COVID-19 Emergency Management: From the Reorganization of the Endoscopy Service to the Verification of the Reprocessing Efficacy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Evaluation of the Effectiveness of the Reprocessing Process
2.3. Virological Analysis
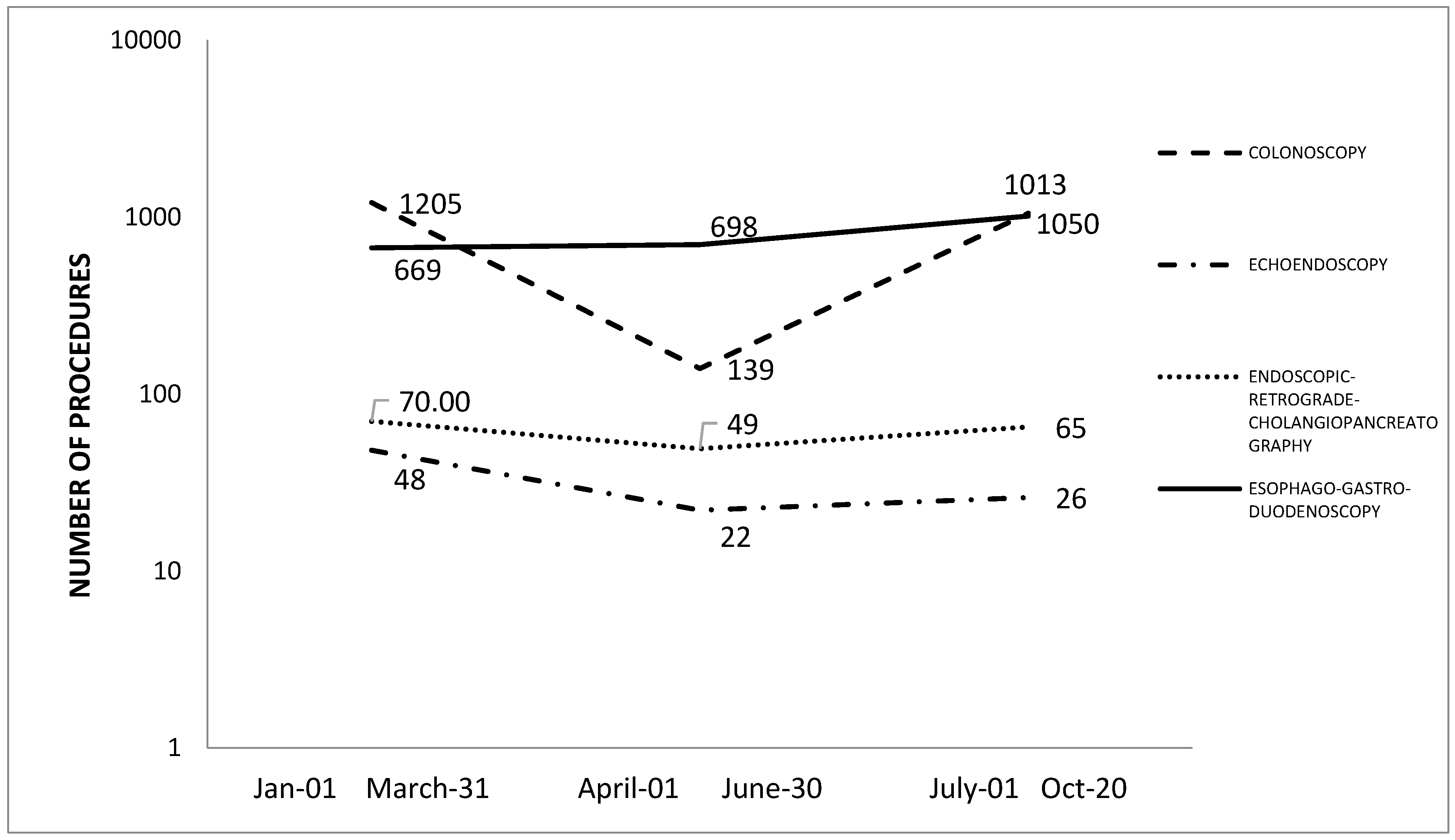
3. Results
How the Endoscopy Service Was Reorganized during the COVID-19 Pandemic
- The identification of endoscopes (colonoscopes, gastroscopes, and bronchoscopes), endoscopy columns, and electrosurgical units dedicated for use on COVID-19 patients. Columns were covered by a disposable sheath and at the end of each procedure, the column was subjected to sanitization in accordance with the manufacturer’s instructions. For the disinfection of compatible surfaces, 0.5% sodium hypochlorite, prepared at the moment, was used, while for the monitor, disposable wipes impregnated with 70% isopropyl alcohol were used. Wipes were chosen on the basis of their compliance with the EN 14476:2019 standard [18]. Each column was equipped with a single-patient irrigation bottle and accessory tubing. The transport of the endoscopes in the reprocessing room was organized in dedicated containers and identified with an appropriate color code and risk identification. Care was taken to ensure that the exterior of the containers was not contaminated when loading.
