Pediatrics Consequences of Caesarean Section—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Search Strategy
- Population: newborns and children who have delivered via cesarean section
- Intervention: cesarean section
- Comparison: any mode of delivery where reported
- Outcomes: respiratory diseases, asthma, obesity, overweight, diabetes mellitus type 1, neurological disorders
2.2. Statistical Analyses
3. Results
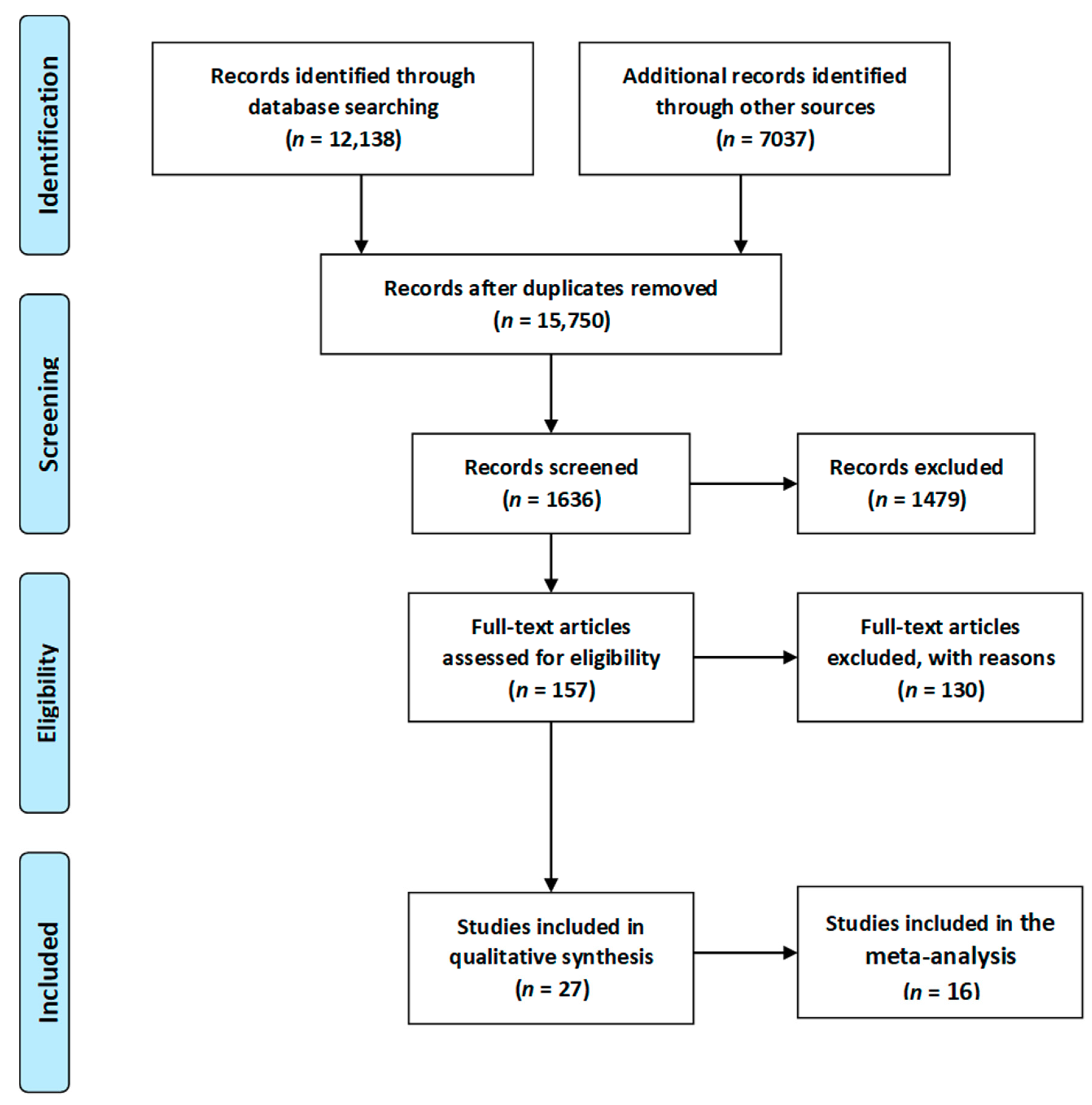
3.1. Study Selection Process
3.2. General Characteristics of the Studies Included
3.3. Caesarean Section and Respiratory Tract Infections—Meta-Analysis
3.4. Caesarean Section and Asthma—Meta-Analysis
3.5. Caesarean Section and Diabetes Mellitus Type 1—Meta-Analysis
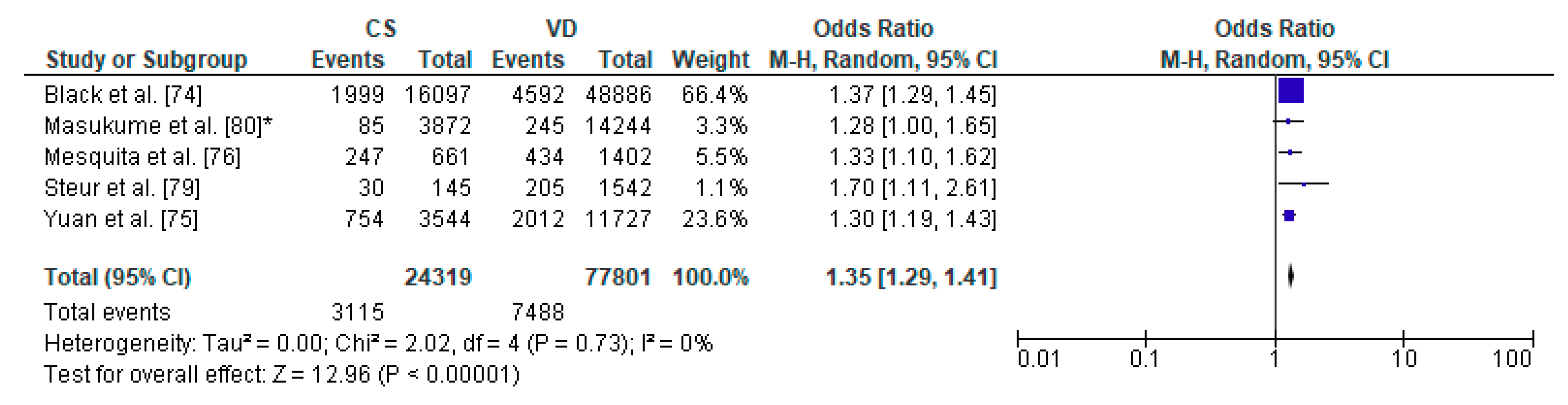
3.6. Caesarean Section and Increased Body Weight—Meta-Analysis
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Betrán, A.P.; Ye, J.; Moller, A.-B.; Zhang, J.; Gülmezoglu, A.M.; Torloni, M.R. The Increasing Trend in Caesarean Section Rates: Global, Regional and National Estimates: 1990–2014. PLoS ONE 2016, 11, e0148343. [Google Scholar] [CrossRef] [PubMed]
