]
- Rustan, A.C.; Drevon, C.A. Fatty Acids: Structures and Properties. eLS 2005, 1–7. [Google Scholar] [CrossRef]
- Lucio, G.C.; Vittorio, F. Joint FAO/WHO Expert Consultation on the Risks and Benefits of Fish Consumption; FAO: Rome, Italy, 2010; p. 63. [Google Scholar]
- Strungaru, S.-A.; Nicoarã, M.; Rãu, M.A.; Plãvan, G.; Micu, D. Do you like to eat fish? An overview of the benefits of fish consumption and risk of mercury poisoning. Biol. Anim. 2015, 61, 117–123. [Google Scholar]
- Lunn, J.; Theobald, H.E. The health effects of dietary unsaturated fatty acids. Nutr. Bull. 2006, 31, 178–224. [Google Scholar] [CrossRef]
- Djazayery, A.; Jazayery, S. ω-3 fatty acids in physical and mental health and disease. In Wild-Type Food in Health Promotion and Disease Prevention; De Meester, F., Watson, R.R., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2008; pp. 309–321. [Google Scholar]
- Harris, W.S. The omega-3 index as a risk factor for coronary heart disease. Am. J. Clin. Nutr. 2008, 87, 1997S–2002S. [Google Scholar] [CrossRef]
- Das, U.N. Biological significance of essential fatty acids. J. Assoc. Phys. India 2006, 54, 309–319. [Google Scholar]
- Lorente-Cebrián, S.; Costa, A.G.V.; Navas-Carretero, S.; Zabala, M.; Martínez, J.A.; Moreno-Aliaga, M.J. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: A review of the evidence. J. Physiol. Biochem. 2013, 69, 633–651. [Google Scholar] [CrossRef] [PubMed]
- Ristić-Medić, D.; Vučić, V.; Takić, M.; Karadžić, I.; Glibetić, M. Polyunsaturated fatty acids in health and disease. J. Serb. Chem. Soc. 2013, 78, 1269–1289. [Google Scholar] [CrossRef]
- Calder, P.C. Very long chain omega-3 (n-3) fatty acids and human health. Eur. J. Lipid Sci. Technol. 2014, 116, 1280–1300. [Google Scholar] [CrossRef]
- Kim, K.-B.; Nam, Y.A.; Kim, H.S.; Hayes, A.W.; Lee, B.-M. α-Linolenic acid: Nutraceutical, pharmacological and toxicological evaluation. Food Chem. Toxicol. 2014, 70, 163–178. [Google Scholar] [CrossRef]
- Bowen, K.J.; Harris, W.S.; Kris-Etherton, P.M. Omega-3 Fatty Acids and Cardiovascular Disease: Are There Benefits? Curr. Treat. Options Cardiovasc. Med. 2016, 18, 69. [Google Scholar] [CrossRef]
- Yang, Z.-H.; Emma-Okon, B.; Remaley, A.T. Dietary marine-derived long-chain monounsaturated fatty acids and cardiovascular disease risk: A mini review. Lipids Health Dis. 2016, 15, 1–9. [Google Scholar] [CrossRef]
- Figuiredo, P.S.; Inada, A.C.; Marcelino, G.; Lopes Cardozo, C.M.; de Cássia Freitas, K.; de Cássia Avellaneda Guimarães, R.; de Castro, A.P.; do Nascimento, V.A.; Hiane, P.A. Fatty Acids Consumption: The Role Metabolic Aspects Involved in Obesity and Its Associated Disorders. Nutrients 2017, 9, 1158. [Google Scholar] [CrossRef]
- Olgunoglu, I.A. Review on omega-3 (n-3) fatty acids in fish and seafood. J. Biol. Agric. Healthc. 2017, 7, 37–45. [Google Scholar]
- Łuczyńska, J.; Paszczyk, B.; Nowosad, J.; Łuczyński, M.J. Mercury, Fatty Acids Content and Lipid Quality Indexes in Muscles of Freshwater and Marine Fish on the Polish Market. Risk Assessment of Fish Consumption. Int. J. Environ. Res. Public Health 2017, 14, 1120. [Google Scholar] [CrossRef] [PubMed]
- Łuczyńska, J.; Paszczyk, B.; Łuczyński, M.J. Fish as a bioindicator of heavy metals pollution in aquatic ecosystem of Pluszne Lake, Poland, and risk assessment for consumer’s health. Ecotoxicol. Environ. Saf. 2018, 153, 60–67. [Google Scholar] [CrossRef]
- Łuczyńska, J.; Paszczyk, B. Health Risk Assessment of Heavy Metals and Lipid Quality Indexes in Freshwater Fish from Lakes of Warmia and Mazury Region, Poland. Int. J. Environ. Res. Public Health 2019, 16, 3780. [Google Scholar] [CrossRef]
