Levels of Anti-Citrullinated Protein Antibodies and Rheumatoid Factor, Including IgA Isotypes, and Articular Manifestations in Ulcerative Colitis and Crohn’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Laboratory Measurements
2.3. Statistical Analysis
3. Results
3.1. General Characteristics
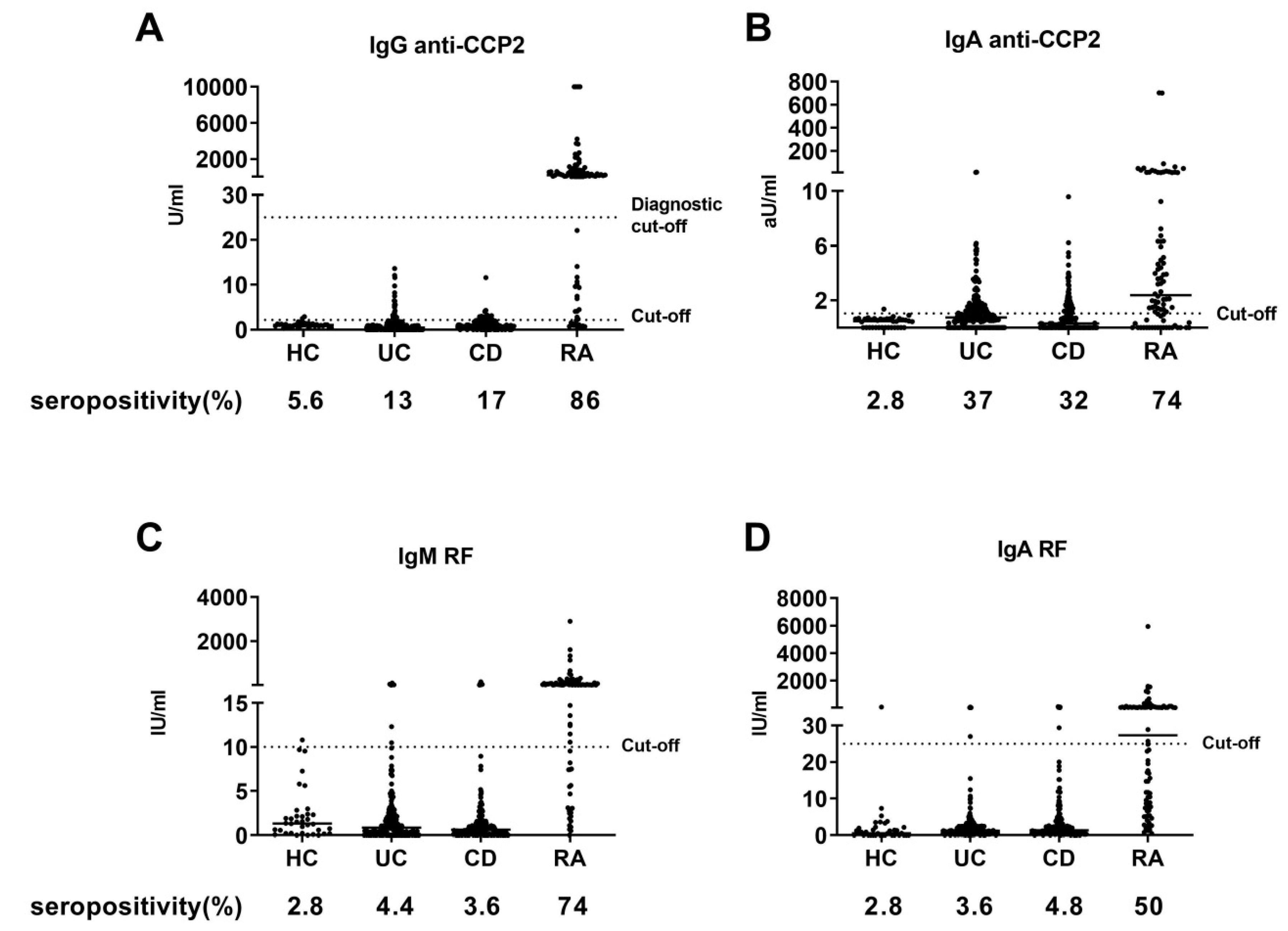
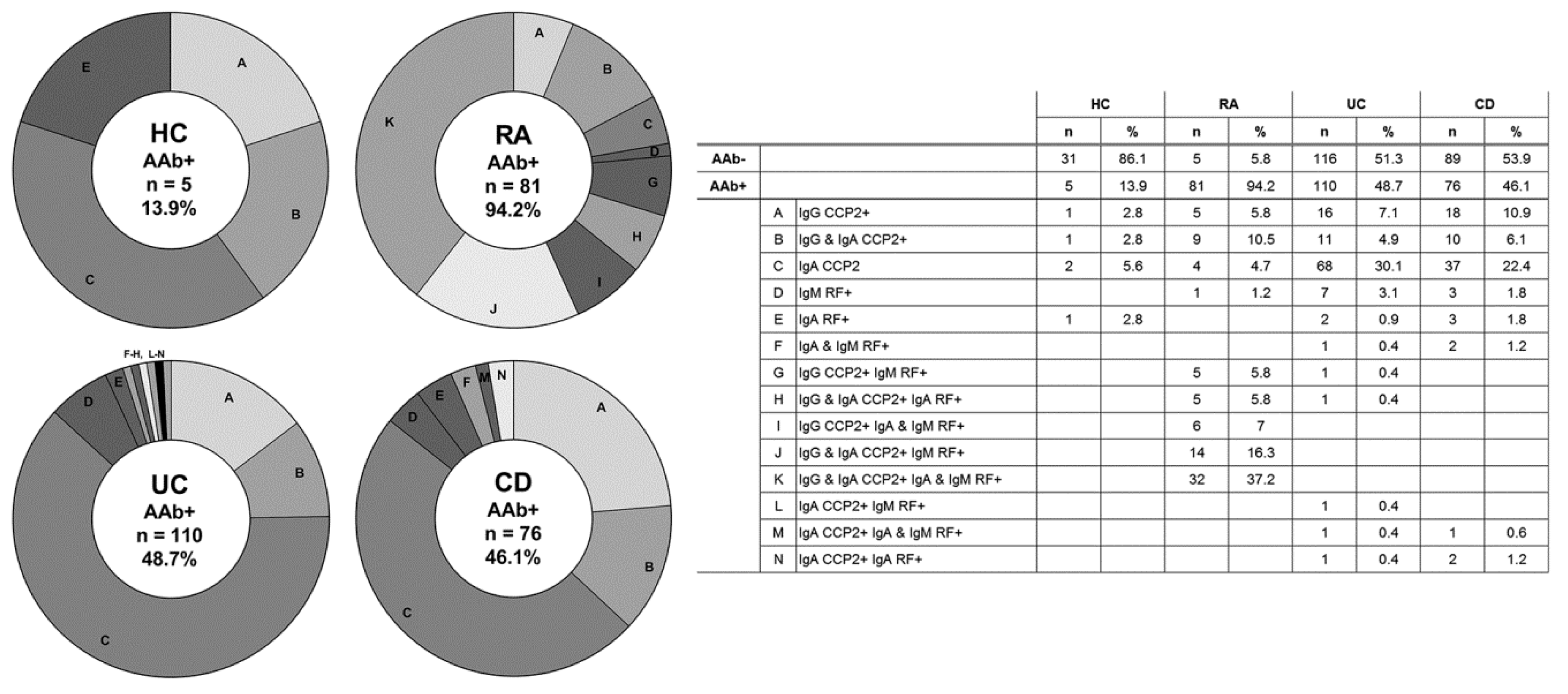
3.2. Autoantibody Seropositivity
3.3. Rheumatic Manifestations and Arthritis Autoantibody Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hendrickson, B.A.; Gokhale, R.; Cho, J.H. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin. Microbiol. Rev. 2002, 15, 79–94. [Google Scholar] [CrossRef]
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Blanchard, J.F.; Rawsthorne, P.; Yu, N. The prevalence of extraintestinal diseases in inflammatory bowel disease: A population-based study. Am. J. Gastroenterol. 2001, 96, 1116–1122. [Google Scholar] [CrossRef]
- Algaba, A.; Guerra, I.; Ricart, E.; Iglesias, E.; Mañosa, M.; Gisbert, J.P.; Guardiola, J.; Mínguez, M.; Castro, B.; De Francisco, R.; et al. Extraintestinal manifestations in patients with inflammatory bowel disease: Study based on the ENEIDA registry. Dig. Dis. Sci. 2020. Online ahead of print. [Google Scholar] [CrossRef]
- Quinton, J.F.; Sendid, B.; Reumaux, D.; Duthilleul, P.; Cortot, A.; Grandbastien, B.; Charrier, G.; Targan, S.R.; Colombel, J.F.; Poulain, D. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: Prevalence and diagnostic role. Gut 1998, 42, 788–791. [Google Scholar] [CrossRef]
- Elkadri, A.A.; Stempak, J.M.; Walters, T.D.; Lal, S.; Griffiths, A.M.; Steinhart, A.H.; Silverberg, M.S. Serum antibodies associated with complex inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 1499–1505. [Google Scholar] [CrossRef]
- Torres, J.; Petralia, F.; Sato, T.; Wang, P.; Telesco, S.E.; Choung, R.S.; Strauss, R.; Li, X.J.; Laird, R.M.; Gutierrez, R.L.; et al. Serum biomarkers identify patients who will develop inflammatory bowel diseases up to 5 years before diagnosis. Gastroenterology 2020, 159, 96–104. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J.S. Diagnosis and management of rheumatoid arthritis: A review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef]
- Van der Helm-van Mil, A.H.; Verpoort, K.N.; Breedveld, F.C.; Toes, R.E.M.; Huizinga, T.W.J. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res. Ther. 2005, 7, R949–R958. [Google Scholar] [CrossRef]
