COVID-19: Short and Long-Term Effects of Hospitalization on Muscular Weakness in the Elderly
Abstract
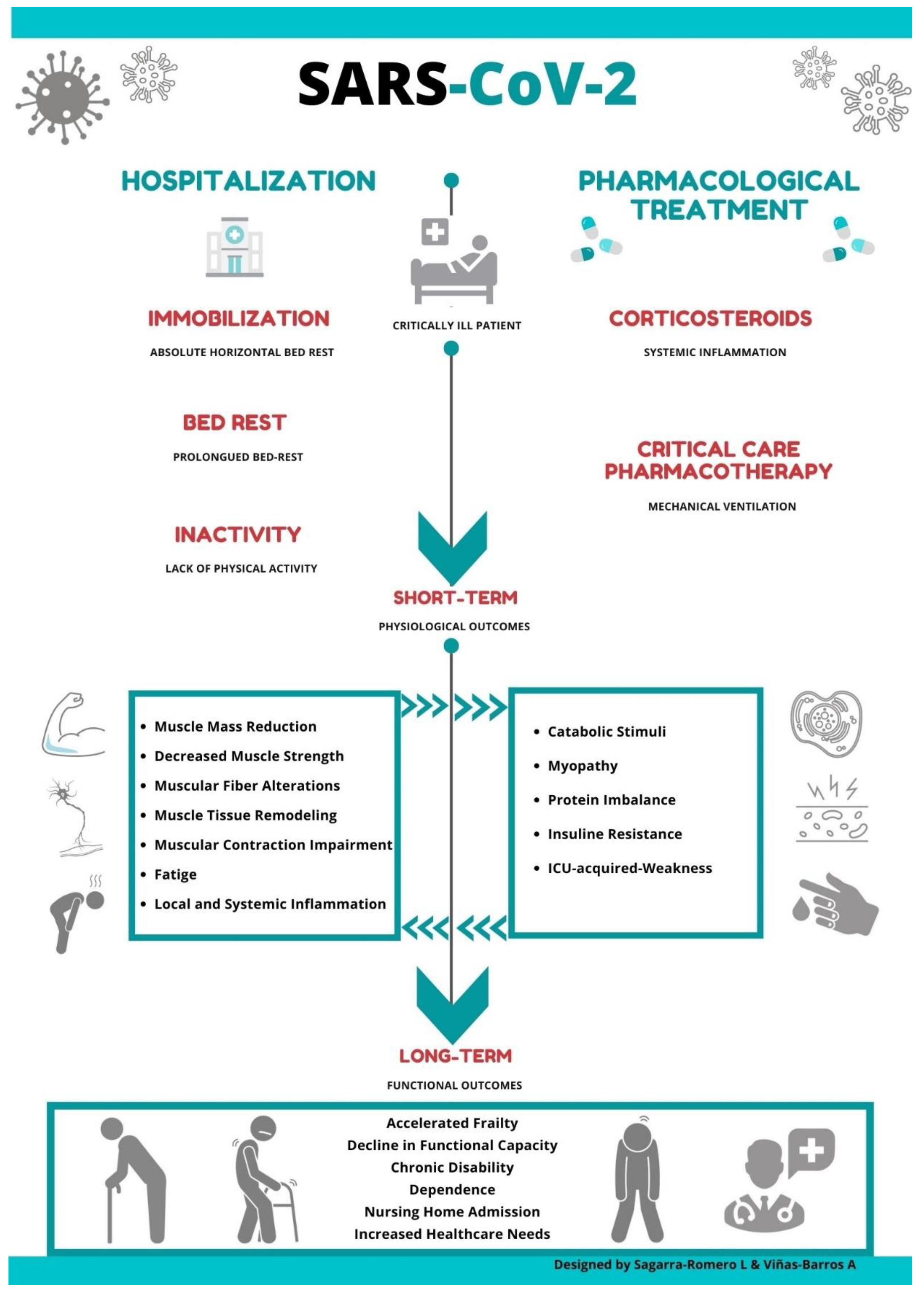
:1. Introduction
2. COVID-19: Impact of Pharmacological Treatment on Muscle Metabolism
3. COVID-19: Impact of Hospitalization and ICU on the Musculoskeletal System
4. COVID-19: Non-Pharmacological Strategies
4.1. Early Strength Intervention
4.2. Neuromuscular Electrical Stimulation
4.3. Heat Therapy
5. COVID-19: Long-Term Consequences
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Landi, F.; Barillaro, C.; Bellieni, A.; Brandi, V.; Carfì, A.; D’Angelo, M.; Fusco, D.; Landi, G.; Monaco, R.L.; Martone, A.; et al. The New Challenge of Geriatrics: Saving Frail Older People from the SARS-COV-2 Pandemic Infection. J. Nutr. Health Aging 2020, 24, 466–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.H.; Xue, X.B.; Zhi, Z.; Epidemiology Working Group for Ncip Epidemic Response, Chinese Center for Disease Control and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Eur. PMC 2020, 41, 145–151. [Google Scholar] [CrossRef]
- Ayoub, H.H.; Chemaitelly, H.; Seedat, S.; Mumtaz, G.R.; Makhoul, M.; Abu-Raddad, L.J. Age could be driving variable SARS-CoV-2 epidemic trajectories worldwide. PLoS ONE 2020, 15, e0237959. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Ranucci, M.; Ballotta, A.; Di Dedda, U.; Bayshnikova, E.; Poli, M.D.; Resta, M.; Falco, M.; Albano, G.; Menicanti, L. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemost. 2020, 18, 1747–1751. [Google Scholar] [CrossRef]
- Kiekens, C.; Boldrini, P.; Andreoli, A.; Avesani, R.; Gamna, F.; Grandi, M.; Lombardi, F.; Lusuardi, M.; Molteni, F.; Perboni, A.; et al. Rehabilitation and respiratory management in the acute and early post-acute phase. “Instant paper from the field” on rehabilitation answers to the COVID-19 emergency. Eur. J. Phys. Rehabil. Med. 2020, 56. [Google Scholar] [CrossRef]
- Bloomfield, S. Changes in musculoskeletal structure and function with prolonged bed rest. Med. Sci. Sports Exerc. 1997, 29, 197–206. [Google Scholar] [CrossRef]
- Stainsby, B.E.; Howitt, S.; Porr, J. Neuromusculoskeletal disorders following SARS: A case series. J. Can. Chiropr. Assoc. 2011, 55, 32–39. [Google Scholar]
- Ofori-Asenso, R.; Chin, K.L.; Mazidi, M.; Zomer, E.; Ilomaki, J.; Zullo, A.R.; Gasevic, D.; Ademi, Z.; Korhonen, M.J.; Logiudice, D.; et al. Global Incidence of Frailty and Prefrailty among Community-Dwelling Older Adults. JAMA Netw. Open 2019, 2, e198398. [Google Scholar] [CrossRef] [Green Version]
- Abbatecola, A.M.; Antonelli-Incalzi, R. COVID-19 Spiraling of Frailty in Older Italian Patients. J. Nutr. Health Aging 2020, 24, 453–455. [Google Scholar] [CrossRef]
