Development of a Prediction Model to Identify Children at Risk of Future Developmental Delay at Age 4 in a Population-Based Setting
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and population
2.2. Outcome Measure
2.3. Candidate Predictors
2.4. Statistical Analyses
2.5. Ethical Consideration
3. Results
3.1. Population Characteristics
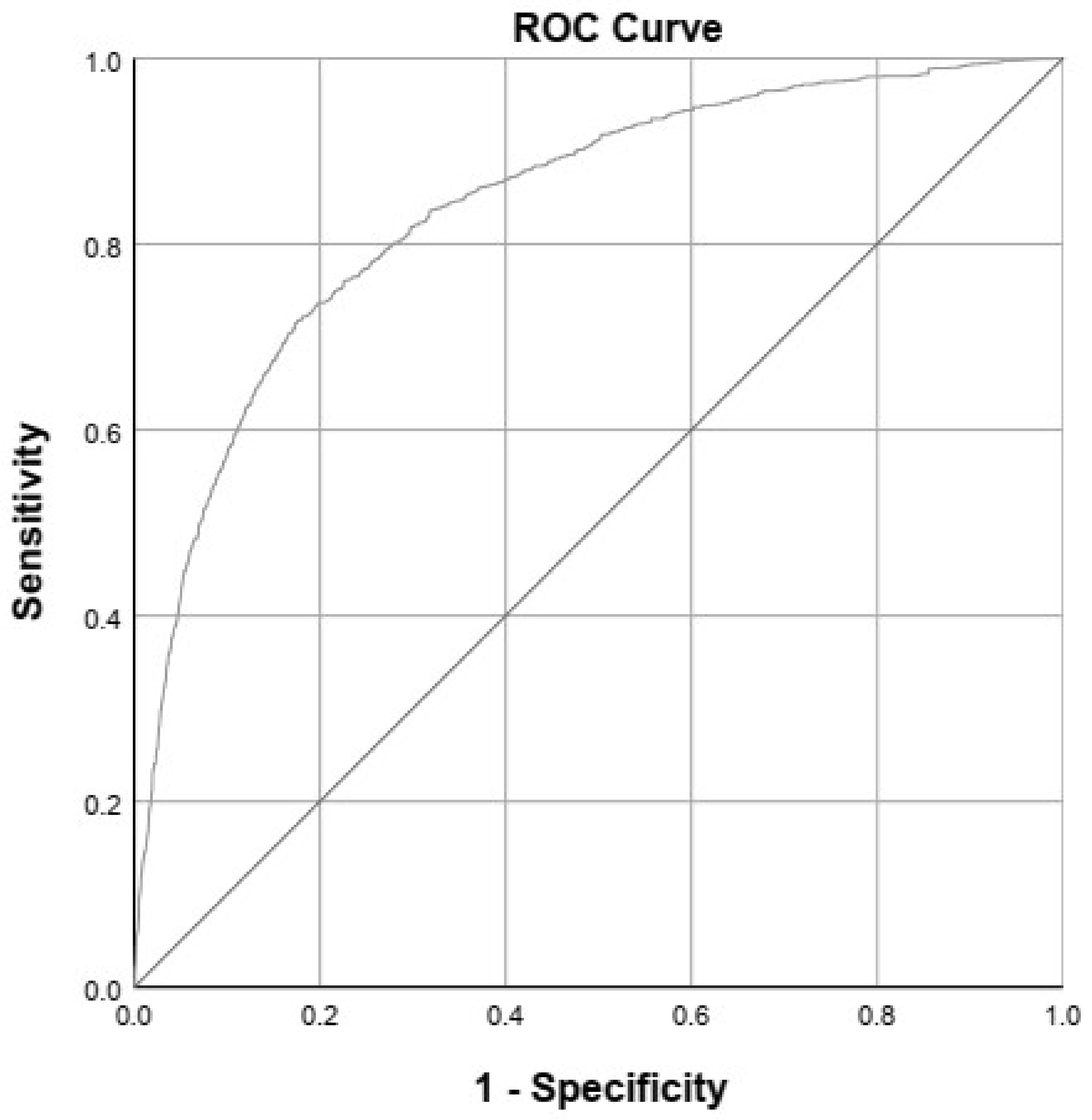
3.2. Prediction Model
3.3. Example
4. Discussion
4.1. Strengths and Limitations
4.2. Implications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Research on Developmental Disabilities Collaborators. Developmental disabilities among children younger than 5 years in 195 countries and territories, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet Glob. Health 2018, 6, e1100–e1121. [Google Scholar] [CrossRef]
- Boyle, C.A.; Boulet, S.; Schieve, L.A.; Cohen, R.A.; Blumberg, S.J.; Yeargin-Allsopp, M.; Visser, S.; Kogan, M.D. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics 2011, 127, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Valla, L.; Wentzel-Larsen, T.; Hofoss, D.; Slinning, K. Prevalence of suspected developmental delays in early infancy: Results from a regional population-based longitudinal study. BMC Pediatr. 2015, 15, 215. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.K.; Jones, P.B.; Kuh, D.; Richards, M. Infant developmental milestones and subsequent cognitive function. Ann. Neurol. 2007, 62, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Taanila, A.; Murray, G.K.; Jokelainen, J.; Isohanni, M.; Rantakallio, P. Infant developmental milestones: A 31-year follow-up. Dev. Med. Child. Neurol. 2005, 47, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Flensborg-Madsen, T.; Mortensen, E.L. Infant developmental milestones and adult intelligence: A 34-year follow-up. Early Hum. Dev. 2015, 91, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.M.; Shinn, C.; Fullilove, M.T.; Scrimshaw, S.C.; Fielding, J.E.; Normand, J.; Carande-Kulis, V.G.; Task Force on Community Preventive Services. The effectiveness of early childhood development programs. A systematic review. Am. J. Prev. Med. 2003, 24, 32–46. [Google Scholar] [CrossRef]
- McCormick, M.C.; Brooks-Gunn, J.; Buka, S.L.; Goldman, J.; Yu, J.; Salganik, M.; Scott, D.T.; Bennett, F.C.; Kay, L.L.; Bernbaum, J.C.; et al. Early intervention in low birth weight premature infants: Results at 18 years of age for the infant health and development program. Pediatrics 2006, 117, 771–780. [Google Scholar] [CrossRef]
- Spittle, A.J.; Orton, J.; Doyle, L.W.; Boyd, R. Early developmental intervention programs post hospital discharge to prevent motor and cognitive impairments in preterm infants. Cochrane Database Syst. Rev. 2007, CD005495. [Google Scholar] [CrossRef]