- Nielen, M.M.J.; Van Schaardenburg, D.; Reesink, H.W.; Van De Stadt, R.J.; Van Der Horst-Bruinsma, I.E.; De Koning, M.H.M.T.; Habibuw, M.R.; Vandenbroucke, J.P.; Dijkmans, B.A.C. Specific autoantibodies precede the symptoms of rheumatoid arthritis: A study of serial measurements in blood donors. Arthritis Rheum. 2004, 50, 380–386. [Google Scholar] [CrossRef]
- Makrygiannakis, D.; Klint, E.A.; Lundberg, I.E.; Lofberg, R.; Ulfgren, A.-K.; Klareskog, L.; Catrina, A.I. Citrullination is an inflammation-dependent process. Ann. Rheum. Dis. 2006, 65, 1219–1222. [Google Scholar] [CrossRef]
- Dragoni, G.; De Hertogh, G.; Vermeire, S. The role of citrullination in inflammatory bowel disease: A neglected player in triggering inflammation and fibrosis? Inflamm. Bowel Dis. 2020, izaa095, Online ahead of print. [Google Scholar] [CrossRef]
- Demoruelle, M.K.; Deane, K.D.; Holers, V.M. When and where does inflammation begin in rheumatoid arthritis? Curr. Opin. Rheumatol. 2014, 26, 64–71. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 2013, 5, 178ra40. [Google Scholar] [CrossRef]
- Rangel-Moreno, J.; Hartson, L.; Navarro, C.; Gaxiola, M.; Selman, M.; Randall, T.D. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J. Clin. Investig. 2006, 116, 3183–3194. [Google Scholar] [CrossRef]
- Haga, H.J.; Palm, Ø.; Peen, E. Prevalence of IgA class antibodies to cyclic citrullinated peptide in patients with inflammatory bowel disease (IBD). Clin. Rheumatol. 2011, 30, 955–957. [Google Scholar] [CrossRef]
- Valesini, G.; Gerardi, M.C.; Iannuccelli, C.; Pacucci, V.A.; Pendolino, M.; Shoenfeld, Y. Citrullination and autoimmunity. Autoimmun. Rev. 2015, 14, 490–497. [Google Scholar] [CrossRef]
- Koutroubakis, I.E.; Bourikas, L.; Kouroumalis, E.A.; Drygiannakis, I.; Drygiannakis, D.; Karmiris, K. Antibodies against cyclic citrullinated peptide (CCP) in inflammatory bowel disease patients with or without arthritic manifestations. Inflamm. Bowel Dis. 2007, 13, 504–505. [Google Scholar] [CrossRef]
- Papamichael, K.; Tsirogianni, A.; Papasteriades, C.; Mantzaris, G.J. Low prevalence of antibodies to cyclic citrullinated peptide in patients with inflammatory bowel disease regardless of the presence of arthritis. Eur. J. Gastroenterol. Hepatol. 2010, 22, 705–709. [Google Scholar] [CrossRef]