- Molina, G.; Weiser, T.G.; Lipsitz, S.R.; Esquivel, M.M.; Uribe-Leitz, T.; Azad, T.; Shah, N.; Semrau, K.; Berry, W.R.; Gawande, A.A.; et al. Relationship Between Cesarean Delivery Rate and Maternal and Neonatal Mortality. JAMA 2015, 314, 2263–2270. [Google Scholar] [CrossRef] [PubMed]
- Betran, A.P.; Torloni, M.R.; Zhang, J.J.; Gulmezoglu, A.M. WHO Working Group on Caesarean Section WHO Statement on Caesarean Section Rates. BJOG Int. J. Obstet. Gynaecol. 2015, 123, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Betran, A.P.; Vela, M.G.; Souza, J.P.; Zhang, J. Searching for the Optimal Rate of Medically Necessary Cesarean Delivery. Birth 2014, 41, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Betran, A.P.; Torloni, M.R.; Zhang, J.; Ye, J.; Mikolajczyk, R.; Deneux-Tharaux, C.; Oladapo, O.T.; Souza, J.P.; Tunçalp, Ö.; Vogel, J.P.; et al. What is the optimal rate of caesarean section at population level? A systematic review of ecologic studies. Reprod. Health 2015, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization. Available online: www.who.int/reproductivehealth/ (accessed on 6 September 2020).