- For the phase involving pre-cleaning of the endoscope, which is conducted at the patient’s bed immediately after the procedure, and for the manual cleaning phase performed in the reprocessing room, the use of enzymatic detergent with disinfectant activity was recommended. The certification of reduction of the viral load according to the EN 14476:2019 standard was required in order to reduce the level of the endoscope contamination, and consequently, to prevent the formation of infectious aerosols, in particular, in the irrigation and brushing of the internal channels phases [1,10]. Disposable valves were already in use before the COVID-19 emergency. The staff were equipped with the appropriate PPE, which was set up inside the dedicated disposable kit. Due to the limited availability of PPE, face shields were disinfected after use by applying 0.5% sodium hypochlorite, prepared at the moment. In the cleaning phase, the automatic irrigation pump was not recommended in order to avoid contamination since the endoscopy unit did not yet have sterilizable fittings for endoscopes that were potentially contaminated with SARS-CoV-2.
- The leak testing was not recommended in wet conditions due to the risk of aerosol generation. For this reason, dry leak testing was performed in the procedure room while staff were still wearing PPE. Confirmation of a leak test was obtained subsequently within the washer-disinfector cycle.
- Two washer-disinfectors (Soluscope series 4, Soluscope, Aubagne, France) were dedicated to the high-disinfection phase. These machines comply with ISO 15883-1 and ISO 15883-4; therefore, they also guarantee efficacy (high disinfection) against viruses, in particular, on adenovirus type 5, poliovirus type 1, and bovine parvovirus Haden strain [19,20]. The manufacturer also provided the certification of virucidal activity according to the EN 14476+A1:2015 standard [18]. The intensive high-disinfection cycle was recommended for use. It involves double-washing with enzymatic detergent and high disinfection with peracetic acid at 900 ppm for 3 min at 40 °C. Regarding bronchoscopes, based on their compatibility, hydrogen peroxide gas plasma sterilization (59% H2O2, 55 min, 45 °C) was recommended.
- For the storage phase, endoscope storage drying cabinets (DSC8000-Soluscope, Soluscope Aubagne, France) that conformed to ISO 16442:201519 were identified as suitable because they allowed for better drying of the internal channels of the endoscopes and were equipped with a UV-C system (254 nm), which guarantees the hygienic quality of the storage environment [26]. Specific drawers and connectors were identified for endoscopes that were dedicated to COVID-19 patients.
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gralnek, I.M.; Hassan, C.; Beilenhoff, U.; Antonelli, G.; Ebigbo, A.; Pellisè, M.; Arvanitakis, M.; Bhandari, P.; Bisschops, R.; van Hooft, J.E.; et al. ESGE and ESGENA Position Statement on gastrointestinal endoscopy and the COVID-19 pandemic. Endoscopy 2020, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.M.; Midgley, C.M.; Dratch, A.; Fenstersheib, M.; Haupt, T.; Holshue, M.; Ghinai, I.; Jarashow, M.C.; Lo, J.; McPherson, T.D.; et al. Active monitoring of persons exposed to patients with confirmed COVID-19—United States, January–February 2020. MMWR Morb. Mortal. Wkly. Rep. 2020. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for patients with Suspected or Confirmed Disease (COVID-19) in Healthcare settings. Available online: https://www.cdc.gov/coronavirus/2019-ncov/html (accessed on 28 July 2020).