- Xu, X.; Ong, Y.K.; Wang, D.Y. Role of adjunctive treatment strategies in COVID-19 and a review of international and national clinical guidelines. Mil. Med. Res. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Köstenberger, M.; Hasibeder, W.; Dankl, D.; Germann, R.; Hörmann, C.; Joannidis, M.; Markstaller, K.; Müller-Muttonen, S.-O.; Neuwersch-Sommeregger, S.; Schaden, E.; et al. SARS-CoV-2: Recommendations for treatment in intensive care medicine. Wien. Klin. Wochenschr. 2020, 132, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- The Recovery Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19—Preliminary Report. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Monreal, E.; de la Maza, S.S.; Natera-Villalba, E.; Beltrán-Corbellini, Á.; Rodríguez-Jorge, F.; Fernández-Velasco, J.I.; Walo-Delgado, P.; Muriel, A.; Zamora, J.; Alonso-Canovas, A.; et al. High versus standard doses of corticosteroids in severe COVID-19: A retrospective cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Hui, D.S.; Joynt, G.M.; Wong, K.T.; Gomersall, C.D.; Li, T.S.; Antonio, G.; Ko, F.W.; Chan, M.C.; Chan, D.P.; Tong, M.W.; et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax 2005, 60, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Schakman, O.; Gilson, H.; Thissen, J.-P. Mechanisms of glucocorticoid-induced myopathy. J. Endocrinol. 2008, 197, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bowyer, S.L.; Lamothe, M.P.; Hollister, J. Steroid myopathy: Incidence and detection in a population with asthma. J. Allergy Clin. Immunol. 1985, 76, 234–242. [Google Scholar] [CrossRef]
- Haran, M.; Schattner, A.; Kozak, N.; Máté, A.; Berrebi, A.; Shvidel, L. Acute steroid myopathy: A highly overlooked entity. QJM Int. J. Med. 2018, 111, 307–311. [Google Scholar] [CrossRef]
- Hanson, P.; Dive, A.; Brucher, J.-M.; Bisteau, M.; Dangoisse, M.; Deltombe, T. Acute corticosteroid myopathy in intensive care patients. Muscle Nerve 1997, 20, 1371–1380. [Google Scholar] [CrossRef]
- Yang, T.; Li, Z.; Jiang, L.; Wang, Y.; Xi, X. Risk factors for intensive care unit-acquired weakness: A systematic review and meta-analysis. Acta Neurol. Scand. 2018, 138, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Jiang, L.; Zhang, S.; Guervilly, C.; Zhang, M.; Feng, X.; Ding, J. Neuromuscular blocking agents for acute respiratory distress syndrome: An updated meta-analysis of randomized controlled trials. Respir. Res. 2020, 21, 23. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Kim, L.; Whitaker, M.; O’Halloran, A.; Cummings, C.; Holstein, R.; Prill, M.; Chai, S.J.; Kirley, P.D.; Alden, N.B.; et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019—COVID-NET, 14 States, March 1–30, 2020; MMWR Morbidity Mortality Weekly Report; CDC: Atlanta, GA, USA, 2020; Volume 69, pp. 458–464. [CrossRef]