- Wax, J.R.; Cartin, A.; Pinette, M.G.; Blackstone, J. Patient Choice Cesarean: An Evidence-Based Review. Obstet. Gynecol. Surv. 2004, 59, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Ecker, J. Elective Cesarean Delivery on Maternal Request. JAMA 2013, 309, 1930–1936. [Google Scholar] [CrossRef]
- UN-IGME. Levels and Trends in Child Mortality: Report 2018. Estimates Developed by the UN Inter-Agency Group for Child Mortality Estimation. New York, NY: UN Children’s Fund. 2018. Available online: https://www.unicef.org/publications/files/Child_Mortality_Report_2018.pdf (accessed on 6 September 2020).
- Bishop, D.; Dyer, R.A.; Maswime, S.; Rodseth, R.N.; Van Dyk, D.; Kluyts, H.-L.; Tumukunde, J.T.; Madzimbamuto, F.D.; Elkhogia, A.M.; Ndonga, A.K.N.; et al. Maternal and neonatal outcomes after caesarean delivery in the African Surgical Outcomes Study: A 7-day prospective observational cohort study. Lancet Glob. Health 2019, 7, e513–e522. [Google Scholar] [CrossRef]
- Curran, E.A.; Dalman, C.; Kearney, P.M.; Kenny, L.C.; Cryan, J.F.; Dinan, T.G.; Khashan, A.S. Association Between Obstetric Mode of Delivery and Autism Spectrum Disorder. JAMA Psychiatry 2015, 72, 935–942. [Google Scholar] [CrossRef]
- O’Neill, S.M.; Curran, E.A.; Dalman, C.; Kenny, L.C.; Kearney, P.M.; Clarke, G.; Cryan, J.F.; Dinan, T.G.; Khashan, A.S. Birth by Caesarean Section and the Risk of Adult Psychosis: A Population-Based Cohort Study. Schizophr. Bull. 2015, 42, 633–641. [Google Scholar] [CrossRef]
- Hyde, M.J.; Modi, N. The long-term effects of birth by caesarean section: The case for a randomised controlled trial. Early Hum. Dev. 2012, 88, 943–949. [Google Scholar] [CrossRef]
- Thavagnanam, S.; Fleming, J.; Bromley, A.; Shields, M.; Cardwell, C. A meta-analysis of the association between Caesarean section and childhood asthma. Clin. Exp. Allergy 2008, 38, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Dahlen, H.G.; Downe, S.; Wright, M.L.; Kennedy, H.P.; Taylor, J.Y. Childbirth and consequent atopic disease: Emerging evidence on epigenetic effects based on the hygiene and EPIIC hypotheses. BMC Pregnancy Childbirth 2016, 16, 4. [Google Scholar] [CrossRef]
- Decker, E.; Engelmann, G.; Findeisen, A.; Gerner, P.; Laass, M.; Ney, D.; Posovszky, C.; Hoy, L.; Hornef, M.W. Cesarean Delivery Is Associated With Celiac Disease but Not Inflammatory Bowel Disease in Children. Pediatrics 2010, 125, e1433–e1440. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, C.; Stene, L.C.; Joner, G.; Cinek, O.; Svensson, J.; Goldacre, M.J.; Parslow, R.C.; Pozzilli, P.; Brigis, G.; Stoyanov, D.; et al. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: A meta-analysis of observational studies. Diabetologia 2008, 51, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Sevelsted, A.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H. Cesarean Section and Chronic Immune Disorders. Obstet. Gynecol. Surv. 2015, 70, 303–305. [Google Scholar] [CrossRef]
- Sinha, A.; Bewley, S. The harmful consequences of prelabour caesarean section on the baby. Obstet. Gynaecol. Reprod. Med. 2012, 22, 54–56. [Google Scholar] [CrossRef]