- Bai, Y.; Yao, L.; Wei, T.; Tian, F.; Jin, D.; Chen, L.; Wang, M. Presumed asymptomatic carrier transmission of COVID-19. JAMA 2020, 323, 1406–1407. [Google Scholar] [CrossRef] [PubMed]
- Rothe, C.; Schunk, M.; Sothmann, P.; Bretzel, G.; Froeschl, G.; Wallrauch, C.; Zimmer, T.; Thiel, V.; Janke, C.; Guggemos, W.; et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020, 382, 970–971. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, D.R.; Lim, W.S.; Rintoul, R.; Navani, N.; Fuller, L.; Woolhouse, I.; Evison, M.; Booton, R.; Janes, S.; Thakrar, R.; et al. Recommendations for Day Case Bronchoscopy Services during the COVID-19 Pandemic. Version 2: Services during the Restoration and Recovery COVID-19 Endemic Phase. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjKtbTTlujsAhUOWa0KHdhmCFQQFjAAegQIBBAC&url=https%3A%2F%2Fwww.brit-thoracic.org.uk%2Fdocument-library%2Fquality-improvement%2Fcovid-19%2Fbronchoscopy-services-during-the-covid-pandemic%2F&usg=AOvVaw1NixS3HoloRQp0Jf95HAoT (accessed on 4 November 2020).
- American Association for Bronchology and Interventional Pulmonology (AABIP): Statement on the Use of Bronchoscopy and Respiratory Specimen Collection in Patients with Suspected or Confirmed COVID-19 Infection. Available online: https://aabronchology.org/wp-content/uploads/2020/03/2020-AABIP-Statement-on-Bronchoscopy-COVID.GAE-updated-Version.pdf (accessed on 16 October 2020).
- Perisetti, A.; Gajendran, M.; Boregowda, U.; Bansal, P.; Goyal, H. COVID-19 and gastrointestinal endoscopies: Current insights and emergent strategies. Dig. Endosc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, D.; Yang, P.; Poon, L.L.M.; Wang, Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020, 20, 411–412. [Google Scholar] [CrossRef]
- Repici, A.; Maselli, R.; Colombo, M.; Gabbiadini, R.; Spadaccini, M.; Anderloni, A.; Carrara, S.; Fugazza, A.; di Leo, M.; Galtieri, P.A.; et al. Coronavirus (COVID-19) outbreak: What the department of endoscopy should know. Gastrointest. Endosc. 2020, 92, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Johnston, E.R.; Habib-Bein, N.; Dueker, J.M.; Quiroz, B.; Corsaro, E.; Ambrogio, M.; Kingsley, M.; Papachristou, G.I.; Kreiss, C.; Khalid, A. Risk of bacterial exposure to the endoscopists face during endoscopy. Gastrointest. Endosc. 2019, 89, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Siegel, J.D.; Rhinehart, E.; Chiarello, L.; Health Care Infection Control Practices Advisory Committee Health Care Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: Preventing transmission of infectious agents in health care settings. Am. J. Infect. Control. 2007, 35, S65. [Google Scholar] [CrossRef] [PubMed]
- UNI EN 14885: 2019 Disinfettanti Chimici ed Antisettici—Applicazione delle Norme Europee per i Disinfettanti Chimici e gli Antisettici. Available online: https://translate.googleusercontent.com/translate_c?depth=1&hl=en&prev=search&pto=aue&rurl=translate.google.com&sl=it&sp=nmt4&u=https://www.certifico.com/normazione/358-news-normazione/10589-uni-en-14885-2019-norme-europee-per-i-disinfettanti-chimici&usg=ALkJrhih9fUIUAW2SbI41JOvlUc4UDCCPg (accessed on 4 April 2020).
- Ofstead, C.L.; Hopkins, K.M.; Buro, B.L.; Eiland, J.E.; Wetzler, H.P. Challenges in achieving effective high-level disinfection in endoscope reprocessing. Am. J. Infect. Control. 2020, 48, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.B.; Muscarella, L.F. Current issues in endoscope reprocessing and infection control during gastrointestinal endoscopy. World J. Gastroenterol. 2006, 12, 3953–3964. [Google Scholar] [CrossRef] [PubMed]
- Kovaleva, J. Infectious complications in gastrointestinal endoscopy and their prevention. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 689–704. [Google Scholar] [CrossRef] [PubMed]
- Beilenhoff, U.; Biering, H.; Blum, R.; Brljak, J.; Cimbro, M.; Dumonceau, J.M.; Hassan, C.; Jung, M.; Kampf, B.; Neumann, C.; et al. Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA)—Update 2018. Endoscopy 2018, 50, 1205–1234. [Google Scholar] [CrossRef] [PubMed]
- UNI EN 14476: 2019 Chemical Disinfectants and Antiseptics. Quantitative Suspension Test for the Evaluation of Virucidal Activity in the Medical Area. Test Method and Requirements (Phase 2/Step 1). Available online: https://standards.iteh.ai/catalog/standards/cen/10065fd9-2d40-4f02-bccd-48206b72f108/en-14476-2013a2-2019 (accessed on 4 April 2020).