- Al-Jarallah, K.F.; Shehab, D.; Al-Attiyah, R.; Al-Azmi, W.; Al-Fadli, A.; Haider, M.Z.; Panaccione, R.; Ghosh, S. Antibodies to mutated citrullinated vimentin and anti-cyclic citrullinated peptide antibodies in inflammatory bowel disease and related arthritis. Inflamm. Bowel Dis. 2012, 18, 1655–1662. [Google Scholar] [CrossRef]
- Van De Stadt, L.A.; Van Der Horst, A.R.; De Koning, M.H.M.T.; Bos, W.H.; Wolbink, G.J.; Van De Stadt, R.J.; Pruijn, G.J.M.; Dijkmans, B.A.C.; Van Schaardenburg, D.; Hamann, D. The extent of the anti-citrullinated protein antibody repertoire is associated with arthritis development in patients with seropositive arthralgia. Ann. Rheum. Dis. 2010, 70, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Janssen, K.M.J.; De Smit, M.J.; Brouwer, E.; de Kok, F.A.; Kraan, J.; Altenburg, J.; Verheul, M.K.; Trouw, L.A.; Van Winkelhoff, A.J.; Vissink, A.; et al. Rheumatoid arthritis–associated autoantibodies in non–rheumatoid arthritis patients with mucosal inflammation: A case–control study. Arthritis Res. Ther. 2015, 17, 174. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, M.A.; Westra, J.; Van Riel, P.; Limburg, P.C.; Van Rijswijk, M.H. IgM, IgA, and IgG rheumatoid factors in early rheumatoid arthritis predictive of radiological progression? Scand. J. Rheumatol. 1995, 24, 146–153. [Google Scholar] [CrossRef]
- Harvey, G.P.; Fitzsimmons, T.R.; Dhamarpatni, A.A.; Marchant, C.; Haynes, D.R.; Bartold, M. Expression of peptidylarginine deiminase-2 and -4, citrullinated proteins and anti-citrullinated protein antibodies in human gingiva. J. Periodontal. Res. 2012, 48, 252–261. [Google Scholar] [CrossRef]
- Nesse, W.; Westra, J.; Wal, J.E.; Abbas, F.; Nicholas, A.P.; Vissink, A.; Brouwer, E. The periodontium of periodontitis patients contains citrullinated proteins which may play a role in ACPA (anti-citrullinated protein antibody) formation. J. Clin. Periodontol. 2012, 39, 599–607. [Google Scholar] [CrossRef]
- Nielsen, S.F.; Bojesen, S.E.; Schnohr, P.; Nordestgaard, B.G. Elevated rheumatoid factor and long term risk of rheumatoid arthritis: A prospective cohort study. BMJ 2012, 345, e5244. [Google Scholar] [CrossRef]
- Palosuo, T.; Tilvis, R.; Strandberg, T.; Aho, K. Filaggrin related antibodies among the aged. Ann. Rheum. Dis. 2003, 62, 261–263. [Google Scholar] [CrossRef]
- Vermeire, S.; Van Assche, G.; Rutgeerts, P. The role of C-reactive protein as an inflammatory marker in gastrointestinal diseases. Nat. Clin. Pr. Gastroenterol. Hepatol. 2005, 2, 580–586. [Google Scholar] [CrossRef]
