Cumene Contamination in Groundwater: Observed Concentrations, Evaluation of Remediation by Sulfate Enhanced Bioremediation (SEB), and Public Health Issues
Abstract
1. Introduction
2. Background
2.1. Cumene as a Groundwater Contaminant
2.1.1. Environmental Concentrations of Cumene in Groundwater
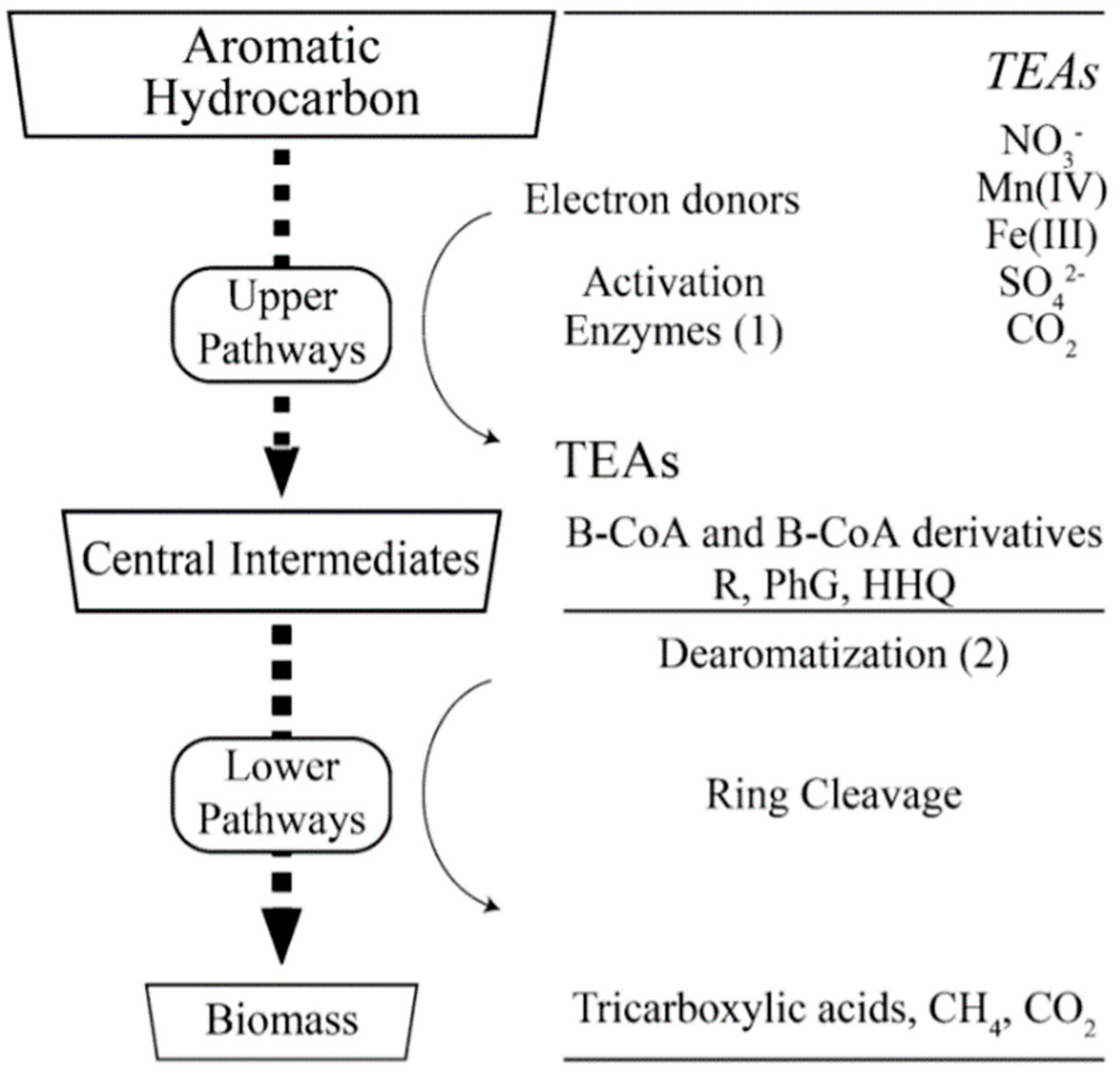
2.1.2. Cumene Remediation in Groundwater
2.2. Cumene in Fuels
2.3. Current Cumene Regulations
2.4. Sulfate Enhanced Biodegradation (SEB)
3. Materials and Methods
3.1. Baseline Cumene in Modern Fuels
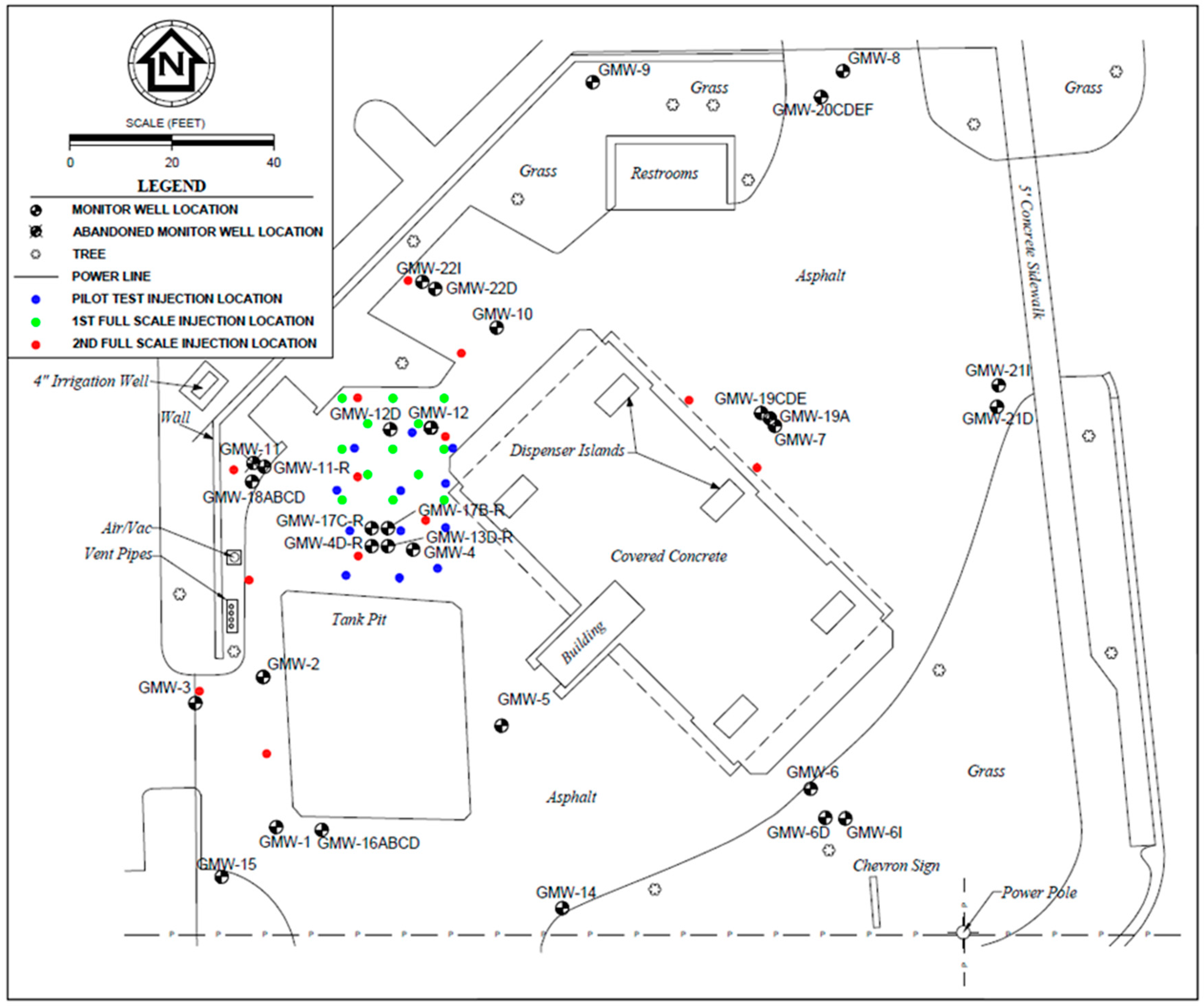
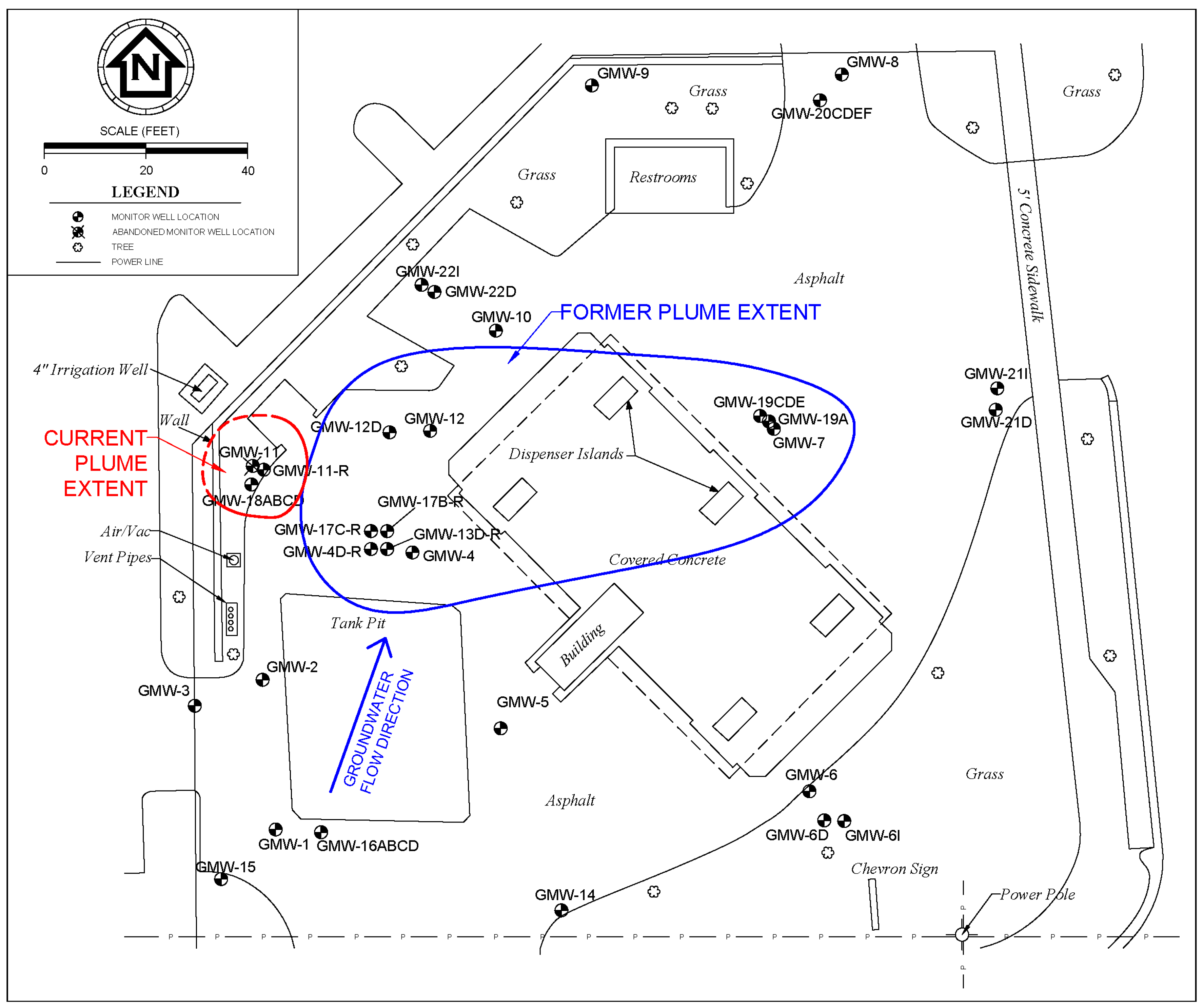
3.2. Field Case Study
3.3. Health Risk Assessment of Cumene