- UNI EN ISO 15883-1:2014 Washer-Disinfectors—Part 1: General Requirements, Terms and Definitions and Tests. Available online: https://infostore.saiglobal.com/en-us/Standards/UNI-EN-ISO-15883-1-2014-1080156_SAIG_UNI_UNI_2516495/ (accessed on 10 April 2020).
- UNI EN ISO 15883-4:2019 Washer-disinfectors—Part 4: Requirements and Tests for Washer-Disinfectors Employing Chemical Disinfection for Thermolabile Endoscopes. Available online: https://www.iso.org/standard/63696.html (accessed on 15 April 2020).
- Sinonquel, P.; Roelandt, P.; Demedts, I.; van Gerven, L.; Vandenbriele, C.; Wilmer, A.; van Wijngaerden, E.; Bisschops, R. COVID-19 and gastrointestinal endoscopy: What should be taken into account? Dig. Endosc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Duodenoscope Surveillance Sampling & Culturing Reducing the Risks of Infection. Available online: https://www.fda.gov/downloads/MedicalDevices/ProductsandMedicalProcedures/ReprocessingofReusableMedicalDevices/UCM597949.pdf (accessed on 11 September 2020).
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.W.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Word Health Organization (WHO). Collecting, Preserving and Shipping Specimens for the Diagnosis of Avian Influenza A(H5N1) Virus Infection. Guide for Field Operations. 2015. Available online: https://www.who.int/csr/resources/publications/surveillance/WHO_CDS_EPR_ARO_2006_1/en/ (accessed on 13 June 2020).
- Word Health Organization (WHO). Cleaning and Disinfection of Environmental Surfaces in the Context of COVID-19. Available online: https://www.who.int/publications/i/item/cleaning-and-disinfection-of-environmental-surfaces-inthe-context-of-covid-19 (accessed on 21 March 2020).
- UNI EN 16442: 2015: Controlled Environment Storage Cabinet for Processed Thermolabile Endoscopes. Available online: https://standards.iteh.ai/catalog/standards/cen/73067757-7a82-4998-858c-0b0dd1d644a7/en-16442-2015 (accessed on 16 April 2020).
- NHS England and Improvement. Advice to Trusts on Maintaining Cancer Treatment during the COVID-19 Response. Available online: https://www.england.nhs.uk/coronavirus/publication/advice-to-trusts-on-maintaining-cancer-treatment-during-the-covid-19-response/ (accessed on 14 October 2020).
- Birnie, G.G.; Quigley, E.M.; Clements, G.B.; Follet, E.A.; Watkinson, G. Endoscopic transmission of hepatitis B virus. Gut 1983, 24, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.R.; Fout, G.S.; Johnson, C.H.; White, K.M.; Parshionikar, S.U. Propidium monoazide reverse transcriptase PCR and RT-qPCR for detecting infectious enterovirus and norovirus. J. Virol. Methods 2015, 219, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Valeriani, F.; Agodi, A.; Casini, B.; Karen, M.W.; Sandhya, U.P. Potential testing of reprocessing procedures by real-time polymerase chain reaction: A multicenter study of colonoscopy devices. Am. J. Infect. Control. 2018, 46, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Covid-19: Disinfectants and sanitisers are changing microbiomes. BMJ 2020, 370. [Google Scholar] [CrossRef] [PubMed]
| Brand | Type of Endoscope and Model | Age | Date of Use | Date of Reprocessing | Date of Sampling | Sites of Sampling | SARS-CoV-2 RNA |
|---|---|---|---|---|---|---|---|
| Olympus | Gastroscope | 13 | 24.04.20 | 24.04.20 | 24.04.20 | eSwab COPAN 1 | Negative |
| GIF800 | eSwab COPAN 2 | Negative | |||||
| GIF800 | |||||||
| Olympus | Gastroscope | 13 | 30.04.20 | 30.04.20 | 30.04.20 | eSwab COPAN 1 | Negative |
| GIF800 | eSwab COPAN 2 | Negative | |||||
| Liquid | Negative | ||||||