- Klareskog, L.; Rönnelid, J.; Lundberg, K.; Padyukov, L.; Alfredsson, L. Immunity to citrullinated proteins in rheumatoid arthritis. Annu. Rev. Immunol. 2008, 26, 651–675. [Google Scholar] [CrossRef]
- Kang, J.; Jeong, S.H.; Lee, K.; Park, N.; Jung, H.; Lee, K.; Ju, J.H. Exacerbation of symptomatic arthritis by cigarette smoke in experimental arthritis. PLoS ONE 2020, 15, e0230719. [Google Scholar] [CrossRef]
- Mortensen, J.H.; Godskesen, L.E.; Jensen, M.D.; Van Haaften, W.T.; Klinge, L.; Olinga, P.; Dijkstra, G.; Kjeldsen, J.; Karsdal, M.A.; Bay-Jensen, A.C.; et al. Fragments of citrullinated and MMP-degraded vimentin and MMP-degraded type III collagen are novel serological biomarkers to differentiate crohn’s disease from ulcerative colitis. J. Crohns Colitis 2015, 9, 863–872. [Google Scholar] [CrossRef]
- Klareskog, L.; Stolt, P.; Lundberg, K.; Källberg, H.; Bengtsson, C.; Grunewald, J.; Harris, H.E.; Ulfgren, A.-K.; Rantapää-Dahlqvist, S.; Eklund, A.; et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA–DR (shared epitope)–restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006, 54, 38–46. [Google Scholar] [CrossRef]
- Hensvold, A.H.; Magnusson, P.K.E.; Joshua, V.; Hansson, M.; Israelsson, L.; Ferreira, R.; Jakobsson, P.J.; Holmdahl, R.; Hammarström, L.; Malmström, V.; et al. Environmental and genetic factors in the development of anticitrullinated protein antibodies (ACPAs) and ACPA-positive rheumatoid arthritis: An epidemiological investigation in twins. Ann. Rheum. Dis. 2013, 74, 375–380. [Google Scholar] [CrossRef]
- Van Heemst, J.; Jansen, D.T.S.L.; Polydorides, S.; Moustakas, A.K.; Bax, M.; Feitsma, A.L.; Bontrop-Elferink, D.G.; Baarse, M.; Van Der Woude, D.; Wolbink, G.J.; et al. Crossreactivity to vinculin and microbes provides a molecular basis for HLA-based protection against rheumatoid arthritis. Nat. Commun. 2015, 6, 6681. [Google Scholar] [CrossRef]
- Ahmad, T.; Marshall, S.E.; Jewell, D. Genetics of inflammatory bowel disease: The role of the HLA complex. World J. Gastroenterol. 2006, 12, 3628–3635. [Google Scholar] [CrossRef]
- Ashton, J.J.; Latham, K.; Beattie, R.M.; Ennis, S. Review article: The genetics of the human leucocyte antigen region in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2019, 50, 885–900. [Google Scholar] [CrossRef]
- Dedmon, L.E. The genetics of rheumatoid arthritis. Rheumatology 2020, 59, 2661–2670. [Google Scholar] [CrossRef]