4. Results
4.1. Baseline Cumene in Modern Fuels
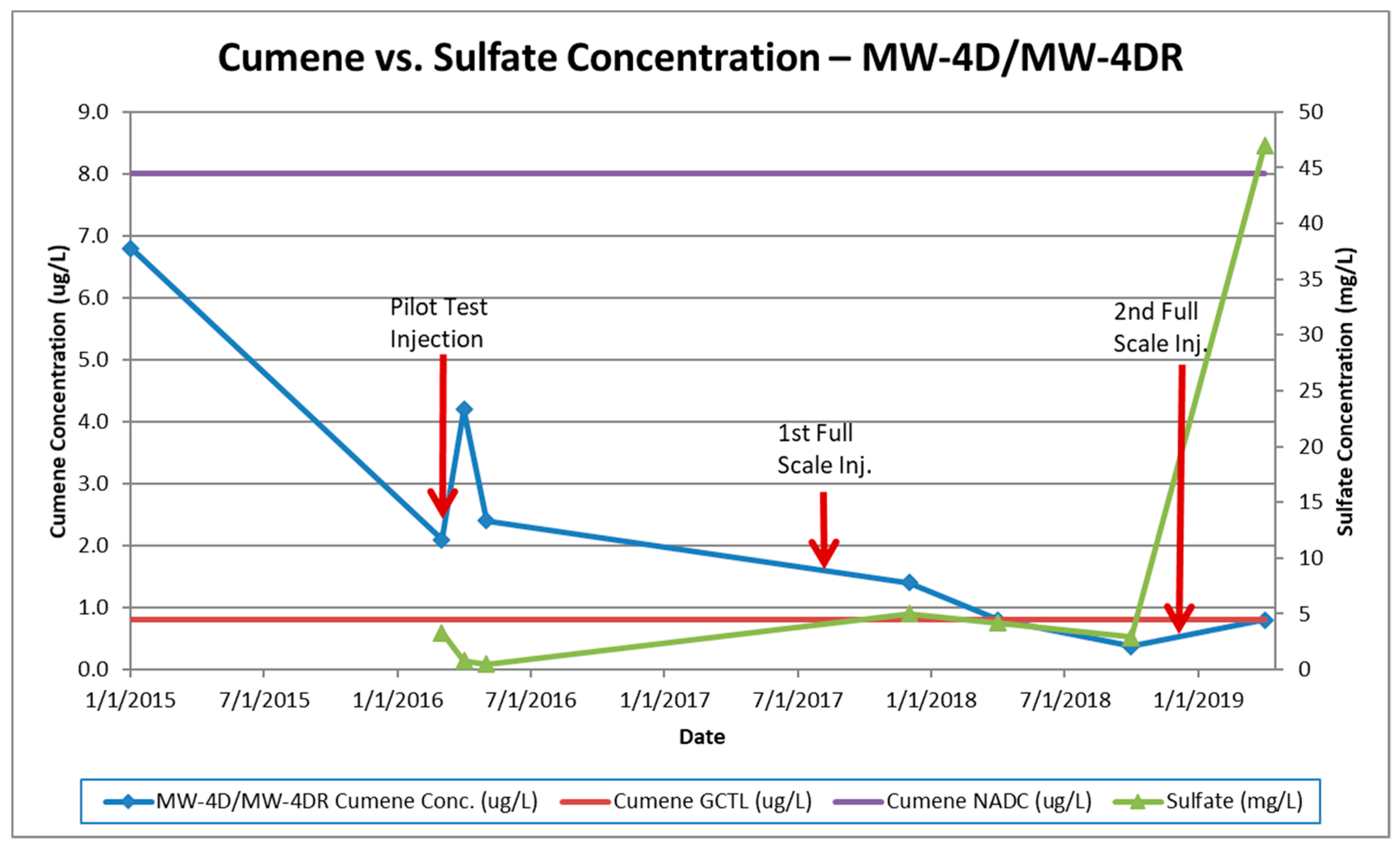
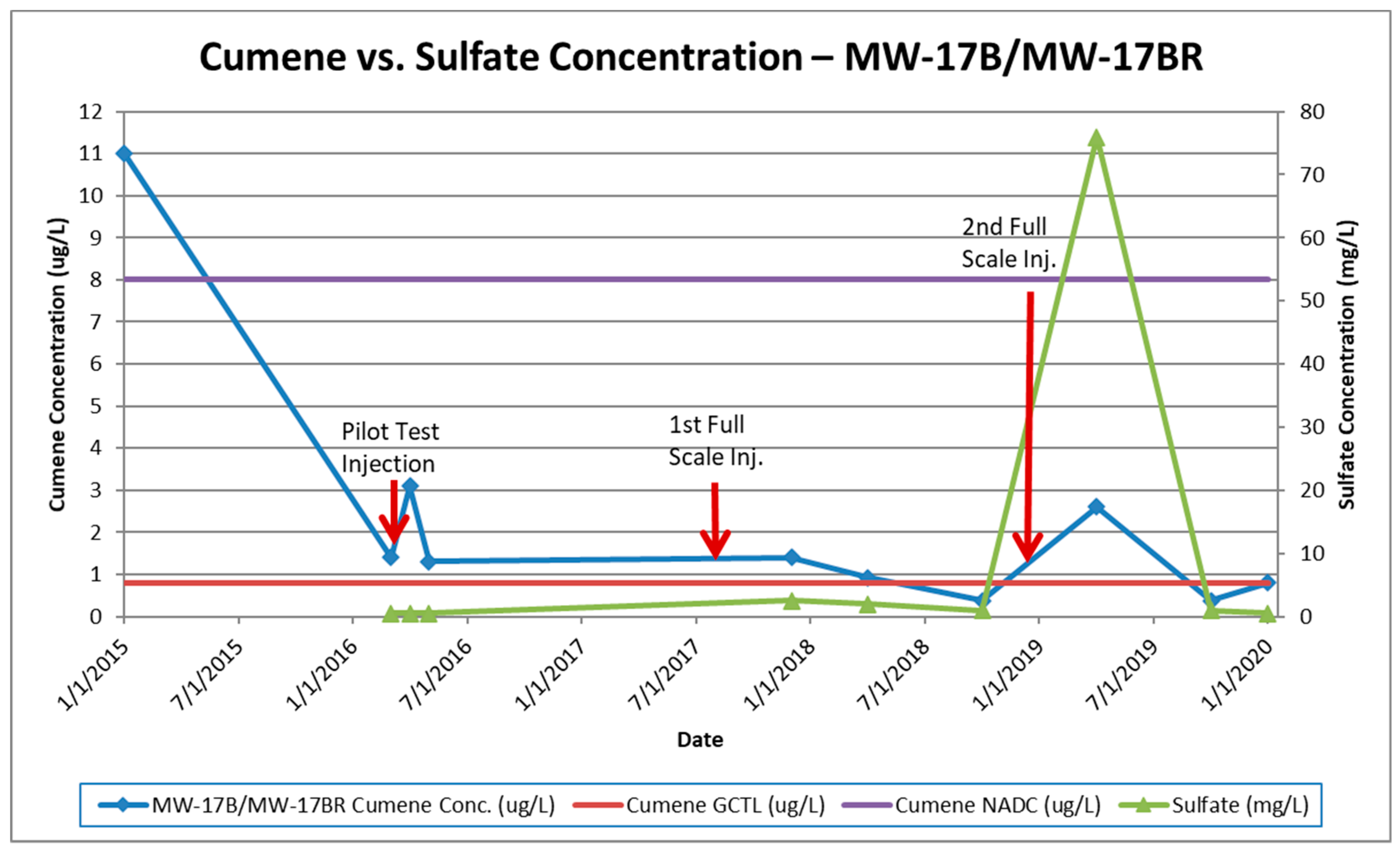
4.2. Case Study
4.3. A Review of Health Impacts of Cumene
5. Discussion
5.1. Baseline Cumene in Modern Fuels
5.2. Effectiveness of Enhanced Bioremediation with Sulfate
5.3. Public Health Issues and Mandated Cumene Remediation in Florida
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sivaranjani, T.; Xavier, S.; Periandy, S. NMR, FT-IR, FT-Raman, UV spectroscopic, HOMO–LUMO and NBO analysis of cumene by quantum computational methods. J. Mol. Struct. 2015, 1083, 39–47. [Google Scholar] [CrossRef]