- Kristensen, K.; Henriksen, L. Cesarean section and disease associated with immune function. J. Allergy Clin. Immunol. 2016, 137, 587–590. [Google Scholar] [CrossRef]
- Elbay, A.; Celik, U.H.; Celik, B.; Ozer, O.F.; Kilic, G.; Akkan, J.C.U.; Bayraktar, B.T.; Kaymak, N.Z. Intraocular pressure in infants and its association with hormonal changes with vaginal birth versus cesarean section. Int. Ophthalmol. 2016, 36, 855–860. [Google Scholar] [CrossRef]
- Liao, S.-L.; Tsai, M.-H.; Yao, T.-C.; Hua, M.-C.; Yeh, K.-W.; Chiu, C.-Y.; Su, K.-W.; Huang, S.-Y.; Kao, C.-C.; Lai, S.-H.; et al. Caesarean Section is associated with reduced perinatal cytokine response, increased risk of bacterial colonization in the airway, and infantile wheezing. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Keag, O.E.; Norman, J.; Stock, S.J. Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: Systematic review and meta-analysis. PLoS Med. 2018, 15, e1002494. [Google Scholar] [CrossRef]
- Nemati, B.; Atmodjo, W.; Gagnon, S.; Humes, D.; McKerlie, C.; Kaplan, F.; Sweezey, N.B. Glucocorticoid receptor disruption delays structural maturation in the lungs of newborn mice. Pediatr. Pulmonol. 2007, 43, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Rog-Zielinska, E.A.; Thomson, A.; Kenyon, C.J.; Brownstein, D.G.; Moran, C.M.; Szumska, D.; Michailidou, Z.; Richardson, J.; Owen, E.; Watt, A.; et al. Glucocorticoid receptor is required for fetal heart maturation. Hum. Mol. Genet. 2013, 22, 3269–3282. [Google Scholar] [CrossRef]
- Schuller, C.; Känel, N.; Müller, O.; Kind, A.B.; Tinner, E.M.; Hösli, I.; Zimmermann, R.; Surbek, D. Stress and pain response of neonates after spontaneous birth and vacuum-assisted and cesarean delivery. Am. J. Obstet. Gynecol. 2012, 207, 416.e1–416.e6. [Google Scholar] [CrossRef] [PubMed]
- Mears, K.; McAuliffe, F.; Grimes, H.; Morrison, J.J. Fetal cortisol in relation to labour, intrapartum events and mode of delivery. J. Obstet. Gynaecol. 2004, 24, 129–132. [Google Scholar] [CrossRef]
- Vogl, S.E.; Worda, C.; Egarter, C.; Bieglmayer, C.; Szekeres, T.; Huber, J.; Husslein, P. Mode of delivery is associated with maternal and fetal endocrine stress response. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Słabuszewska-Jóźwiak, A.; Włodarczyk, M.; Kilian, K.; Rogulski, Z.; Ciebiera, M.; Szymańska-Majchrzak, J.; Zaręba, K.; Szymański, J.K.; Raczkiewicz, D.; Włodarczyk, M.; et al. Does the Caesarean Section Impact on 11β HSD2 and Fetal Cortisol? Int. J. Environ. Res. Public Health 2020, 17, 5566. [Google Scholar] [CrossRef]
- Sano, Y.; Doi, T.; Kikuchi, S.; Kawai, K.; Tanaka, M. Correlations between stress hormone levels in umbilical cord blood and duration of delivery. J Pak. Med. Assoc. 2015, 65, 782–784. [Google Scholar]
- Celebi, M.; Alan, S.; Kahvecioglu, D.; Cakir, U.; Yildiz, D.; Erdeve, O.; Arsan, S.; Atasay, B. Impact of Prophylactic Continuous Positive Airway Pressure on Transient Tachypnea of the Newborn and Neonatal Intensive Care Admission in Newborns Delivered by Elective Cesarean Section. Am. J. Perinatol. 2015, 33, 99–106. [Google Scholar] [CrossRef]