- Chen, J.; Qi, T.; Liu, L.; Ling, Y.; Qian, Z.; Li, T.; Li, F.; Xu, Q.; Zhang, Y.; Xu, S.; et al. Clinical progression of patients with COVID-19 in Shanghai, China. J. Infect. 2020, 80, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yu, S.; Zha, X.; Wang, N.; Pang, Q.; Li, D.; Li, A. Clinical characteristics and durations of hospitalized patients with COVID-19 in Beijing: A retrospective cohort study. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Parry, S.M.; Puthucheary, Z.A. The impact of extended bed rest on the musculoskeletal system in the critical care environment. Extrem. Physiol. Med. 2015, 4, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGavock, J.M.; Hastings, J.L.; Snell, P.G.; McGuire, D.K.; Pacini, E.L.; Levine, B.D.; Mitchell, J.H. A Forty-Year Follow-Up of the Dallas Bed Rest and Training Study: The Effect of Age on the Cardiovascular Response to Exercise in Men. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2009, 64, 293–299. [Google Scholar] [CrossRef]
- Kortebein, P.; Symons, T.B.; Ferrando, A.; Paddon-Jones, D.; Ronsen, O.; Protas, E.; Conger, S.; Lombeida, J.; Wolfe, R.; Evans, W.J. Functional Impact of 10 Days of Bed Rest in Healthy Older Adults. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2008, 63, 1076–1081. [Google Scholar] [CrossRef] [Green Version]
- Calero-García, M.J.; Ortega, A.R.; Navarro, E.; Calero, M.D. Relationship between hospitalization and functional and cognitive impairment in hospitalized older adults patients. Aging Ment. Health 2016, 21, 1164–1170. [Google Scholar] [CrossRef]
- English, K.L.; Paddon-Jones, D. Protecting muscle mass and function in older adults during bed rest. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 34–39. [Google Scholar] [CrossRef]
- Dittmer, D.K.; Teasell, R. Complications of immobilization and bed rest. Part 1: Musculoskeletal and cardiovascular complications. Can. Fam. Physician Med. Fam. Can. 1993, 39, 1428–1432, 1435–1437. [Google Scholar]
- Greenleaf, J.E.; Kozlowski, S. Physiological Consequences of Reduced Physical Activity during Bed Rest. Exerc. Sport Sci. Rev. 1982, 10, 84–119. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Dickerson, R.N.; Moore, F.A.; Paddon-Jones, D.; Weijs, P.J.M. Protein Turnover and Metabolism in the Elderly Intensive Care Unit Patient. Nutr. Clin. Pr. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2017, 32 (Suppl. 1), 112S–120S. [Google Scholar] [CrossRef] [PubMed]
- Vanhorebeek, I.; Latronico, N.; Berghe, G.V.D. ICU-acquired weakness. Intensiv. Care Med. 2020, 46, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Capelli, C.; Antonutto, G.; Kenfack, M.A.; Cautero, M.; Lador, F.; Moia, C.; Tam, E.; Ferretti, G. Factors determining the time course of VO2(max) decay during bedrest: Implications for VO2(max) limitation. Eur. J. Appl. Physiol. 2006, 98, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.; Hussain, S.N.A.; Mathur, S.; Picard, M.; Herridge, M.; Correa, J.; Bain, A.; Guo, Y.; Advani, A.; Advani, S.L.; et al. Mechanisms of Chronic Muscle Wasting and Dysfunction after an Intensive Care Unit Stay. A Pilot Study. Am. J. Respir. Crit. Care Med. 2016, 194, 821–830. [Google Scholar] [CrossRef] [Green Version]