- National Toxicology Program. Report on Carcinogens: Monograph on Cumene; United States Department of Health and Human Services: Washington, DC, USA, 2013. Available online: https://ntp.niehs.nih.gov/ntp/roc/thirteenth/genotoxstudies/cumenegtox_508.pdf (accessed on 5 April 2019).
- O’Keefe, W.F. Letter and Attachment to Toxic Substances Control Act Interagency Testing Committee; American Petroleum Institute: Washington, DC, USA, 1984. [Google Scholar]
- World Health Organization (WHO), Cumene. Concise International Chemical Assessment Document 18; Joint Publication of the United Nations Environmental Programme, the International Labour Organisation, and the World Health Organization; World Health Organization: Geneva, Switzerland, 1999; Available online: https://www.who.int/ipcs/publications/cicad/cicad18_rev_1.pdf (accessed on 5 April 2019).
- Glickman, A.H.; Alexander, H.C.; Buccafusco, R.J.; Morris, C.R.; Francis, B.O.; Suprenant, D.C.; Ward, T.J. An evaluation of the aquatic hazard of cumene (isopropoyl benzene). Ecotoxicol. Environ. Saf. 1995, 31, 287–289. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency (EHCA). Cumene: Substance Infracard; European Chemical Agency: Helsinki, Finland, 2019; Available online: https://www.echa.europa.eu/substance-information/-/substanceinfo/100.002.458 (accessed on 5 April 2019).