- Domingo, J.L. Omega-3 fatty acids and the benefits of fish consumption: Is all that glitters gold? Environ. Int. 2007, 33, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). Food and Nutrition Paper. Fats and Fatty Acids in Human Nutrition; Report of an Expert Consultation; FAO: Rome, Italy, 2010. [Google Scholar]
- Łuczyńska, J.; Łuczyński, M.J.; Paszczyk, B.; Tońska, E. Concentration of mercury in muscles of predatory and non-predatory fish from lake Pluszne (Poland). J. Vet. Res. 2016, 60, 43–47. [Google Scholar] [CrossRef]
- Christie, W.W. The isolation of lipids from tissues. Recommended Procedures. Chloroform-methanol (2:1, v/v) extraction and “Folch” wash. In Lipid Analysis. Isolation, Separation, Identification and Structural Analysis of Lipids; Christie, W.W., Ed.; Pergamon Press: Oxford, UK, 1973; pp. 39–40. [Google Scholar]
- Hamid, M.A.; Mansor, M.; Nor, S.A.M. Length-weight Relationship and Condition Factor of Fish Populations in Temengor Reservoir: Indication of Environmental Health. Sains Malays. 2015, 44, 61–66. [Google Scholar] [CrossRef]
- Sadekarpawar, S.; Parikh, P. Gonadosomatic and hepatosomatic indices of freshwater fish Oreochromis mossambicus in response to a plant nutrient. World J. Zool. 2013, 8, 110–118. [Google Scholar] [CrossRef]
- Usero, J.; Gonzalez-Regalado, E.G.; Gracia, I. Trace metals in bivalve molluscs Chamelea gallina from the Atlantic coast of southern Spain. Mar. Pollut. Bull. 1996, 32, 305–310. [Google Scholar] [CrossRef]
- Usero, J.; Regalado, E.G.; Gracia, I. Trace metals in bivalve molluscs Ruditapes decussatus and Ruditapes philippinarum from the Atlantic coast of southern Spain. Environ. Int. 1997, 23, 291–298. [Google Scholar] [CrossRef]
- Abdel-Khalek, A.A.; Elhaddad, E.; Mamdouh, S.; Saed Marie, M.-A. Assessment of metal pollution around Sabal Drainage in River Nile and its impacts on bioaccumulation level, metals correlation and human risk hazard using Oreochromis niloticus as a bioindicator. Turk. J. Fish. Aquat. Sci. 2016, 16, 227–239. [Google Scholar] [CrossRef]
- Lau, S.; Mohamed, M.; Yen, A.T.C.; Su’Ut, S. Accumulation of heavy metals in freshwater molluscs. Sci. Total Environ. 1998, 214, 113–121. [Google Scholar] [CrossRef]
- Ulbricht, T.; Southgate, D. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Garaffo, M.A.; Vassallo-Agius, R.; Nengas, Y.; Lembo, E.; Rando, R.; Maisano, R.; Dugo, G.; Giuffrida, D. Fatty Acids Profile, Atherogenic (IA) and Thrombogenic (IT) Health Lipid Indices, of Raw Roe of Blue Fin Tuna (Thunnus thynnus L.) and Their Salted Product “Bottarga”. Food Nutr. Sci. 2011, 2, 736–743. [Google Scholar] [CrossRef]
- Telahigue, K.; Hajji, T.; Rabeh, I.; El Cafsi, M. The changes of fatty acid composition in sun dried, oven dried and frozen hake (Merluccius merluccius) and sardinella (Sardinella aurita). Afr. J. Biochem. Res. 2013, 7, 158–164. [Google Scholar] [CrossRef]
- Abrami, G.; Natiello, F.; Bronzi, P.; McKenzie, D.; Bolis, L.; Agradi, E. A comparison of highly unsaturated fatty acid levels in wild and farmed eels (Anguilla Anguilla). Comp. Biochem. Phys 1992, 101, 79–81. [Google Scholar] [CrossRef]
- Senso, L.; Suárez, M.; Ruiz-Cara, T.; García-Gallego, M. On the possible effects of harvesting season and chilled storage on the fatty acid profile of the fillet of farmed gilthead sea bream (Sparus aurata). Food Chem. 2007, 101, 298–307. [Google Scholar] [CrossRef]
- Statistical Yearbook of Agriculture. Food Economy, Consumption. 2015. Available online: http://stat.gov.pl/en/topics/statistical-yearbooks/statistical-yearbooks/statistical-yearbook-of-agriculture-2015,6,10.html (accessed on 6 October 2020).
- United States Environmental Protection Agency (US EPA). Regional Screening Level (RSL) Summary Table; EPA Headquarters: Washington, DC, USA, 2017.