- Siderius, E.J.; Carmiggelt, B.; Rijn, C.S.; Heerkens, Y.F. Preventive child health care within the framework of the dutch health care system. J. Pediatr. 2016, 177S, S138–S141. [Google Scholar] [CrossRef]
- Schonhaut, L.; Armijo, I.; Pérez, M. Gestational age and developmental risk in moderately and late preterm and early term infants. Pediatrics 2015, 135, e835–e841. [Google Scholar] [CrossRef] [PubMed]
- Kirkegaard, I.; Obel, C.; Hedegaard, M.; Henriksen, T.B. Gestational age and birth weight in relation to school performance of 10-year-old children: A follow-up study of children born after 32 completed weeks. Pediatrics 2006, 118, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Holditch-Davis, D.; Miles, M.S. Effects of gender on the health and development of medically at-risk infants. JOGNN 2010, 39, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Urben, S.; Van Hanswijck De Jonge, L.; Barisnikov, K.; Pizzo, R.; Monnier, M.; Lazeyras, F.; Borradori Tolsa, C.; Hüppi, P.S. Gestational age and gender influence on executive control and its related neural structures in preterm-born children at 6 years of age. Child Neuropsychol. 2017, 23, 188–207. [Google Scholar] [CrossRef]
- Romeo, D.M.; Brogna, C.; Sini, F.; Romeo, M.G.; Cota, F.; Ricci, D. Early psychomotor development of low-risk preterm infants: Influence of gestational age and gender. Eur. J. Paediatr. Neurol. 2016, 20, 518–523. [Google Scholar] [CrossRef]
- Van Wassenaer, A. Neurodevelopmental consequences of being born SGA. Pediatr. Endocrinol. Rev. 2005, 2, 372–377. [Google Scholar]
- Kerstjens, J.M.; De Winter, A.F.; Sollie, K.M.; Bocca-Tjeertes, I.F.; Potijk, M.R.; Reijneveld, S.A.; Bos, A.F. Maternal and pregnancy-related factors associated with developmental delay in moderately preterm-born children. J. Obstet. Gynecol. 2013, 121, 727–733. [Google Scholar] [CrossRef]
- Potijk, M.R.; Kerstjens, J.M.; Bos, A.F.; Reijneveld, S.A.; De Winter, A.F. Developmental delay in moderately preterm-born children with low socioeconomic status: Risks multiply. J. Pediatr. 2013, 163, 1289–1295. [Google Scholar] [CrossRef]
- Smith, L.K.; Draper, E.S.; Evans, T.A.; Field, D.J.; Johnson, S.J.; Manktelow, B.N.; Seaton, S.E.; Marlow, N.; Petrou, S.; Boyle, E.M. Associations between late and moderately preterm birth and smoking, alcohol, drug use and diet: A population-based case-cohort study. Arch. Dis. Child Fetal Neonatal Ed. 2015, 100, F486–F491. [Google Scholar] [CrossRef]
- Simon, L.; Nusinovici, S.; Flamant, C.; Cariou, B.; Rouger, V.; Gascoin, G.; Darmaun, D.; Rozé, J.; Hanf, M. Post-term growth and cognitive development at 5 years of age in preterm children: Evidence from a prospective population-based cohort. PLoS ONE 2017, 12, e0174645. [Google Scholar] [CrossRef][Green Version]