- Florida Department of Environmental Protection (FDEP). Contaminated Site Cleanup. Florida Administrative Code, Chapter 62–780, 2 February 2017. Available online: https://www.flrules.org/gateway/ChapterHome.asp?Chapter=62-780 (accessed on 10 April 2019).
- Florida Department of Environmental Protection (FDEP). Contaminant Cleanup Target Levels. Florida Administrative Code, Chapter 62–777, 17 April 2005. Available online: https://www.flrules.org/gateway/ChapterHome.asp?Chapter=62-777 (accessed on 10 April 2019).
- Teply, J.; Dressler, M. Direct determination of organic compounds in water using steam–solid chromatography. J. Chromatogr. 1980, 191, 221–229. [Google Scholar] [CrossRef]
- Botta, D.; Castellani Pirri, L.; Mantica, E. Ground water pollution by organic solvents and their microbial degradation products. In Analysis of Organic Micro-Pollutants in Water: Proceedings of the 3rd European Symposium, Oslo, Norway, 19–21 September 1983; Angeletti, G., Bjorseth, A., Eds.; Reidel Publishing Co.: Boston, MA, USA, 1984; pp. 261–275. [Google Scholar]
- Montz, W.E.; Puyear, R.L.; Brammer, J.D. Identification and quantification of water-soluble hydrocarbons generated by two cycle outboard motors. Arch. Environ. Contam. Toxicol. 1984, 11, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Steurmer, D.H.; Ng, D.J.; Morris, C.J. Organic contaminants in groundwater near an underground coal gasification site in northeastern Wyoming. Environ. Sci. Technol. 1982, 16, 582–587. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine. Cumene. Hazardous Substances Database; NSBD, Complete Update: 2005 Last Reviewed: 2000; National Library of Medicine: Bethesda, MD, USA, 2005. Available online: https://www.nlm.nih.gov/toxnet/index.html (accessed on 9 September 2020).
- U.S. Environmental Protection Agency (USEPA). How to Evaluate Alternative Cleanup Technologies for Underground Storage Tank Sites: A Guide for Corrective Action Plan Reviewers; EPA 510-B-17-003; U.S. Environmental Protection Agency: Washington, DC, USA, 2017. Available online: https://www.epa.gov/ust/how-evaluate-alternative-cleanup-technologies-underground-storage-tank-sites-guide-corrective (accessed on 29 September 2020).
- Fontenot, K.K.; Bush, E.W.; Portier, R.J.; Meyer, B.A.; Beasley, J.S.; Walsh, M.M. Determining an optimum tree species for the phytoremediation of Cumene and 4-Cumylphenol in groundwater. J. Environ. Hortic. 2008, 32, 196–202. [Google Scholar] [CrossRef]
- Dutton, G.D.; Miller, H.D. Georgia-Pacific successfully completes one of the first large-scale hazardous waste bioremediation projects in the southeastern United States. In Proceedings HMC-South’91 Conference and Exhibition; International Atomic Energy Agency (IAEA): Vienna, Austria, 1991. [Google Scholar]
- Walker, J.D.; Austin, H.F.; Colwell, R.R. Utilization of mixed hydrocarbon substrate by petroleum-degrading microorganisms. J. Gen. Appl. Microbiol. 1975, 21, 27–39. [Google Scholar] [CrossRef]
- Walker, J.D.; Calomiris, J.J.; Herbert, T.L.; Colwell, R.R. Petroleum hydrocarbons: Degradation and growth potential for Atlantic Ocean sediment bacteria. Mar. Biol. 1976, 34, 1–9. [Google Scholar] [CrossRef]
- Mandelbaum, R.T.; Shati, M.R.; Ronen, D. In situ microcosms in aquifer bioremediation studies. FEMS Microbiol. Rev. 1997, 20, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Acton, D.W.; Barker, J.F. In situ biodegradation potential of aromatic hydrocarbons in anaerobic groundwaters. J. Contam. Hydrol. 1992, 9, 325–352. [Google Scholar] [CrossRef]
- Beasley, K.K.; Grieg, L.M.; Suflita, J.M.; Nanny, M.A. Polarizability and spin density correlate with the relative anaerobic biodegradability of alkylaromatic hydrocarbons. Environ. Sci. Technol. 2009, 43, 4995–5000. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Fushinobu, S.; Fukuda, E.; Terada, T.; Nakamura, S.; Shimizu, K.; Nojiri, H.; Omori, T.; Shoun, H.; Wakagi, T. Crystal structure of the terminal oxygenase component of cumene dioxygenase from Pseudomonas fluorescens IP01. J. Bacteriol. 2005, 187, 2483–2490. [Google Scholar] [CrossRef] [PubMed]
- U.S. Oil & Refining Co. Material Safety Data Sheet: Gasoline. MSDS No. 953. 2012. Available online: http://www.usor.com/files/pdf/4/Gasoline%20-%20SDS934%20120820.pdf (accessed on 10 November 2019).
- Canada Imperial Oil Limited, An Affiliate of Exxon Mobil Corporation. Material Safety Data Sheet: Unleaded Gasoline for Export. Product Code 12443. 2009. Available online: http://www.nglenergypartners.com/wp-content/uploads/ExxonMobil.GasolineUnleadedEtOH_03.30.2009.pdf (accessed on 10 November 2019).
- Gulf Oil Limited Partnership. Safety Data Sheet: Unleaded Gasoline, Non-Oxygenated, All Grades. 2018. Available online: https://msds.gulfoil.com/PDF/2018%20PDF/Gulf%20SDS%20Gasoline%20%20Non-Oxygenated.pdf (accessed on 10 November 2019).