- Gizzi, C.; Klifa, R.; Pattumelli, M.; Massenzi, L.; Taveira, M.; Shankar-Aguilera, S.; De Luca, D. Continuous Positive Airway Pressure and the Burden of Care for Transient Tachypnea of the Neonate: Retrospective Cohort Study. Am. J. Perinatol. 2015, 32, 939–943. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Maughan, H.; Guttman, D.S.; Field, C.J.; Chari, R.S.; Sears, M.R.; Becker, A.B.; Scott, J.A.; Kozyrskyj, A.L. Child Study Investigators. Gut microbiota of healthy Canadian infants: Profiles by mode of delivery and infant diet at 4 months. CMAJ 2013, 185, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P.; et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019, 574, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Makino, H.; Kushiro, A.; Ishikawa, E.; Kubota, H.; Gawad, A.; Sakai, T.; Oishi, K.; Martin, R.; Ben-Amor, K.; Knol, J.; et al. Mother-to-Infant Transmission of Intestinal Bifidobacterial Strains Has an Impact on the Early Development of Vaginally Delivered Infant’s Microbiota. PLoS ONE 2013, 8, e78331. [Google Scholar] [CrossRef] [PubMed]
- Sakwinska, O.; Foata, F.; Berger, B.; Brüssow, H.; Combremont, S.; Mercenier, A.; Dogra, S.; Soh, S.E.; Yen, J.; Heong, G.; et al. Does the maternal vaginal microbiota play a role in seeding the microbiota of neonatal gut and nose? Benef. Microbes 2017, 8, 763–778. [Google Scholar] [CrossRef]
- Fujimura, K.E.; Slusher, N.A.; Cabana, M.D.; Lynch, S.V. Role of the gut microbiota in defining human health. Expert Rev. Anti- Infect. Ther. 2010, 8, 435–454. [Google Scholar] [CrossRef]
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An Immunomodulatory Molecule of Symbiotic Bacteria Directs Maturation of the Host Immune System. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef]
- Olszak, T.; An, D.; Zeissig, S.; Vera, M.P.; Richter, J.; Franke, A.; Glickman, J.N.; Siebert, R.; Baron, R.M.; Kasper, D.L.; et al. Microbial Exposure During Early Life Has Persistent Effects on Natural Killer T Cell Function. Science 2012, 336, 489–493. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; DeRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nat. Cell Biol. 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Hapfelmeier, S.; Lawson, M.A.E.; Slack, E.; Kirundi, J.K.; Stoel, M.; Heikenwalder, M.; Cahenzli, J.; Velykoredko, Y.; Balmer, M.L.; Endt, K.; et al. Reversible Microbial Colonization of Germ-Free Mice Reveals the Dynamics of IgA Immune Responses. Science 2010, 328, 1705–1709. [Google Scholar] [CrossRef]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1897. [Google Scholar] [CrossRef]
- Young, V.B. The intestinal microbiota in health and disease. Curr. Opin. Gastroenterol. 2012, 28, 63–69. [Google Scholar] [CrossRef]
- Maynard, C.L.; Elson, C.O.; Hatton, R.D.; Weaver, C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nat. Cell Biol. 2012, 489, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Jang, S.-O.; Kim, B.-J.; Song, Y.-H.; Kwon, J.-W.; Kang, M.-J.; Choi, W.-A.; Jung, H.-D.; Hong, S.-J. The Effects of Lactobacillus rhamnosus on the Prevention of Asthma in a Murine Model. Allergy Asthma Immunol. Res. 2010, 2, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-Y.; Ni, Y.-H. Gut microbiota and the development of pediatric diseases. J. Gastroenterol. 2015, 50, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Luoto, R.; Kalliomäki, M.; Laitinen, K.; Delzenne, N.M.; Cani, P.D.; Salminen, S.; Isolauri, E. Initial Dietary and Microbiological Environments Deviate in Normal-weight Compared to Overweight Children at 10 Years of Age. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 90–95. [Google Scholar] [CrossRef]