- European Food Safety Authority (EFSA) Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J. 2012, 10, 2985. [Google Scholar] [CrossRef]
- Khemis, I.B.; Aridh, N.B.; Hamza, N.; M’Hetli, M.; Sadok, S. Heavy metals and minerals contents in pikeperch (Sander lucioperca), carp (Cyprinus carpio) and flathead grey mullet (Mugil cephalus) from Sidi Salem Reservoir (Tunisia): Health risk assessment related to fish consumption. Environ. Sci. Pollut. Res. 2017, 24, 19494–19507. [Google Scholar] [CrossRef]
- Kareem, O.K.; Ajani, E.K.; Orisasona, O.; Olanrewaju, A.N. The Sex Ratio, Gonadosomatic Index, Diet Composition and Fecundity of African Pike, Hepsetus odoe (Bloch, 1794) in Eleyele Lake, Nigeria. J. Fish. Livest. Prod. 2015, 3, 1–4. [Google Scholar] [CrossRef]
- Pieterse, G.N. Histopathological Changes in the Testis of Oreochromis Mossambicus (Cichlidae) as a Biomarker of Heavy Metal Pollution. Ph.D. Thesis, Faculty of Science, Rand Afrikaans University, Johannesburg, South Africa, 2004. [Google Scholar]
- Tsoumani, M.; Liasko, R.; Moutsaki, P.; Kagalou, I.; Leonardos, I. Length-weight relationships of an invasive cyprinid fish (Carassius gibelio) from 12 Greek lakes in relation to their trophic states. J. Appl. Ichthyol. 2006, 22, 281–284. [Google Scholar] [CrossRef]
- Kuklina, I.; Kouba, A.; Buřič, M.; Horká, I.; Ďuriš, Z.; Kozák, P. Accumulation of Heavy Metals in Crayfish and Fish from Selected Czech Reservoirs. BioMed Res. Int. 2014, 2014, 306103. [Google Scholar] [CrossRef] [PubMed]
- Moiseenko, T.I.; Gashkina, N.A. Bioaccumulation of mercury in fish as indicator of water pollution. Geochem. Int. 2016, 54, 485–493. [Google Scholar] [CrossRef]
- Voegborlo, R.B.; Adimado, A.A. Total Mercury Distribution in Different Fish Species Representing Different Trophic levels from the Atlantic Coast of Ghana. J. Sci. Technol. 2010, 30, 109. [Google Scholar] [CrossRef]