- Sunoco, Inc. Safety Data Sheet: Sunoco Regular Gasoline with 10% Ethanol. 2014. Available online: https://static1.squarespace.com/static/51e0d2dde4b03a4e2198c2f0/t/55b6f6d1e4b05525c7002c5b/1438054097738/Gasoline+SDS.pdf (accessed on 10 November 2019).
- Citgo Petroleum Corporation. Safety Data Sheet: Citgo Gasolines, All Grades Unleaded. 2018. Available online: http://www.docs.citgo.com/msds_pi/UNLEAD.pdf (accessed on 10 November 2019).
- Marathon Petroleum Company LP. Safety Data Sheet: Marathon Petroleum Gasolines-All Grades Unleaded. 2018. Available online: https://www.marathonbrand.com/content/documents/brand/sds/0127MAR019.pdf (accessed on 10 November 2019).
- Petrocom Energy Group, LLC. Material Safety Data Sheet: Gasoline, MSDS No. PEG-UNL. 2008. Available online: http://msds.mkap.com/LiquidFuelProducts/UnleadedGasoline.pdf (accessed on 10 November 2019).
- Valero Marketing & Supply Company and Affiliates. Safety Data Sheet: Unleaded Gasoline. 2014. Available online: https://www.marathonbrand.com/content/documents/brand/sds/0127MAR019.pdf (accessed on 10 November 2019).
- CRC Handbook of Chemistry and Physics. Available online: http://www.hbcponline.com/ (accessed on 10 November 2019).
- Center for Disease Control and Prevention (CDC)-NIOSH Pocket Guide to Chemical Hazards—Cumene. Centers for Disease Control and Prevention. 2019. Available online: https://www.cdc.gov/niosh/npg/npgd0159.html (accessed on 10 November 2019).
- Zambrano, J.; Stoner, S. Ambient Water Quality Standards and Guidance Values and Groundwater Effluent Limitations. 1998. Available online: https://www.dec.ny.gov/docs/water_pdf/togs1112.pdf (accessed on 12 November 2019).
- Florida Department of Environmental Protection. Groundwater and Surface Water Cleanup Target Levels. Available online: https://floridadep.gov/sites/default/files/2-GroundwaterandSurfaceWaterCleanupTargetLevels_3.pdf (accessed on 12 November 2019).
- State of Maryland Department of the Environment. Cleanup Standards for Soil and Groundwater. 2018. Available online: https://mde.state.md.us/programs/LAND/MarylandBrownfieldVCP/Documents/www.mde.state.md.us/assets/document/MDE Soil and Groundwater Cleanup Standards 10-2018 Interim Final Update 3-2.pdf (accessed on 12 November 2019).
- Delaware Department of Natural Resources and Environmental Control. Remediation Standards Guidance Under the Delaware Hazardous Substance Cleanup Act. 1999. Available online: http://www.dnrec.state.de.us/DNREC2000/Divisions/AWM/sirb/DOCS/PDFS/Misc/RemStnd.pdf (accessed on 12 November 2019).
- North Carolina Department of Environmental Quality. Subchapter 2L-Groundwater Classification and Standards. 2019. Available online: http://reports.oah.state.nc.us/ncac/title 15a-environmental quality/chapter 02-environmental management/subchapter l/subchapter l rules.pdf (accessed on 12 November 2019).
- Minnesota Department of Health. Comparison of State Water Guidance and Federal Drinking Water Standards. 2019. Available online: https://www.health.state.mn.us/communities/environment/risk/guidance/waterguidance.html (accessed on 12 November 2019).
- Maine Department of Environmental Protection. Maine Remedial Action Guidelines (RAGs) for Sites Contaminated with Hazardous Substances. 2018. Available online: https://www.maine.gov/dep/spills/publications/guidance/rags/ME-Remedial-Action-Guidelines-10-19-18cc.pdf (accessed on 11 November 2019).
- Kansas Department of Health and Environment. Risk-Based Standards for Kansas. 2015. Available online: http://www.kdheks.gov/remedial/download/RSK_Manual_15.pdf (accessed on 12 November 2019).
- Illinois Environmental Protection Agency. Section 620.410 Groundwater Quality Standards for Class I: Potable Resource Groundwater. 2012. Available online: http://www.ilga.gov/commission/jcar/admincode/035/035006200D04100R.html (accessed on 12 November 2019).
- Iowa Department of Natural Resources. Statewide Standards for a Protected Groundwater Source, Statewide Standards-Cumulative Risk Calculator. 2016. Available online: https://programs.iowadnr.gov/riskcalc/Home/statewidestandards (accessed on 12 November 2019).
- New Jersey Department of Environmental Protection. Ground Water Quality Standards-Class IIA by Constituent. 2019. Available online: https://www.nj.gov/dep/standards/ground water.pdf (accessed on 12 November 2019).
- Michigan Department of Environmental Quality. Part 22 Groundwater Quality-Standards List. 2019. Available online: https://www.michigan.gov/documents/deq/wrd-groundwater-p22-standards_564955_7.pdf (accessed on 12 November 2019).
- New Hampshire Department of Environmental Services. NHDES Risk Characterization and Management Policy (Section 7.4)-Table 2, Method 1 Groundwater Standards. 2018. Available online: https://www.des.nh.gov/organization/divisions/waste/hwrb/documents/rcmp.pdf (accessed on 12 November 2019).