- Di Rosa, G.; Cavallaro, T.; Alibrandi, A.; Marseglia, L.; Lamberti, M.; Giaimo, E.; Nicotera, A.; Bosignore, M.; Gagliano, A. Predictive role of early milestones-related psychomotor profiles and long-term neurodevelopmental pitfalls in preterm infants. Early Hum. Dev. 2016, 101, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Källén, K.; Serenius, F.; Westgren, M.; Maršál, K. Impact of obstetric factors on outcome of extremely preterm births in Sweden: Prospective population-based observational study (EXPRESS). Acta Obstet. Gynecol. Scand. 2015, 94, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Lodha, A.; Sauvé, R.; Chen, S.; Tang, S.; Christianson, H. Clinical risk index for babies score for the prediction of neurodevelopmental outcomes at 3 years of age in infants of very low birthweight. Dev. Med. Child Neurol. 2009, 51, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Yeo, K.T.; Safi, N.; Wang, Y.A.; Le Marsney, R.; Schindler, T.; Bolisetty, S.; Haslam, R.; Lui, K. Prediction of outcomes of extremely low gestational age newborns in Australia and New Zealand. BMJ Paediatr. Open. 2017, 1, e000205. [Google Scholar] [CrossRef]
- Nelson, B.B.; Dudovitz, R.N.; Coker, T.R.; Barnet, E.S.; Biely, C.; Li, N.; Sziagyi, P.G.; Larson, K.; Halfon, N.; Zimmerman, F.J.; et al. Predictors of poor school readiness in children without developmental delay at age 2. Pediatrics 2016, 138, e20154477. [Google Scholar] [CrossRef]
- Kerstjens, J.M.; Bos, A.F.; Ten Vergert Elisabeth, M.J.; De Meer, G.; Butcher, P.R.; Reijneveld, S.A. Support for the global feasibility of the ages and stages questionnaire as developmental screener. Early Hum. Dev. 2009, 85, 443–447. [Google Scholar] [CrossRef]
- Kloosterman, G.J. On intrauterine growth: The significance of prenatal care. Int. J. Gynaecol. Obstet. 1970, 1, 895–912. [Google Scholar] [CrossRef]
- Fredriks, A.M.; Van Buuren, S.; Burgmeijer, R.J.; Meulmeester, J.F.; Beuker, F.J.; Brugman, E.; Roede, M.J.; Verloove-Vanhorick, S.P.; Wit, J.M. Continuing positive secular growth change in the Netherlands 1955–1997. Pediatr. Res. 2000, 47, 316–323. [Google Scholar] [CrossRef]
- Moons, K.G.M.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.A.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Fansohoff, D.F.; Collins, G.S. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): Explanation and elaboration. Ann. Intern. Med. 2015, 162, 1. [Google Scholar] [CrossRef]