- Kalisinska, E.; Lanocha-Arendarczyk, N.; Kosik-Bogacka, D.; Budis, H.; Pilarczyk, B.; Tomza-Marciniak, A.; Podlasinska, J.; Cieslik, L.; Popiolek, M.; Pirog, A.; et al. Muscle mercury and selenium in fishes and semiaquatic mammals from a selenium-deficient area. Ecotoxicol. Environ. Saf. 2017, 136, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Djikanovic, V.; Skorić, S.; Gacic, Z. Concentrations of metals and trace elements in different tissues of nine fish species from the Medjuvrsje reservoir (West Morava River Basin, Serbia). Arch. Biol. Sci. 2016, 68, 811–819. [Google Scholar] [CrossRef]
- Łuczyńska, J.; Łuczyński, M.J.; Paszczyk, B. Assessment of mercury in muscles, liver and gills of marine and freshwater fish. J. Elem. 2016, 21, 113–129. [Google Scholar] [CrossRef]
- Rakocevic, J.; Sukovic, D.; Maric, D. Distribution and relationships of eleven trace elements in muscle of six fish species from Skadar Lake (Montenegro). Turk. J. Fish. Aquat. Sci. 2018, 18, 647–657. [Google Scholar] [CrossRef]
- Łuczyńska, J. The influence of weight and length on the mercury content in the muscle tissue of fish from four lakes in the Olsztyn Lake District (Poland). Arch. Pol. Fish. 2005, 13, 51–61. [Google Scholar]
- Kimakova, T.; Kuzmová, L.; Nevolná, Z.; Bencko, V. Fish and fish products as risk factors of mercury exposure. Ann. Agric. Environ. Med. 2018, 1–6. [Google Scholar] [CrossRef]
- Di Lena, G.; Casini, I.; Caproni, R.; Fusari, A.; Orban, E. Total mercury levels in commercial fish species from Italian fishery and aquaculture. Food Addit. Contam. Part B 2017, 1–18. [Google Scholar] [CrossRef]
- Olmedo, P.; Pla, A.; Hernández, A.F.; Barbier, F.; Ayouni, L.; Gil, F. Determination of toxic elements (mercury, cadmium, lead, tin and arsenic) in fish and shellfish samples. Risk assessment for the consumers. Environ. Int. 2013, 59, 63–72. [Google Scholar] [CrossRef]
- Strandberg, U.; Palviainen, M.; Eronen, A.; Piirainen, S.; Laurén, A.; Akkanen, J.; Kankaala, P. Spatial variability of mercury and polyunsaturated fatty acids in the European perch (Perca fluviatilis)—Implications for risk-benefit analyses of fish consumption. Environ. Pollut. 2016, 219, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Polak-Juszczak, L.; Komar-Szymczak, K. Fatty acid profiles and fat contents of commercially important fish from Vistula Lagoon. Pol. J. Food Nutr. Sci. 2009, 59, 225–229. [Google Scholar]