- Pennsylvania Department of Environmental Protection. Standards/Action Levels for Confirmatory Samples Collected at Closure Site Assessments. 2011. Available online: http://files.dep.state.pa.us/EnvironmentalCleanupBrownfields/StorageTanks/StorageTanksPortalFiles/attachment-5.pdf (accessed on 12 November 2019).
- Ladino-Orjuela, G.; Gomes, E.; Silva, R.D.; Salt, C.; Parsons, J.R. Metabolic pathways for degradation of aromatic hydrocarbons by bacteria. Rev. Environ. Contam. Toxicol. 2016, 237, 105–121. [Google Scholar] [PubMed]
- Suthersan, S.; Houston, K.; Schnobrich, M.; Horst, J. Engineered anaerobic bio-oxidation systems for petroleum hydrocarbon residual source zones with soluble sulfate application. Groundw. Monit. Remediat. 2011, 31. [Google Scholar] [CrossRef]
- Wiedermeier, T.H.; Rifai, H.S.; Newell, C.J.; Wilson, J.T. Natural Attenuation of Fuels and Chlorinated Solvents in the Subsurface; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Hussain, A.; Hasan, A.; Javid, A.; Qazi, J.I. Exploited application of sulfate-reducing bacteria for concomitant treatment of metallic and non-metallic wastes: A mini review. 3 Biotech 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (USEPA). 2018 Edition of the Drinking Water Standards and Health Advisories Tables, EPA 822-F-18-001. 2018. Available online: https://www.epa.gov/sites/production/files/2014-03/documents/tum_ch1.pdf (accessed on 29 September 2020).
- United States Environmental Protection Agency (USEPA). Method 8260B Volatile Organic Compounds by Gas. Chromatography/Mass Spectometry (GC/MS). 1996. Available online: https://19january2017snapshot.epa.gov/sites/production/files/2015-12/documents/8260b.pdf (accessed on 15 March 2019).
- U.S. Environmental Protection Agency (USEPA). Toxicological Review of Cumene. In Support. of Summary Information on the Integrated Risk Information System. June 1997. Available online: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0306tr.pdf (accessed on 30 September 2010).
- Hazardous Substances DataBank (HSDB). Isopropylbenzene. 2017. Available online: https://toxnet.nlm.nih.gov/ (accessed on 15 September 2020).
- Occupational Safety and Health Administration (OSHA). OSHA Annotated Table Z-1; United States Department of Labor: Washington, DC, USA, 2015. Available online: https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1000TABLEZ1 (accessed on 15 September 2020).
- Florida Department of Health Environmental Health Bureau of Environmental Health, Water Programs. Maximum Contaminant Levels and Health Advisory Levels. Available online: http://www.floridahealth.gov/environmental-health/drinking-water/_documents/hal-list.pdf (accessed on 15 September 2020).
- U.S. Environmental Protection Agency (USEPA). Regional Screening Levels Generic Tables. May 2020. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 15 September 2020).
- U.S. Environmental Protection Agency (USEPA). 2018 Edition of the Drinking Water Standards and Health Advisories, March 2018; EPA 822-F-18-001; Office of Water, USEPA: Washington, DC, USA, 2018. Available online: https://www.epa.gov/sites/production/files/2018-03/documents/dwtable2018.pdf (accessed on 15 September 2020).
- U.S. Environmental Protection Agency (USEPA). Vapor Intrusion Screening Level (VISL) Calculator Version 3.5 for June 2017 RSLs. Version 3.5.2 Updated 18 October 2017. Available online: https://www.epa.gov/vaporintrusion/vapor-intrusion-screening-level-calculator (accessed on 15 September 2020).
- International Agency for Research on Cancer (IARC). Some Chemicals Present in Industrial and Consumer Products, Food, and Drinking Water; Cumene Monograph 101-009; World Health Organization/IARC: Lyon, France, 2013; Volume 101, p. 325. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA). Vapor Intrusion Screening Level (VISL) Calculator User’s Guide. May 2014. Available online: https://www.epa.gov/vaporintrusion/vapor-intrusion-screening-level-calculator (accessed on 15 September 2020).