- Buso, A.; Comelli, M.; Picco, R.; Isola, M.; Magnesa, B.; Pišot, R.; Rittweger, J.; Salvadego, D.; Šimunič, B.; Grassi, B.; et al. Mitochondrial Adaptations in Elderly and Young Men Skeletal Muscle Following 2 Weeks of Bed Rest and Rehabilitation. Front. Physiol. 2019, 10, 474. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Sobenin, I.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. Mitochondrial Aging and Age-Related Dysfunction of Mitochondria. BioMed Res. Int. 2014, 2014, 238463. [Google Scholar] [CrossRef] [Green Version]
- Leung, T.; Wong, K.S.; Hui, A.C.; To, K.F.; Lai, S.T.; Ng, W.F.; Ng, H.K. Myopathic Changes Associated with Severe Acute Respiratory Syndrome. Arch. Neurol. 2005, 62, 1113–1117. [Google Scholar] [CrossRef]
- Chan, K.H.; Farouji, I.; Abu Hanoud, A.; Slim, J. Weakness and elevated creatinine kinase as the initial presentation of coronavirus disease 2019 (COVID-19). Am. J. Emerg. Med. 2020, 38, 1548.e1–1548.e3. [Google Scholar] [CrossRef]
- Lee, N.; Hui, D.; Wu, A.; Chan, P.; Cameron, P.; Joynt, G.M.; Ahuja, A.; Yung, M.Y.; Leung, C.; To, K.; et al. A Major Outbreak of Severe Acute Respiratory Syndrome in Hong Kong. N. Engl. J. Med. 2003, 348, 1986–1994. [Google Scholar] [CrossRef]
- Bruyère, O.; Beaudart, C.; Reginster, J.-Y.; Buckinx, F.; Schoene, D.; Hirani, V.; Cooper, C.; Kanis, J.; Rizzoli, R.; McCloskey, E.; et al. Assessment of muscle mass, muscle strength and physical performance in clinical practice: An international survey. Eur. Geriatr. Med. 2016, 7, 243–246. [Google Scholar] [CrossRef]
- Beaudart, C.; McCloskey, E.; Bruyère, O.; Cesari, M.; Rolland, Y.; Rizzoli, R.; De Carvalho, I.A.; Thiyagarajan, J.A.; Bautmans, I.; Bertière, M.-C.; et al. Sarcopenia in daily practice: Assessment and management. BMC Geriatr. 2016, 16, 170. [Google Scholar] [CrossRef] [PubMed]
- Formenti, P.; Umbrello, M.; Coppola, S.; Froio, S.; Chiumello, D. Clinical review: Peripheral muscular ultrasound in the ICU. Ann. Intensiv. Care 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stringer, H.J.; Wilson, D. The Role of Ultrasound as a Diagnostic Tool for Sarcopenia. J. Frailty Aging 2018, 7, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Gentil, P.; De Lira, C.A.B.; Souza, D.; Jimenez, A.; Mayo, X.; de Fátima Pinho Lins Gryschek, A.; Pereira, E.G.; Alcaraz, P.; Bianco, A.; Paoli, A.; et al. Resistance Training Safety during and after the SARS-Cov-2 Outbreak: Practical Recommendations. BioMed Res. Int. 2020, 2020, 3292916. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, M.; Cè, E.; Paneroni, M.; Guazzi, M.; Lippi, G.; Paoli, A.; Baldari, C.; Schena, F.; Esposito, F. Safety procedures for exercise testing in the scenario of COVID-19: A position statement of the Società Italiana Scienze Motorie e Sportive. Sport Sci. Health 2020, 16, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Kleinpell, R.M.; Fletcher, K.; Jennings, B.M. Reducing Functional Decline in Hospitalized Elderly. In Patient Safety and Quality: An Evidence-Based Handbook for Nurses; Hughes, R.G., Ed.; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2008. [Google Scholar]