- Akagawa, S.; Tsuji, S.; Onuma, C.; Akagawa, Y.; Yamaguchi, T.; Yamagishi, M.; Yamanouchi, S.; Kimata, T.; Sekiya, S.-I.; Ohashi, A.; et al. Effect of Delivery Mode and Nutrition on Gut Microbiota in Neonates. Ann. Nutr. Metab. 2019, 74, 132–139. [Google Scholar] [CrossRef]
- Montoya-Williams, D.; Lemas, D.J.; Spiryda, L.; Patel, K.; Carney, O.O.; Neu, J.; Carson, T.L. The Neonatal Microbiome and Its Partial Role in Mediating the Association between Birth by Cesarean Section and Adverse Pediatric Outcomes. Neonatology 2018, 114, 103–111. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef]
- Begum, M.; Pilkington, R.; Chittleborough, C.; Lynch, J.; Penno, M.; Smithers, L.G. Caesarean section and risk of type 1 diabetes: Whole-of-population study. Diabet. Med. 2019, 36, 1686–1693. [Google Scholar] [CrossRef]
- Yajnik, C.S. Early Life Origins of Insulin Resistance and Type 2 Diabetes in India and Other Asian Countries. J. Nutr. 2004, 134, 205–210. [Google Scholar] [CrossRef]
- Barker, D.J.P. The developmental origins of chronic adult disease. Acta Paediatr. 2004, 93, 26–33. [Google Scholar] [CrossRef]
- Painter, R.C.; Roseboom, T.J.; Bleker, O.P. Prenatal exposure to the Dutch famine and disease in later life: An overview. Reprod. Toxicol. 2005, 20, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Beedle, A.S. Early life events and their consequences for later disease: A life history and evolutionary perspective. Am. J. Hum. Biol. 2007, 19, 1–19. [Google Scholar] [CrossRef]
- St Clair, D.; Xu, M.; Wang, P.; Yu, Y.; Fang, Y.; Zhang, F.; Zheng, X.; Gu, N.; Feng, G.; Sham, P.; et al. Rates of adult schizophrenia. Following prenatal exposure to the Chinese famine of 1959–1961. JAMA 2005, 294, 557–562. [Google Scholar] [CrossRef]
- Dahlen, H.; Kennedy, H.; Anderson, C.; Bell, A.; Clark, A.; Foureur, M.; Ohm, J.; Shearman, A.; Taylor, J.; Wright, M.; et al. The EPIIC hypothesis: Intrapartum effects on the neonatal epigenome and consequent health outcomes. Med. Hypotheses 2013, 80, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Moarii, M.; Boeva, V.; Vert, J.-P.; Reyal, F. Changes in correlation between promoter methylation and gene expression in cancer. BMC Genom. 2015, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Becket, E.; Chopra, S.; Duymich, C.E.; Lin, J.J.; You, J.S.; Pandiyan, K.; Nichols, P.W.; Siegmund, K.D.; Charlet, J.; Weisenberger, D.J.; et al. Identification of DNA Methylation–Independent Epigenetic Events Underlying Clear Cell Renal Cell Carcinoma. Cancer Res. 2016, 76, 1954–1964. [Google Scholar] [CrossRef] [PubMed]
- Odom, L.N.; Taylor, H.S. Environmental induction of the fetal epigenome. Expert Rev. Obstet. Gynecol. 2010, 5, 657–664. [Google Scholar] [CrossRef][Green Version]
- Oberlander, T.F.; Weinberg, J.; Papsdorf, M.; Grunau, R.; Misri, S.; Devlin, A.M. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics 2008, 3, 97–106. [Google Scholar] [CrossRef]