- Van Erp, S.J.H.; Verheul, M.K.; Nivine Levarht, E.W.; van der Reijden, J.J.; van der Heijde, D.; van Gaalen, F.A.; Hommes, D.W.; Norman, G.L.; Shums, Z.; Mahler, M.; et al. Absence of serological rheumatoid arthritis biomarkers in inflammatory bowel disease patients with arthropathies. Eur. J. Gastroenterol. Hepatol. 2017, 29, 345–348. [Google Scholar] [CrossRef]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef]
- Pittayanon, R.; Lau, J.T.; Leontiadis, G.I.; Tse, F.; Yuan, Y.; Surette, M.; Moayyedi, P. Differences in gut microbiota in patients with vs without inflammatory bowel diseases: A systematic review. Gastroenterol. 2020, 158, 930–946. [Google Scholar] [CrossRef]
- Schirmer, M.; Garner, A.; Vlamakis, H.; Xavier, R.J. Microbial genes and pathways in inflammatory bowel disease. Nat. Rev. Genet. 2019, 17, 497–511. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, W.; Lan, P.; Mou, X. The microbiome in inflammatory bowel diseases: From pathogenesis to therapy. Protein Cell 2020, 1–15. [Google Scholar] [CrossRef]
- Reyes-Castillo, Z.; Valdés-Miramontes, E.; Llamas-Covarrubias, M.; Muñoz-Valle, J.F. Troublesome friends within us: The role of gut microbiota on rheumatoid arthritis etiopathogenesis and its clinical and therapeutic relevance. Clin. Exp. Med. 2020. Online ahead of print. [Google Scholar] [CrossRef]
- Rosenstein, E.D.; Greenwald, R.A.; Kushner, L.J.; Weissmann, G. Hypothesis: The Humoral Immune Response to Oral Bacteria Provides a Stimulus for the Development of Rheumatoid Arthritis. Inflamm. 2004, 28, 311–318. [Google Scholar] [CrossRef]
- Scher, J.U.; Ubeda, C.; Equinda, M.; Khanin, R.; Buischi, Y.; Viale, A.; Littman, D.R.; Pamer, E.G.; Bretz, W.A.; Abramson, S.B. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum. 2012, 64, 3083–3094. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef]
- Muñoz-Atienza, E.; Flak, M.B.; Sirr, J.; Paramonov, N.; Aduse-Opoku, J.; Pitzalis, C.; Curtis, M. The P. gingivalis autocitrullinome is not a target for ACPA in early rheumatoid arthritis. J. Dent. Res. 2020, 99, 456–462. [Google Scholar] [CrossRef]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2013, 2, e01202. [Google Scholar] [CrossRef]
| Characteristics | Healthy Controls (HC) (n = 36) | Rheumatoid Arthritis (RA) Patients (n = 86) | Ulcerative Colitis (UC) Patients (n = 226) | Crohn’s Disease (CD) Patients (n = 165) | UC vs. CD p Value |