- Sándor, Z.; Papp, Z.G.; Csengeri, I.; Jeney, Z. Fish meat quality and safety. Tehnol. Mesa. 2011, 52, 97–105. [Google Scholar]
- Vasconi, M.; Caprino, F.; Bellagamba, F.; Busetto, M.L.; Bernardi, C.; Puzzi, C.; Moretti, V.M. Fatty Acid Composition of Freshwater Wild Fish in Subalpine Lakes: A Comparative Study. Lipids 2015, 50, 283–302. [Google Scholar] [CrossRef]
- Łuczyńska, J.; Borejszo, Z.; Łuczyński, M.J. The Composition of Fatty Acids in Muscles of Six Freshwater Fish Species from the Mazurian Great Lakes (Northeastern Poland). Arch. Pol. Fish. 2008, 16, 167–178. [Google Scholar] [CrossRef]
- Kainz, M.J.; Hager, H.H.; Rasconi, S.; Kahilainen, K.K.; Amundsen, P.-A.; Hayden, B. Polyunsaturated fatty acids in fishes increase with total lipids irrespective of feeding sources and trophic position. Ecosphere 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Woźniak, M.; Poczyczyński, P.; Kozłowski, K. The nutritional value of selected species of fish from lake and fish farm of north-eastern Poland. Pol. J. Nat. Sci. 2013, 28, 295–304. [Google Scholar]
- Linhartová, Z.; Krejsa, J.; Zajic, T.; Masilko, J.; Sampels, S.; Mraz, J. Proximate and fatty acid composition of 13 important freshwater fish species in central Europe. Aquac. Int. 2018, 26, 695–711. [Google Scholar] [CrossRef]
- Sousa, Á.B.B.D.; Santos Júnior, O.D.O.; Visentainer, J.V.; De Almeida, N.M. Total lipid nutritional quality of the adipose tissue from the orbital cavity in Nile tilapia from continental aquaculture. Acta Sci. Anim. Sci. 2017, 39, 335. [Google Scholar] [CrossRef][Green Version]
- Pleadin, J.; Lešić, T.; Krešić, G.; Barić, R.; Bogdanović, T.; Oraić, D.; Vulić, A.; Legac, A.; Zrnčić, S. Nutritional quality of different fish species farmed in the Adriatic Sea. Ital. J. Food Sci. 2017, 29, 537–549. [Google Scholar]
| Perch (Perca Fluviatilis L.) (n = 9) | Roach (Rutilus Rutilus L.) (n = 10) | |
|---|---|---|
| Weight (g) | 236.22 ± 81.69 | 207.20 ± 38.17 |
| Length (cm) | 25.66 ± 2.36 | 26.05 ± 1.66 |
| FCF | 1.337 ± 0.168 a | 1.165 ± 0.105 b |