- Breton, C.V.; Byun, H.-M.; Wenten, M.; Pan, F.; Yang, A.; Gilliland, F.D. Prenatal Tobacco Smoke Exposure Affects Global and Gene-specific DNA Methylation. Am. J. Respir. Crit. Care Med. 2009, 180, 462–467. [Google Scholar] [CrossRef]
- Schlinzig, T.; Johansson, S.; Gunnar, A.; Ekström, T.J.; Norman, M. Epigenetic modulation at birth–altered DNA-methylation in white blood cells after Caesarean section. Acta Paediatr. 2009, 98, 1096–1099. [Google Scholar] [CrossRef] [PubMed]
- Słabuszewska-Jóźwiak, A.; Włodarczyk, M.; Ciebiera, M.; Zwolińska, J.; Wojtyła, C.; Nowicka, G.; Jakiel, G.; Raczkiewicz, D. Placental DNA methylation in caesarean sections–A pilot study. Arch. Med. Sci. 2020, 16. [Google Scholar] [CrossRef]
- Franz, M.B.; Poterauer, M.; Elhenicky, M.; Stary, S.; Birner, P.; Vinatzer, U.; Husslein, P.; Streubel, B.; Husslein, H. Global and single gene DNA methylation in umbilical cord blood cells after elective caesarean: A pilot study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 179, 121–124. [Google Scholar] [CrossRef]
- Hansen, A.K.; Wisborg, K.; Uldbjerg, N.; Henriksen, T.B. Risk of respiratory morbidity in term infants delivered by elective caesarean section: Cohort study. BMJ 2007, 336, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Yael, B.; Walfisch, A.; Wainstock, T.; Segal, I.; Sergienko, R.; Landau, D.; Sheiner, E. Elective cesarean delivery at term and the long-term risk for respiratory morbidity of the offspring. Eur. J. Nucl. Med. Mol. Imaging 2018, 177, 1653–1659. [Google Scholar] [CrossRef]
- Magnus, M.C.; Håberg, S.E.; Stigum, H.; Nafstad, P.; London, S.J.; Vangen, S.; Nystad, W. Delivery by Cesarean Section and Early Childhood Respiratory Symptoms and Disorders: The Norwegian Mother and Child Cohort Study. Am. J. Epidemiol. 2011, 174, 1275–1285. [Google Scholar] [CrossRef]
- Almqvist, C.; Cnattingius, S.; Lichtenstein, P.; Lundholm, C. The impact of birth mode of delivery on childhood asthma and allergic diseases—A sibling study. Clin. Exp. Allergy 2012, 42, 1369–1376. [Google Scholar] [CrossRef]
- Van Berkel, A.C.; Dekker, H.T.D.; Jaddoe, V.W.V.; Reiss, I.; Gaillard, R.; Hofman, A.; De Jongste, J.C.; Duijts, L. Mode of delivery and childhood fractional exhaled nitric oxide, interrupter resistance and asthma: The Generation R study. Pediatr. Allergy Immunol. 2015, 26, 330–336. [Google Scholar] [CrossRef]
- Khashan, A.S.; Kenny, L.C.; Lundholm, C.; Kearney, P.M.; Gong, T.; Almqvist, C. Mode of Obstetrical Delivery and Type 1 Diabetes: A Sibling Design Study. Pediatrics 2014, 134, e806–e813. [Google Scholar] [CrossRef]
- Ajslev, T.A.; Andersen, C.S.; Gamborg, M.; Sorensen, T.I.; Jess, T. Childhood overweight after establishment of the gut microbiota: The role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int. J. Obes. 2011, 35, 522–529. [Google Scholar] [CrossRef]