- Martínez-Velilla, N.; Casas-Herrero, A.; Zambom-Ferraresi, F.; De Asteasu, M.L.S.; Lucia, A.; Galbete, A.; García-Baztán, A.; Alonso-Renedo, J.; González-Glaría, B.; Gonzalo-Lázaro, M.; et al. Effect of Exercise Intervention on Functional Decline in Very Elderly Patients during Acute Hospitalization. JAMA Intern. Med. 2019, 179, 28–36. [Google Scholar] [CrossRef]
- Adler, J.; Malone, D. Early Mobilization in the Intensive Care Unit: A Systematic Review. Cardiopulm. Phys. Ther. J. 2012, 23, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Kalisch, B.J.; Lee, S.; Dabney, B.W. Outcomes of inpatient mobilization: A literature review. J. Clin. Nurs. 2013, 23, 1486–1501. [Google Scholar] [CrossRef] [Green Version]
- Courtney, M.; Edwards, H.; Chang, A.; Parker, A.; Mn, K.F.; Hamilton, K. Fewer Emergency Readmissions and Better Quality of Life for Older Adults at Risk of Hospital Readmission: A Randomized Controlled Trial to Determine the Effectiveness of a 24-Week Exercise and Telephone Follow-Up Program. J. Am. Geriatr. Soc. 2009, 57, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Mallery, L.H.; Macdonald, E.A.; Hubley-Kozey, C.L.; Earl, E.M.; Rockwood, K.; Macknight, C. The Feasibility of performing resistance exercise with acutely ill hospitalized older adults. BMC Geriatr. 2003, 3, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-López, L.; Torres-Sánchez, I.; Rodríguez-Torres, J.; Cabrera-Martos, I.; Ortiz-Rubio, A.; Valenza, M.C. Does adding an integrated physical therapy and neuromuscular electrical stimulation therapy to standard rehabilitation improve functional outcome in elderly patients with pneumonia? A randomised controlled trial. Clin. Rehabil. 2019, 33, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-M.; Xie, Y.-X.; Wang, C. Recommendations for respiratory rehabilitation in adults with coronavirus disease 2019. Chin. Med. J. 2020, 133, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Pincherle, A.; Jöhr, J.; Pancini, L.; Leocani, L.; Vecchia, L.D.; Ryvlin, P.; Schiff, N.D.; Diserens, K. Intensive Care Admission and Early Neuro-Rehabilitation. Lessons for COVID-19? Front. Neurol. 2020, 11, 880. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, C.L.; Stiller, K.; Needham, D.M.; Tipping, C.J.; Harrold, M.; Baldwin, C.E.; Bradley, S.J.; Berney, S.; Caruana, L.R.; Elliott, D.; et al. Expert consensus and recommendations on safety criteria for active mobilization of mechanically ventilated critically ill adults. Crit. Care 2014, 18, 658. [Google Scholar] [CrossRef] [Green Version]
- McNeary, L.; Maltser, S.; Verduzco-Gutierrez, M. Navigating Coronavirus Disease 2019 (Covid-19) in Physiatry: A CAN Report for Inpatient Rehabilitation Facilities. PM R J. Inj. Funct. Rehabil. 2020, 12, 512–515. [Google Scholar] [CrossRef]