- Van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate imputation by chained equations in R. J. Stat. Software 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Shah, P.E.; Kaciroti, N.; Richards, B.; Lumeng, J.C. Gestational age and kindergarten school readiness in a national sample of preterm infants. J. Pediatr. 2016, 178, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Kerstjens, J.M.; De Winter, A.F.; Bocca-Tjeertes, I.F.; Bos, A.F.; Reijneveld, S.A. Risk of developmental delay increases exponentially as gestational age of preterm infants decreases: A cohort study at age 4 years. Dev. Med. Child. Neurol. 2012, 54, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Van Dokkum, N.H.; De Kroon, M.L.; Bos, A.F.; Reijneveld, S.A.; Kerstjens, J.M. Attainment of gross motor milestones by preterm children with normal development upon school entry. Early Hum. Dev. 2018, 119, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Council on Children with Disabilities; Section on Developmental Behavioral Pediatrics; Bright Futures Steering Committee; Medical Home Initiatives for Children with Special Needs Project Advisory Committee. Identifying infants and young children with developmental disorders in the medical home: An algorithm for developmental surveillance and screening. Pediatrics 2006, 118, 405–420. [Google Scholar] [CrossRef]
- Welten, M.; Wijga, A.H.; Hamoen, M.; Gehring, U.; Koppelman, G.H.; Twisk, J.W.R.; Raat, H.; Heymans, M.W.; De Kroon, M.L.A. Dynamic prediction model to identify young children at high risk of future overweight: Development and internal validation in a cohort study. Pediatr. Obes. 2020, 15, e12647. [Google Scholar] [CrossRef]
| Candidate Predictors | Descriptive Value | Data Available for n (%) |
|---|---|---|
| Perinatal Factors | ||
| Gestational age at birth in weeks, mean (SD) | 34.3 (4.05) | 1983 (100) |
| Gestational age 24–32 weeks, n (%) | 513 (26) | |
| Gestational age 32–36 weeks, n (%) | 627 (32) | |
| Gestational age 37–42 weeks, n (%) | 543 (27) | |
| Male sex, n (%) | 1065 (54) | 1982 (99.9) |
| SGA, n (%) | 226 (11) | 1983 (100) |
| Multiple birth, n (%) | 443 (22) | 1983 (100) |
| Apgar score at 5 min <7, n (%) | 73 (3.7) | 1326 (67) |
| Parental factors | ||
| Maternal educational level <12 years, n (%) | 1407 (71) | 1973 (99.5) |
| Pre-existing maternal obesity (BMI > 30 kg/m2), n (%) | 94 (4.7) | 770 (39) |
| Maternal smoking during pregnancy, n (%) | 297 (15) | 1338 (68) |
| Growth (0–2 years) | ||
| Weight for height z score 1 year, mean (SD) | −0.08 (1.09) | 1260 (64) |
| Weight for height z score 2 years, mean (SD) | −0.24 (1.06) | 1206 (61) |
| Developmental milestones (0–2 years) | ||
| Smiling, onset age in weeks, mean (SD) | 8.5 (3.51) | 540 (27) |
| Speaking 2 to 3 word sentences, n abnormal (%) | 287 (15) | 1382 (70) |
| Head lifting, n abnormal (%) | 335 (17) | 1019 (51) |
| Standing, n abnormal (%) | 353 (18) | 1088 (55) |
| Walking, onset age in months, mean (SD) | 15.5 (3.12) | 1239 (63) |