| Pentax | Colonscope | 8 | 23.04.20 | 23.04.20 | 24.04.20 | eSwab COPAN 1 | Negative |
| EC878 | eSwab COPAN 2 | Negative | |||||
| Liquid | Negative | ||||||
| Olympus | Colonscope | 15 | 24.04.20 | 24.04.20 | 27.04.20 | eSwab COPAN 1 | Negative |
| CF BIC 094 | eSwab COPAN 2 | Negative | |||||
| Liquid | Negative | ||||||
| Olympus | Gastroscope | 18 | 24.04.20 | 24.04.20 | 27.04.20 | eSwab COPAN 1 | Negative |
| GIF BIC 238 | eSwab COPAN 2 | Negative | |||||
| Liquid | Negative | ||||||
| Pentax | Gastroscope | 8 | 29.04.20 | 29.04.20 | 30.04.20 | eSwab COPAN 1 | Negative |
| EG 040 | eSwab COPAN 2 | Negative | |||||
| Liquid | Negative | ||||||
| FujiFilm | Gastroscope | 1 | 05.05.20 | 05.05.20 | 06.05.20 | eSwab COPAN 1 | Negative |
| FG098 | eSwab COPAN 2 | Negative | |||||
| Liquid | Negative | ||||||
| Pentax | Colonscope | 14 | 06.05.20 | 06.05.20 | 07.05.20 | eSwab COPAN 1 | Negative |
| EC333 | eSwab COPAN 2 | Negative | |||||
| Liquid | Negative | ||||||
| Pentax | Colonscope | 14 | 06.05.20 | 06.05.20 | 07.05.20 | eSwab COPAN 1 | Negative |
| EC 306 | eSwab COPAN 2 | Negative | |||||
| Liquid | Negative | ||||||
| Olympus | Colonscope | 5 | 21.05.20 | 21.05.20 | 22.05.20 | eSwab COPAN 1 | Negative |
| GIF241 | eSwab COPAN 2 | Negative | |||||
| Liquid | Negative | ||||||
| Olympus | Broncoscope | 11 | 24.04.20 | 24.04.20 | 27.04.20 | eSwab COPAN 1 | Negative |
| BF1T180 | eSwab COPAN 2 | Negative | |||||
| Liquid | Negative | ||||||
| Olympus | Broncoscope | 11 | 29.04.20 | 29.04.20 | 30.04.20 | eSwab COPAN 1 | Negative |
| BF1T180 | eSwab COPAN 2 | Negative | |||||
| Liquid | Negative | ||||||
| Olympus | Broncoscope | 11 | 05.05.20 | 05.05.20 | 05.05.20 | eSwab COPAN 1 | Negative |
| BF1T180 | eSwab COPAN 2 | Negative | |||||
| Liquid | Negative | ||||||
| Olympus | Broncoscope | 9 | 20.05.20 | 20.05.20 | 22.05.20 | eSwab COPAN 1 | Negative |
| BF1T180 | eSwab COPAN 2 | Negative | |||||
| Liquid | Negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casini, B.; Tuvo, B.; Maggi, F.; Del Magro, G.; Ribechini, A.; Costa, A.L.; Totaro, M.; Baggiani, A.; Gemignani, G.; Privitera, G. COVID-19 Emergency Management: From the Reorganization of the Endoscopy Service to the Verification of the Reprocessing Efficacy. Int. J. Environ. Res. Public Health 2020, 17, 8142. https://doi.org/10.3390/ijerph17218142
Casini B, Tuvo B, Maggi F, Del Magro G, Ribechini A, Costa AL, Totaro M, Baggiani A, Gemignani G, Privitera G. COVID-19 Emergency Management: From the Reorganization of the Endoscopy Service to the Verification of the Reprocessing Efficacy. International Journal of Environmental Research and Public Health. 2020; 17(21):8142. https://doi.org/10.3390/ijerph17218142
Chicago/Turabian StyleCasini, Beatrice, Benedetta Tuvo, Fabrizio Maggi, Giuliana Del Magro, Alessandro Ribechini, Anna Laura Costa, Michele Totaro, Angelo Baggiani, Giulia Gemignani, and Gaetano Privitera. 2020. "COVID-19 Emergency Management: From the Reorganization of the Endoscopy Service to the Verification of the Reprocessing Efficacy" International Journal of Environmental Research and Public Health 17, no. 21: 8142. https://doi.org/10.3390/ijerph17218142
APA StyleCasini, B., Tuvo, B., Maggi, F., Del Magro, G., Ribechini, A., Costa, A. L., Totaro, M., Baggiani, A., Gemignani, G., & Privitera, G. (2020). COVID-19 Emergency Management: From the Reorganization of the Endoscopy Service to the Verification of the Reprocessing Efficacy. International Journal of Environmental Research and Public Health, 17(21), 8142. https://doi.org/10.3390/ijerph17218142