- Thomas, P.; Baldwin, C.; Bissett, B.; Boden, I.; Gosselink, R.; Granger, C.L.; Hodgson, C.; Jones, A.Y.; Kho, M.E.; Moses, R.; et al. Physiotherapy management for COVID-19 in the acute hospital setting: Clinical practice recommendations. J. Physiother. 2020, 66, 73–82. [Google Scholar] [CrossRef]
- Brower, R.G. Consequences of bed rest. Crit. Care Med. 2009, 37 (Suppl. 10), S422–S428. [Google Scholar] [CrossRef]
- Karlsen, A.; Cullum, C.K.; Norheim, K.L.; Scheel, F.U.; Zinglersen, A.H.; Vahlgren, J.; Schjerling, P.; Kjaer, M.; Mackey, A.L. Neuromuscular Electrical Stimulation Preserves Leg Lean Mass in Geriatric Patients. Med. Sci. Sports Exerc. 2020, 52, 773–784. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, P.O.; Setten, M.; Maskin, L.P.; Bonelli, I.; Vidomlansky, S.R.; Attie, S.; Frosiani, S.L.; Kozima, S.; Valentini, R. Muscle weakness in septic patients requiring mechanical ventilation: Protective effect of transcutaneous neuromuscular electrical stimulation. J. Crit. Care 2012, 27, 319.e1–319.e8. [Google Scholar] [CrossRef]
- Zinglersen, A.H.; Halsteen, M.B.; Kjaer, M.; Karlsen, A.; Kjaer, M. Can electrical stimulation enhance effects of a functional training program in hospitalized geriatric patients? Exp. Gerontol. 2018, 106, 101–108. [Google Scholar] [CrossRef] [PubMed]
- McGorm, H.; Roberts, L.A.; Coombes, J.S.; Peake, J.M. Turning up the Heat: An Evaluation of the Evidence for Heating to Promote Exercise Recovery, Muscle Rehabilitation and Adaptation. Sports Med. 2018, 48, 1311–1328. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Monroe, J.C.; Gavin, T.P.; Roseguini, B.T. Skeletal muscle adaptations to heat therapy. J. Appl. Physiol. 2020, 128, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, K.; Shepherd, S.O.; Strauss, J.A.; Low, D.A.; Cooper, R.J.; Wagenmakers, A.J.M.; Cocks, M. Passive heat therapy in sedentary humans increases skeletal muscle capillarization and eNOS content but not mitochondrial density or GLUT4 content. Am. J. Physiol. Circ. Physiol. 2019, 317, H114–H123. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.M.; Ralph, L.; Look, M.; Erasala, G.N.; Verna, J.L.; Matheson, L.N.; Mooney, V. Treating acute low back pain with continuous low-level heat wrap therapy and/or exercise: A randomized controlled trial. Spine J. Off. J. North Am. Spine Soc. 2005, 5, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Ngai, J.C.; Ko, F.W.; Ng, S.S.; To, K.-W.; Tong, M.; Hui, D.S. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology 2010, 15, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Lau, H.M.-C.; Lee, E.W.-C.; Wong, C.N.-C.; Ng, G.Y.-F.; Jones, A.Y.-M.; Hui, D.S.-C. The Impact of Severe Acute Respiratory Syndrome on the Physical Profile and Quality of Life. Arch. Phys. Med. Rehabil. 2005, 86, 1134–1140. [Google Scholar] [CrossRef] [Green Version]
- Li, J. Rehabilitation management of patients with COVID-19: Lessons learned from the first experience in China. Eur. J. Phys. Rehabil. Med. 2020, 56. [Google Scholar] [CrossRef]