| HSI | 1.497 ± 0.280 a | 0.957 ± 0.514 b |
| GSI | 4.399 ± 6.554 a | 0.963 ± 0.508 b |
| MPI | 0.068 ± 0.021 a | 0.027 ± 0.005 b |
| Mean ± SD | ||
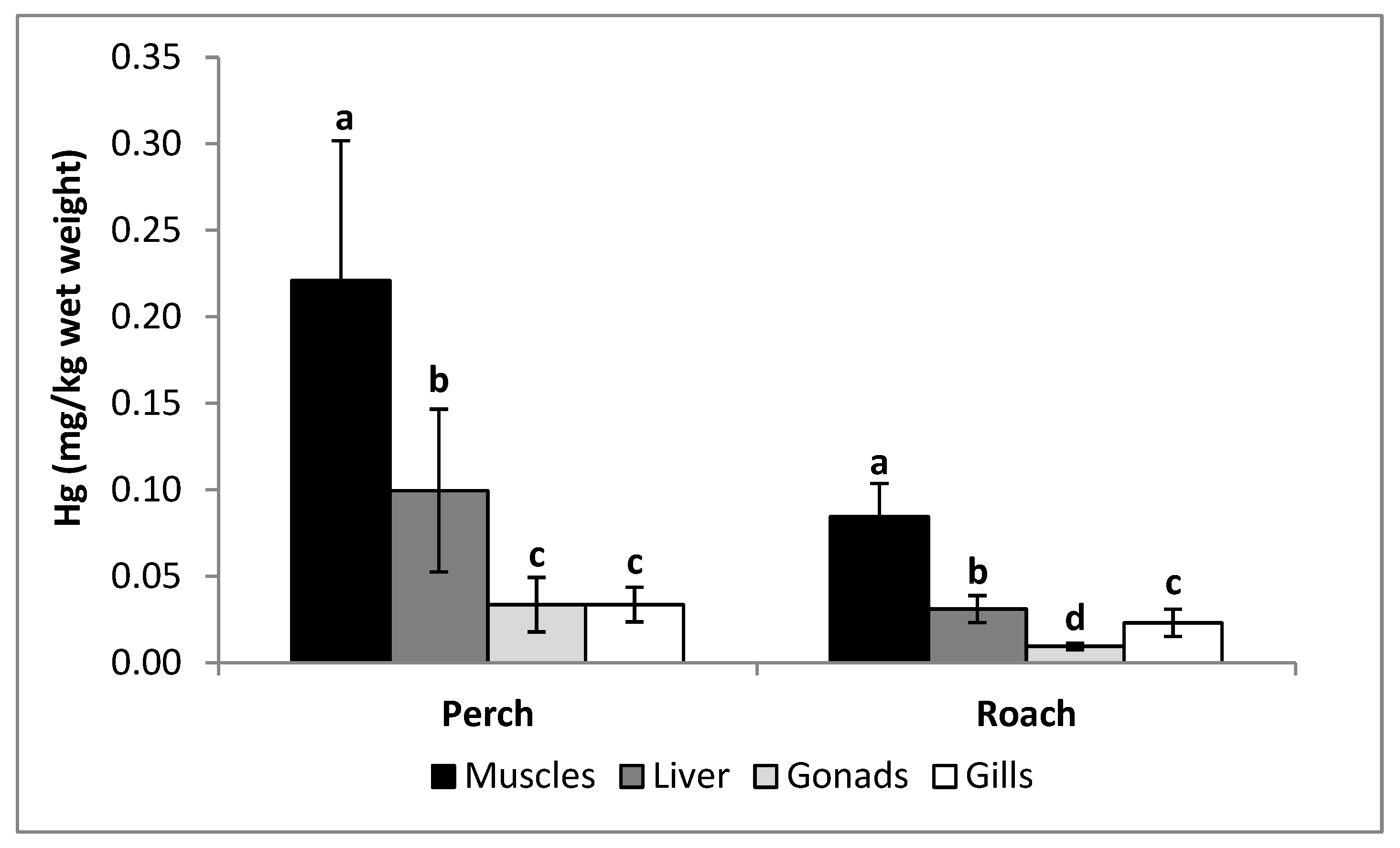
| Muscles | 0.221 ± 0.081 a | 0.085 ± 0.019 b |
| Liver | 0.100 ± 0.047 a | 0.031 ± 0.008 b |
| Gonads | 0.034 ± 0.016 a | 0.009 ± 0.002 b |
| Gills | 0034 ± 0.010 a | 0.023 ± 0.008 b |
| BCF | ||
| Muscles | >11,056.1 ± 4036.3 a | >4229.5 ± 954.1 b |
| Liver | >4976.4 ± 2352.4 a | >1550.5 ± 389.3 b |
| Gonads | >1676.7 ± 787.5 a | >471.0 ± 96.6 b |
| Gills | >1678.9 ± 499.3 a | >1154.0 ± 393.7 b |
| Weight | Length | Muscles | Liver | Gonads | Gills | FCF | |
|---|---|---|---|---|---|---|---|
| Perch (Perca fluviatilis L.) (n = 9) | |||||||
| Weight | |||||||
| Length | 0.978 | ||||||
| p = 0.000 | |||||||
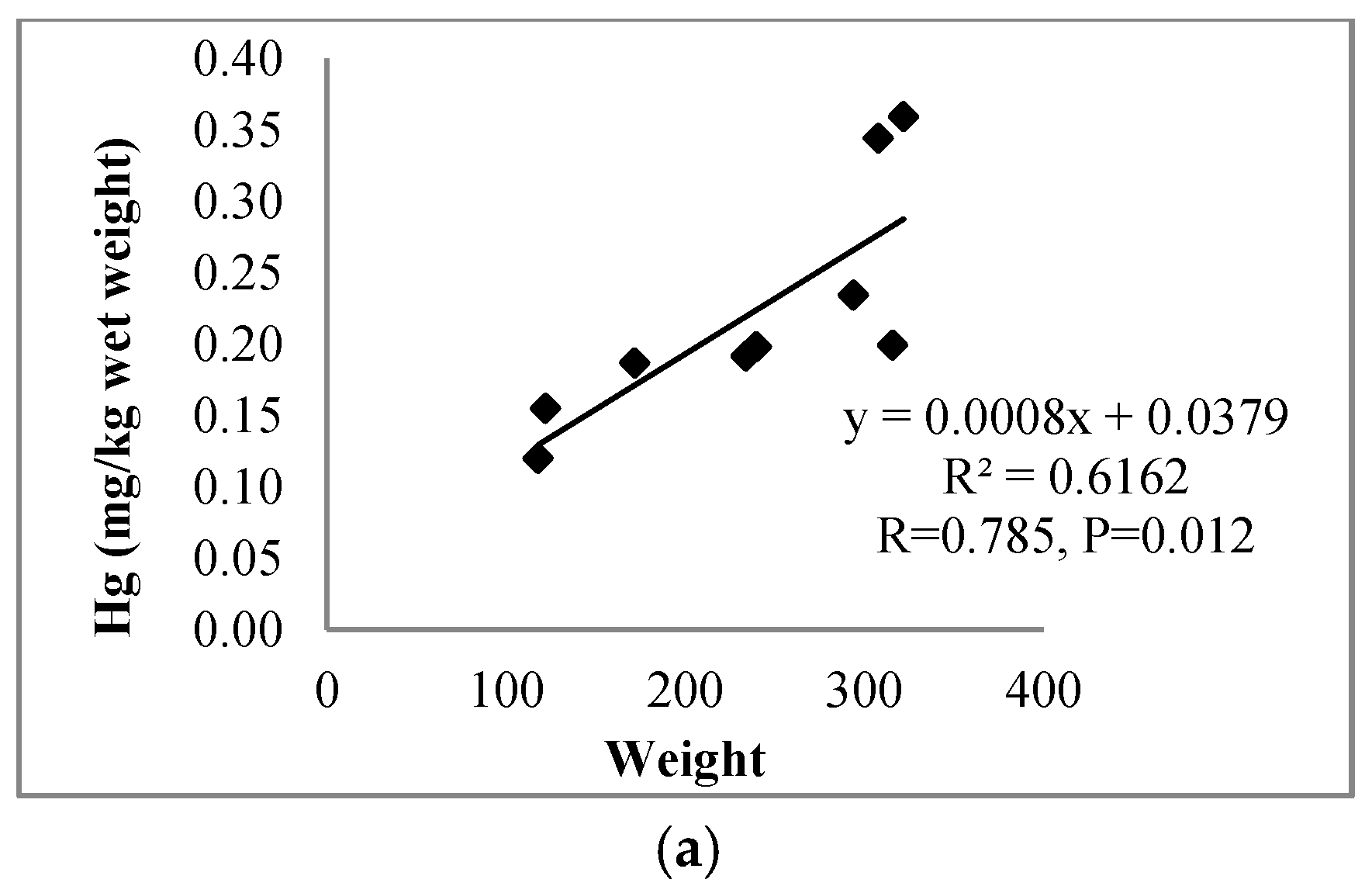
| Muscles | 0.785 | 0.760 | |||||
| p = 0.012 | p = 0.017 | ||||||
| Liver | 0.484 | 0.545 | 0.408 | ||||
| p = 0.187 | p = 0.129 | p = 0.276 | |||||
| Gonads | 0.010 | 0.036 | 0.245 | 0.485 | |||
| p = 0.979 | p = 0.927 | p = 0.526 | p = 0.186 | ||||
| Gills | −0.202 | −0.140 | −0.124 | 0.410 | 0.788 | ||
| p = 0.602 | p = 0.719 | p = 0.751 | p = 0.273 | p = 0.012 | |||
| FCF | 0.919 | 0.835 | 0.695 | 0.369 | 0.024 | −0.184 | |
| p = 0.000 | p = 0.005 | p = 0.038 | p = 0.329 | p = 0.951 | p = 0.635 | ||
| Roach (Rutilus rutilus L.) (n = 10) | |||||||
| Weight | |||||||
| Length | 0.847 | ||||||
| p = 0.002 | |||||||
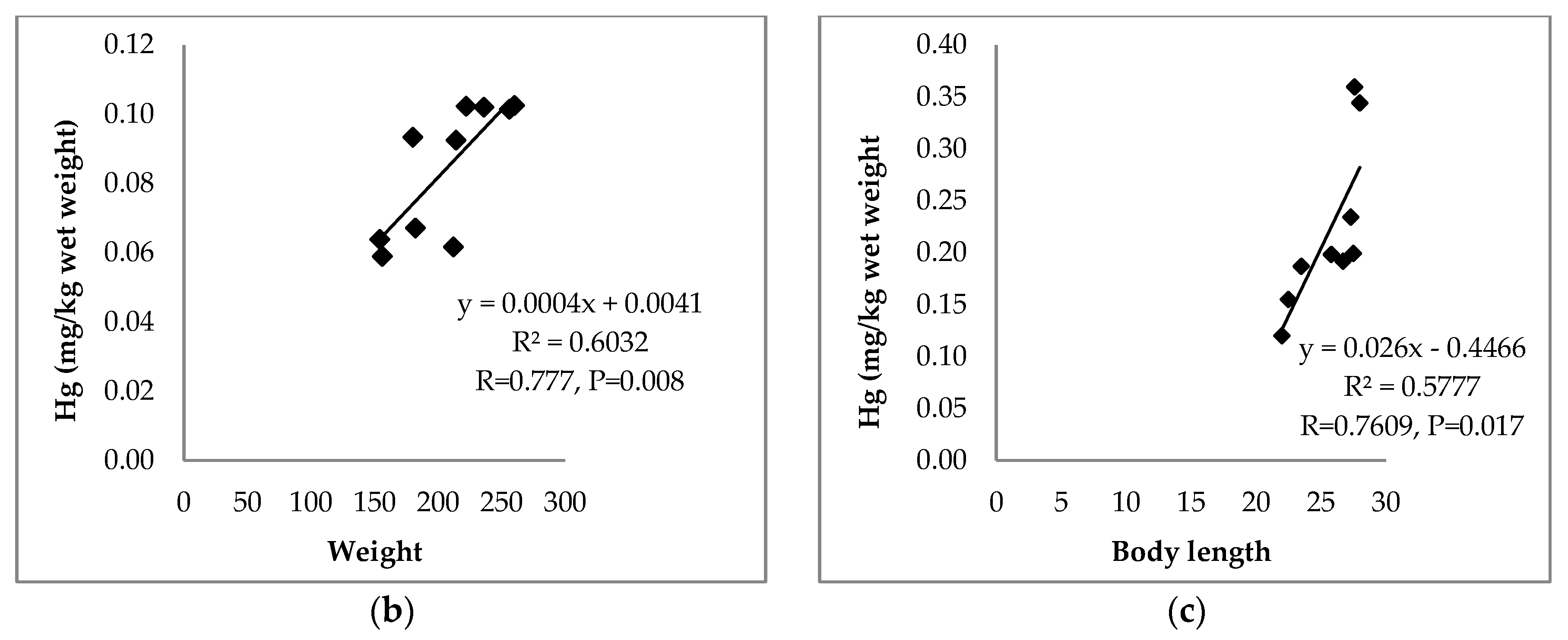
| Muscles | 0.777 | 0.448 | |||||
| p = 0.008 | p = 0.194 | ||||||
| Liver | 0.551 | 0.470 | 0.415 | ||||
| p = 0.099 | p = 0.170 | p = 0.232 | |||||
| Gonads | 0.497 | 0.620 | 0.443 | 0.567 | |||
| p = 0.144 | p = 0.056 | p = 0.200 | p = 0.087 | ||||
| Gills | 0.469 | 0.498 | 0.603 | −0.149 | 0.404 | ||
| p = 0.172 | p = 0.143 | p = 0.065 | p = 0.681 | p = 0.246 | |||
| FCF | 0.291 | −0.259 | 0.616 | 0.181 | −0.151 | −0.048 | |
| p = 0.415 | p = 0.470 | p = 0.058 | p = 0.617 | p = 0.677 | p = 0.896 | ||
| EDI | TWI | %TWI * | %TWI ** | THQ | References | ||
|---|---|---|---|---|---|---|---|