| Predictor | Categories or Unit of Measurement | Regression Coefficient | OR | 95%-CI of OR |
|---|---|---|---|---|
| Sex | 0 = female, 1 = male | 1.2 | 3.5 | 2.3–5.3 |
| Maternal educational level | 0 = 12+ years, 1 = <12 years | 0.8 | 2.3 | 1.4–3.8 |
| Maternal pre-existing obesity | 0 = BMI <30 kg/m2, 1 = BMI >30 kg/m2 | 0.6 | 1.9 | 0.9–4.0 |
| Smiling | Age in weeks | 0.1 | 1.1 | 1.0–1.2 |
| Speaking 2–3 word sentences (2 years) | 0 = yes, 1 = no | 1.7 | 5.5 | 3.4–8.7 |
| Standing (1 year) | 0 = yes, 1 = no | 0.9 | 2.5 | 1.4–4.4 |
| Weight for height 1 year | z score | −0.2 | 0.8 | 0.7–1.0 |
| Cut-off value | Sensitivity (%) | Specificity (%) | NPV (%) | PPV (%) |
|---|---|---|---|---|
| Probability 5% | 86 | 62 | 18 | 98 |
| Probability 10% | 73 | 80 | 27 | 97 |
| Probability 20% | 55 | 91 | 38 | 95 |
| Probability 30% | 38 | 96 | 47 | 94 |
| Probability 40% | 25 | 98 | 51 | 93 |
| Probability 50% | 14 | 99 | 55 | 92 |
| Variable | Unit | |
|---|---|---|
| Weight for height z score 1 year | z score | −0.09 |
| Smiling | Age in weeks | 13 |
| Maternal pre-existing obesity | 0 = no (BMI < 30), 1 = yes (BMI > 30) | 1 |
| Maternal educational level | 0 = 12+ years, 1 = <12 years | 1 |
| Standing | 0 = yes, 1 = no | 1 |
| Sex | 0 = female, 1 = male | 1 |
| Speaking 2–3 word sentences | 0 = yes, 1 = no | 1 |
| Probability (%) | 62.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Dokkum, N.H.; Reijneveld, S.A.; Heymans, M.W.; Bos, A.F.; de Kroon, M.L.A. Development of a Prediction Model to Identify Children at Risk of Future Developmental Delay at Age 4 in a Population-Based Setting. Int. J. Environ. Res. Public Health 2020, 17, 8341. https://doi.org/10.3390/ijerph17228341
van Dokkum NH, Reijneveld SA, Heymans MW, Bos AF, de Kroon MLA. Development of a Prediction Model to Identify Children at Risk of Future Developmental Delay at Age 4 in a Population-Based Setting. International Journal of Environmental Research and Public Health. 2020; 17(22):8341. https://doi.org/10.3390/ijerph17228341
Chicago/Turabian Stylevan Dokkum, Nienke H., Sijmen A. Reijneveld, Martijn W. Heymans, Arend F. Bos, and Marlou L. A. de Kroon. 2020. "Development of a Prediction Model to Identify Children at Risk of Future Developmental Delay at Age 4 in a Population-Based Setting" International Journal of Environmental Research and Public Health 17, no. 22: 8341. https://doi.org/10.3390/ijerph17228341
APA Stylevan Dokkum, N. H., Reijneveld, S. A., Heymans, M. W., Bos, A. F., & de Kroon, M. L. A. (2020). Development of a Prediction Model to Identify Children at Risk of Future Developmental Delay at Age 4 in a Population-Based Setting. International Journal of Environmental Research and Public Health, 17(22), 8341. https://doi.org/10.3390/ijerph17228341