- Xiong, Q.; Xu, M.; Li, J.; Liu, Y.; Zhang, J.; Xu, Y.; Dong, W. Clinical sequelae of COVID-19 survivors in Wuhan, China: A single-centre longitudinal study. Clin. Microbiol. Infect. 2020. [Google Scholar] [CrossRef]
- Bagnato, S.; Boccagni, C.; Marino, G.; Prestandrea, C.; D’Agostino, T.; Rubino, F. Critical illness myopathy after COVID-19. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020, 99, 276–278. [Google Scholar] [CrossRef]
- Herridge, M.S.; Tansey, C.M.; Matté, A.; Tomlinson, G.; Diaz-Granados, N.; Cooper, A.; Guest, C.B.; Mazer, C.D.; Mehta, S.; Stewart, T.E.; et al. Functional Disability 5 Years after Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2011, 364, 1293–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latronico, N.; Bolton, C.F. Critical illness polyneuropathy and myopathy: A major cause of muscle weakness and paralysis. Lancet Neurol. 2011, 10, 931–941. [Google Scholar] [CrossRef]
- Sidiras, G.; Gerovasili, V.; Patsaki, I.; Karatzanos, E.; Papadopoulos, E.; Markaki, V.; Strantzalis, G.; Nanas, S. Short and long term out comes of ICU acquired weakness. Health Sci. J. 2013, 7, 188–200. [Google Scholar]
- Najafpour, Z.; Godarzi, Z.; Arab, M.; Yaseri, M. Risk Factors for Falls in Hospital In-Patients: A Prospective Nested Case Control Study. Int. J. Health Policy Manag. 2019, 8, 300–306. [Google Scholar] [CrossRef]
- Singer, J.P.; Lederer, D.J.; Baldwin, M.R. Frailty in Pulmonary and Critical Care Medicine. Ann. Am. Thorac. Soc. 2016, 13, 1394–1404. [Google Scholar] [CrossRef] [Green Version]
- Van Pelt, D.C.; Milbrandt, E.B.; Qin, L.; Weissfeld, L.A.; Rotondi, A.J.; Schulz, R.; Chelluri, L.; Angus, D.C.; Pinsky, M.R. Informal Caregiver Burden among Survivors of Prolonged Mechanical Ventilation. Am. J. Respir. Crit. Care Med. 2007, 175, 167–173. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagarra-Romero, L.; Viñas-Barros, A. COVID-19: Short and Long-Term Effects of Hospitalization on Muscular Weakness in the Elderly. Int. J. Environ. Res. Public Health 2020, 17, 8715. https://doi.org/10.3390/ijerph17238715
Sagarra-Romero L, Viñas-Barros A. COVID-19: Short and Long-Term Effects of Hospitalization on Muscular Weakness in the Elderly. International Journal of Environmental Research and Public Health. 2020; 17(23):8715. https://doi.org/10.3390/ijerph17238715
Chicago/Turabian StyleSagarra-Romero, Lucía, and Andrea Viñas-Barros. 2020. "COVID-19: Short and Long-Term Effects of Hospitalization on Muscular Weakness in the Elderly" International Journal of Environmental Research and Public Health 17, no. 23: 8715. https://doi.org/10.3390/ijerph17238715
APA StyleSagarra-Romero, L., & Viñas-Barros, A. (2020). COVID-19: Short and Long-Term Effects of Hospitalization on Muscular Weakness in the Elderly. International Journal of Environmental Research and Public Health, 17(23), 8715. https://doi.org/10.3390/ijerph17238715