|---|---|---|---|---|---|
| Female, n (%) | 20 (55.6) | 60 (70) | 123 (54.4) | 105 (63.6) | 0.077 |
| Age, years, mean, (SD) | 34 (15) | 55 (11) | 42 (15) | 41 (15) | 0.606 |
| BMI, kg/m2, mean, (SD) a | – | – | 26.1 (4.6) | 24.8 (4.9) | 0.001 |
| Current or ever smoker, n (%) b | 8 (22.3) | 34 (39) | 129 (57.1) | 108 (67.1) | 0.006 |
| DAS28, median, IQR | – | 2.2 (1.7–2.8) | – | – | – |
| Disease duration, years, (median, IQR) | – | 5.5 (3–10) | 7 (4–13) | 9 (4–16) | 0.037 |
| Montréal classification | |||||
| Age at diagnosis, n (%) c | 0.092 | ||||
| A1 below 16 years | – | – | 20 (9.0) | 26 (16.2) | |
| A2 between 17 and 40 years | – | – | 146 (65.8) | 101 (62.7) | |
| A3 above 40 years | – | – | 56 (25.2) | 34 (21.1) | |
| Disease extent (E) and severity | |||||
| (S) in UC, n (%) d | |||||
| E1 ulcerative proctitis | – | – | 37 (17.0) | – | – |
| E2 left sided UC | – | – | 74 (33.9) | – | – |
| E3 extensive UC | – | – | 107 (49.1) | – | – |
| S0 clinical remission | – | – | 20 (9.3) | – | – |
| S1 mild UC | – | – | 68 (31.8) | – | – |
| S2 moderate UC | – | – | 72 (33.6) | – | – |
| S3 severe UC | – | – | 54 (25.2) | – | – |
| Disease location (L) and | |||||
| behavior (B) in CD, n (%) e | |||||
| L1 ileal | – | – | – | 57 (36.3) | – |
| L2 colonic | – | – | – | 32 (20.4) | – |
| L3 ileocolonic | – | – | – | 67 (42.7) | – |
| L4 isolated upper disease | – | – | – | 1 (0.6) | – |
| B1 non-stricturing, non-penetrating | – | – | – | 83 (51.2) | – |
| B2 stricturing | – | – | – | 56 (34.6) | – |
| B3 penetrating | – | – | – | 23 (14.2) | – |
| P perianal disease modifier | – | – | – | 45 (27.8) | – |
| Ulcerative Colitis | Crohn’s Disease | |||||
|---|---|---|---|---|---|---|
| Characteristics and manifestations | AAb− (n = 116) | AAb+ (n = 110) | p Value | AAb− (n = 89) | AAb+ (n = 76) | p Value |
| Female, n (%) | 67 (57.8) | 56 (50.9) | 0.35 | 55 (61.8) | 50 (65.8) | 0.624 |
| Age, years, mean (SD) | 44.1 (15) | 39.4 (14) | 0.016 | 43.6 (15) | 38.0 (14) | 0.017 |
| Ever smoker, n (%) | 70 (60.3) | 59 (53.6) | 0.348 | 60 (69.0) | 48 (64.9) | 0.616 |
| Active disease, n (%) a | 34 (29.3) | 35 (31.8) | 0.661 | 39 (47.6) | 29 (42.7) | 0.622 |
| Age at diagnosis, n (%) | 0.021 | 0.159 | ||||
| A1 below 16 years | 9 (7.8) | 11 (10.3) | 10 (11.2) | 16 (22.2) | ||
| A2 between 17 and 40 years | 68 (59.1) | 78 (72.9) | 60 (67.4) | 41 (56.9) | ||