| Gasoline Brand Name/Grade | % Cumene Conc. | Reference |
|---|---|---|
| Shell–All Grades Unleaded | 0–0.5% | US Oil MSDS 2012 |
| ExxonMobil–Unleaded with Ethanol | 0–1% | Canada Imperial MSDS 2009 |
| Gulf–All Grades Unleaded | 0–1% | Gulf SDS 2018 |
| Sunoco–87 Unleaded | 0–1% | Sunoco SDS 2015 |
| Citgo–All Grades Unleaded | 0–4% | Citgo SDS 2018 |
| Marathon–All Grades Unleaded | 0–4% | Marathon SDS 2018 |
| Petrocom–All Grades Unleaded | 0–5% | Petrocom MSDS 2008 |
| Valero–All Grades Unleaded | 0–5% | Valero SDS 2014 |
| Flint hills–All Grades Unleaded | 0–10% | Flint Hills MSDS 2012 |
| Property | Value | Reference |
|---|---|---|
| Cas number | 98-82-8 | [31] |
| Molecular formula | C H | [31] |
| Molecular weight | 120.191 g/mol | [31] |
| Color | Clear/Colorless | [32] |
| Odor | Sharp, penetrating, aromatic | [32] |
| Boiling point | 152.4 °C | [31] |
| Flash point | 36 °C | [31] |
| Melting point | −96.01 °C | [31] |
| Vapor pressure (at 25 °C) | 0.61 kPa | [31] |
| Water solubility (at 25 °C) | 0.050 g/kg | [31] |
| Octanol/water partition coefficient, log k | 3.66 | [31] |
| Density (at 20 °C) | 0.8615 g/cm3 | [31] |
| Henry’s law constant, k (at 20 °C) | 1.466 kPa-m3/mol | [31] |
| State | Cumene Criterion (µg/L) | Reference |
|---|---|---|
| New York | 5 | [33] |
| Florida | 8 | [34] |
| Maryland | 45 | [35] |
| Deleware | 66 | [36] |
| North Carolina | 70 | [37] |
| Minnesota | 300 | [38] |
| Maine | 450 | [39] |
| Kansas | 451 | [40] |
| Illinois | 700 | [41] |
| Iowa | 700 | [42] |
| New jersey | 700 | [43] |
| Michigan | 800 | [44] |
| New Hampshire | 800 | [45] |
| Pennsylvania | 840 | [46] |
| Substance | Peak # | R. Time | I. Time | F. Time | Area | Cumene (µg/L) | Cumene (%) |
|---|---|---|---|---|---|---|---|
| Cumene Standard #1 | 1 | 7.585 | 7.515 | 7.660 | 2,105,031 | 2,000,000 | 0.20 |
| 1-Regular Grade | 63 | 7.594 | 7.530 | 7.660 | 852,240 | 809,717 | 0.08 |
| 1-Mid Grade | 58 | 7.593 | 7.475 | 7.655 | 1,295,322 | 1,230,692 | 0.12 |
| 1-Premium Grade | 59 | 7.591 | 7.470 | 7.655 | 1,392,741 | 1,323,250 | 0.13 |
| 2-Regular Grade | 64 | 7.589 | 7.530 | 7.655 | 486,391 | 462,122 | 0.05 |
| 2-Mid Grade | 62 | 7.593 | 7.535 | 7.655 | 494,345 | 469,680 | 0.05 |
| 2-Premium Grade | 55 | 7.591 | 7.530 | 7.655 | 690,231 | 655,792 | 0.07 |
| 2-Diesel | 43 | 7.591 | 7.550 | 7.670 | 211,478 | 200,926 | 0.02 |
| 3-Regular Grade | 64 | 7.587 | 7.465 | 7.650 | 1,127,090 | 1,070,854 | 0.11 |
| 3-Mid Grade | 62 | 7.587 | 7.470 | 7.650 | 1,149,367 | 1,092,019 | 0.11 |
| 3-Premium Grade | 52 | 7.587 | 7.520 | 7.645 | 1,028,558 | 977,238 | 0.10 |
| 3-Ethanol Free | 44 | 7.585 | 7.520 | 7.645 | 905,763 | 860,570 | 0.09 |
| Cumene Standard #2 | 1 | 7.570 | 7.490 | 7.650 | 5,943,042 | 2,000,000 | 0.20 |
| 4-Regular Unleaded | 56 | 7.575 | 7.455 | 7.640 | 1,203,979 | 405,173 | 0.04 |
| 4-Mid Grade | 59 | 7.576 | 7.460 | 7.640 | 1,223,605 | 411,777 | 0.04 |
| 4-Premium | 58 | 7.574 | 7.455 | 7.635 | 1,305,727 | 439,414 | 0.04 |
| 4-Diesel | 49 | 7.576 | 7.535 | 7.660 | 373,823 | 125,802 | 0.01 |
| 5-Regular Unleaded | 62 | 7.573 | 7.455 | 7.640 | 1,405,543 | 473,005 | 0.05 |
| 5-Mid Grade | 62 | 7.571 | 7.455 | 7.635 | 1,313,845 | 442,146 | 0.04 |
| 5-Premium | 58 | 7.571 | 7.450 | 7.630 | 1,353,273 | 455,414 | 0.05 |
| 5-Diesel | 49 | 7.569 | 7.530 | 7.650 | 549,469 | 184,912 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herman, J.P.; Redfern, L.; Teaf, C.; Covert, D.; Michael, P.R.; Missimer, T.M. Cumene Contamination in Groundwater: Observed Concentrations, Evaluation of Remediation by Sulfate Enhanced Bioremediation (SEB), and Public Health Issues. Int. J. Environ. Res. Public Health 2020, 17, 8380. https://doi.org/10.3390/ijerph17228380
Herman JP, Redfern L, Teaf C, Covert D, Michael PR, Missimer TM. Cumene Contamination in Groundwater: Observed Concentrations, Evaluation of Remediation by Sulfate Enhanced Bioremediation (SEB), and Public Health Issues. International Journal of Environmental Research and Public Health. 2020; 17(22):8380. https://doi.org/10.3390/ijerph17228380
Chicago/Turabian StyleHerman, John P., Lauren Redfern, Christopher Teaf, Douglas Covert, Peter R. Michael, and Thomas M. Missimer. 2020. "Cumene Contamination in Groundwater: Observed Concentrations, Evaluation of Remediation by Sulfate Enhanced Bioremediation (SEB), and Public Health Issues" International Journal of Environmental Research and Public Health 17, no. 22: 8380. https://doi.org/10.3390/ijerph17228380
APA StyleHerman, J. P., Redfern, L., Teaf, C., Covert, D., Michael, P. R., & Missimer, T. M. (2020). Cumene Contamination in Groundwater: Observed Concentrations, Evaluation of Remediation by Sulfate Enhanced Bioremediation (SEB), and Public Health Issues. International Journal of Environmental Research and Public Health, 17(22), 8380. https://doi.org/10.3390/ijerph17228380