| RfD (mg/kg/day) | 3 × 10−4 | [56] | |||||
| TWI (for inorganic mercury) | 4 | [57] | |||||
| TWI (for methylmercury) | 1.3 | ||||||
| Perca fluviatilis L. (n = 9) | 0.124 | 0.869 | 21.73 | 66.871 | 0.414 | This study | |
| Rutilus rutilus L. (n = 10) | 0.048 | 0.333 | 8.31 | 25.582 | 0.158 | This study | |
| Rutilus rutilus L. (n = 10) | 0.040 | 0.280 | 7.00 | 21.50 | 0.135 | [45] | |
| Perca fluviatilis L. (n = 10) | 0.091 | 0.637 | 15.92 | 49.00 | 0.303 | [37] | |
| Abramis brama L. (n = 6) | 0.0086 | 0.060 | 1.50 | 4.611 | 0.029 | ||
| Perca fluviatilis L. (n = 5) | 0.0762 | 0.534 | 13.34 | 41.056 | 0.254 | ||
| Leuciscus idus L. (n = 6) | 0.0604 | 0.423 | 10.57 | 32.527 | 0.201 | ||
| Cyprinus carpio L. (n = 5) | 0.0043 | 0.024 | 0.60 | 1.845 | 0.011 | ||
| Oncorhynchus mykiss Walb. (n = 6) | 0.0081 | 0.057 | 1.42 | 4.363 | 0.027 | ||
| Sander lucioperca L. (n = 9) | 4.97 × 10−5 | [58] | |||||
| Cyprinus carpio L. (n = 9) | 1.17 × 10−5 | ||||||
| Fatty Acid | Systematic Name | Trivial Name | Perch (Perca fluviatilis L.) (n = 9) | Roach (Rutilus rutilus L.) (n = 10) |
|---|---|---|---|---|
| Fat (%) | 0.88 ± 0.56 | 0.72 ± 0.26 | ||
| C12:0 | dodecanoic | lauric | 0.11 ± 0.02 a | 0.11 ± 0.02 a |
| C14:0 | tetradecanoic | myristic | 1.94 ± 0.55 a | 1.11 ± 0.37 b |
| C16:0 | hexadecanoic | palmitic | 20.64 ± 0.70 a | 20.63 ± 1.43 a |
| C18:0 | octadecanoic | stearic | 5.21 ± 0.61 b | 5.79 ± 0.54 a |
| C18:1 | octadecenoic | oleic | 14.48 ± 1.44 a | 11.17 ± 2.78 b |
| C18:2(n-6) LA | cis, cis-9,12-octadecadienoic | linoleic | 2.95 ± 0.57 a | 2.35 ± 0.79 a |
| C20:4(n-6) AA | all cis-5,8,11,14-eicosatetraenoic | arachidonic | 7.64 ± 1.16 b | 10.04 ± 1.65 a |
| C18:3(n-3) ALA | all cis-9,12,15-octadecatrienoic | α-linolenic | 1.93 ± 0.67 a | 1.11 ± 0.68 b |
| C20:5(n-3) EPA | all cis-5,8,11,14,17-eicosapentaenoic | eicosapentaenoic | 7.11 ± 0.46 a | 6.11 ± 0.55 b |
| C22:5(n-3) DPA | all cis-7,10,13,16,19-docosapentaenoic | docosapentaenoic | 2.72 ± 0.34 a | 2.83 ± 0.21 a |
| C22:6(n-3) DHA | all cis-4,7,10,13,16,19-docosahexaenoic | docosahexaenoic | 22.78 ± 4.4 a | 23.81 ± 4.27 a |