| A3 above 40 years | 38 (33.0) | 18 (16.8) | 19 (21.3) | 15 (20.8) | ||
| Disease duration, years, median (IQR) | 7 (4–14) | 7 (4–12) | 0.425 | 11 (4–17) | 8.5 (4–15) | 0.264 |
| ESR, mm/h, median (IQR) | 11 (5–18) | 11 (5–18) | 0.974 | 9 (5–20) | 17.5 (8–24) | 0.009 |
| CRP, mg/l, median (IQR) | ≤3 (≤3–3) | ≤3 (≤3–3) | 0.865 | ≤3 (≤3–3) | ≤3 (≤3–8) | 0.008 |
| pANCA, seropositive (%) b | 51 (52.7) | 54 (61.4) | 0.239 | 20 (25.6) | 18 (29.5) | 0.702 |
| ASCA, seropositive (%) c | 24 (25.8) | 24 (27.3) | 0.867 | 48 (61.5) | 40 (69.0) | 0.582 |
| Arthropathies | ||||||
| Enthesitis, n | 0 | 1 | - | 3 | 0 | - |
| Arthritis, n | 0 | 0 | - | 0 | 1 | - |
| Inflammatory backpain, n | 0 | 0 | - | 3 | 0 | - |
| Arthralgia, n (%) d | 13 (14.7) | 21 (22.3) | 0.254 | 26 (32.5) | 24 (34.3) | 0.863 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janssen, K.M.J.; Hop, H.; Vissink, A.; Dijkstra, G.; de Smit, M.J.; Brouwer, E.; Westra, J. Levels of Anti-Citrullinated Protein Antibodies and Rheumatoid Factor, Including IgA Isotypes, and Articular Manifestations in Ulcerative Colitis and Crohn’s Disease. Int. J. Environ. Res. Public Health 2020, 17, 8054. https://doi.org/10.3390/ijerph17218054
Janssen KMJ, Hop H, Vissink A, Dijkstra G, de Smit MJ, Brouwer E, Westra J. Levels of Anti-Citrullinated Protein Antibodies and Rheumatoid Factor, Including IgA Isotypes, and Articular Manifestations in Ulcerative Colitis and Crohn’s Disease. International Journal of Environmental Research and Public Health. 2020; 17(21):8054. https://doi.org/10.3390/ijerph17218054
Chicago/Turabian StyleJanssen, Koen M. J., Hilde Hop, Arjan Vissink, Gerard Dijkstra, Menke J. de Smit, Elisabeth Brouwer, and Johanna Westra. 2020. "Levels of Anti-Citrullinated Protein Antibodies and Rheumatoid Factor, Including IgA Isotypes, and Articular Manifestations in Ulcerative Colitis and Crohn’s Disease" International Journal of Environmental Research and Public Health 17, no. 21: 8054. https://doi.org/10.3390/ijerph17218054
APA StyleJanssen, K. M. J., Hop, H., Vissink, A., Dijkstra, G., de Smit, M. J., Brouwer, E., & Westra, J. (2020). Levels of Anti-Citrullinated Protein Antibodies and Rheumatoid Factor, Including IgA Isotypes, and Articular Manifestations in Ulcerative Colitis and Crohn’s Disease. International Journal of Environmental Research and Public Health, 17(21), 8054. https://doi.org/10.3390/ijerph17218054