| Ʃ SFA | 28.93 ± 0.91 a | 28.72 ± 1.56 a | ||
| Ʃ MUFA | 21.68 ± 3.38 a | 17.90 ± 5.28 a | ||
| Ʃ n-6 PUFA | 13.08 ± 1.47 b | 17.95 ± 1.85 a | ||
| Ʃ n-3 PUFA | 36.31 ± 3.86 a | 35.43 ± 3.35 a | ||
| Ʃ PUFA | 49.39 ± 3.30 b | 53.38 ± 4.36 a | ||
| Ʃ UFA | 71.07 ± 0.91 a | 71.28 ± 1.56 a | ||
| Ʃ n-3 HUFA | 33.64 ± 4.46 a | 34.05 ± 4.03 a | ||
| n-3/n-6 | 2.82 ± 0.48 a | 1.99 ± 0.24 b | ||
| AI | 0.40 ± 1.70 a | 0.35 ± 0.02 b | ||
| TI | 0.22 ± 0.02 a | 0.22 ± 0.02 a | ||
| FLQ | 29.89 ± 4.25 a | 29.92 ± 4.14 a | ||
| OFA | 22.68.41 a | 21.86 ± 1.82 a | ||
| DFA | 76.27 ± 0.50 a | 77.07 ± 1.27 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łuczyńska, J.; Paszczyk, B.; Łuczyński, M.J.; Kowalska-Góralska, M.; Nowosad, J.; Kucharczyk, D. Using Rutilus rutilus (L.) and Perca fluviatilis (L.) as Bioindicators of the Environmental Condition and Human Health: Lake Łańskie, Poland. Int. J. Environ. Res. Public Health 2020, 17, 7595. https://doi.org/10.3390/ijerph17207595
Łuczyńska J, Paszczyk B, Łuczyński MJ, Kowalska-Góralska M, Nowosad J, Kucharczyk D. Using Rutilus rutilus (L.) and Perca fluviatilis (L.) as Bioindicators of the Environmental Condition and Human Health: Lake Łańskie, Poland. International Journal of Environmental Research and Public Health. 2020; 17(20):7595. https://doi.org/10.3390/ijerph17207595
Chicago/Turabian StyleŁuczyńska, Joanna, Beata Paszczyk, Marek Jan Łuczyński, Monika Kowalska-Góralska, Joanna Nowosad, and Dariusz Kucharczyk. 2020. "Using Rutilus rutilus (L.) and Perca fluviatilis (L.) as Bioindicators of the Environmental Condition and Human Health: Lake Łańskie, Poland" International Journal of Environmental Research and Public Health 17, no. 20: 7595. https://doi.org/10.3390/ijerph17207595
APA StyleŁuczyńska, J., Paszczyk, B., Łuczyński, M. J., Kowalska-Góralska, M., Nowosad, J., & Kucharczyk, D. (2020). Using Rutilus rutilus (L.) and Perca fluviatilis (L.) as Bioindicators of the Environmental Condition and Human Health: Lake Łańskie, Poland. International Journal of Environmental Research and Public Health, 17(20), 7595. https://doi.org/10.3390/ijerph17207595