- Chu, S.; Zhang, Y.; Jiang, Y.; Sun, W.; Zhu, Q.; Wang, B.; Jiang, F.; Zhang, J. Cesarean section without medical indication and risks of childhood allergic disorder, attenuated by breastfeeding. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Black, M.; Bhattacharya, S.; Philip, S.; Norman, J.E.; McLernon, D.J. Planned Cesarean Delivery at Term and Adverse Outcomes in Childhood Health. JAMA 2015, 314, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Gaskins, A.J.; Blaine, A.I.; Zhang, C.; Gillman, M.W.; Missmer, S.A.; Field, A.E.; Chavarro, J.E. Association Between Cesarean Birth and Risk of Obesity in Offspring in Childhood, Adolescence, and Early Adulthood. JAMA Pediatr. 2016, 170, e162385. [Google Scholar] [CrossRef]
- Mesquita, D.N.; Barbieri, M.A.; Goldani, H.A.; Cardoso, V.C.; Goldani, M.Z.; Kac, G.; Silva, A.A.; Bettiol, H. Cesarean section is associated with in- creased peripheral and central adiposity in young adulthood: Cohort study. PLoS ONE 2013, 8, e66827. [Google Scholar] [CrossRef]
- Masukume, G.; O’Neill, S.M.; Baker, P.N.; Kenny, L.C.; Morton, S.M.B.; Khashan, A.S. The Impact of Caesarean Section on the Risk of Childhood Overweight and Obesity: New Evidence from a Contemporary Cohort Study. Sci. Rep. 2018, 8, 15113. [Google Scholar] [CrossRef]
- Ahlqvist, V.H.; Persson, M.; Magnusson, C.; Berglind, D. Elective and nonelective cesarean section and obesity among young adult male offspring: A Swedish population–based cohort study. PLoS Med. 2019, 16, e1002996. [Google Scholar] [CrossRef] [PubMed]
- Steur, M.; Smit, H.A.; Schipper, C.M.A.; Scholtens, S.; Kerkhof, M.; De Jongste, J.C.; Haveman-Nies, A.; Brunekreef, B.; Wijga, A.H. Predicting the risk of newborn children to become overweight later in childhood: The PIAMA birth cohort study. Pediatr. Obes. 2011, 6, e170–e178. [Google Scholar] [CrossRef]
- Masukume, G.; Khashan, A.S.; Morton, S.M.B.; Baker, P.N.; Kenny, L.C.; McCarthy, F.P. Caesarean section delivery and childhood obesity in a British longitudinal cohort study. PLoS ONE 2019, 14, e0223856. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.L.; Rm, C.T.; Jong, E.I.F.-D.; Khashan, A.; Tracy, M.; Downe, S.; Rm, E.I.F.J.; Rm, H.G.D. The effect of medical and operative birth interventions on child health outcomes in the first 28 days and up to 5 years of age: A linked data population-based cohort study. Birth 2018, 45, 347–357. [Google Scholar] [CrossRef]
- Curran, E.A.; Khashan, A.S.; Dalman, C.; Kenny, L.C.; Cryan, J.F.; Dinan, T.G.; Kearney, P.M. Obstetric mode of delivery and attention-deficit/hyperactivity disorder: A sibling-matched study. Int. J. Epidemiol. 2016, 45, 532–542. [Google Scholar] [CrossRef]
- Sheiner, E.; Wainstock, T.; Segal, I.; Sergienko, R.; Landau, D.; Walfisch, A.; Yael, B. Elective Cesarean Delivery at Term and the Long-Term Risk for Neurological Morbidity of the Offspring. Am. J. Perinatol. 2018, 35, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, M.; Sarecka-Hujar, B.; Kopyta, I. Evaluation of Risk Factors for Epilepsy in Pediatric Patients with Cerebral Palsy. Brain Sci. 2020, 10, 481. [Google Scholar] [CrossRef]
- Pistiner, M.; Gold, D.R.; Abdulkerim, H.; Hoffman, E.B.; Celedón, J.C. Birth by cesarean section, allergic rhinitis, and allergic sensitization among children with a parental history of atopy. J. Allergy Clin. Immunol. 2008, 122, 274–279. [Google Scholar] [CrossRef]
- Roduit, C.; Scholtens, S.; De Jongste, J.C.; Wijga, A.H.; Gerritsen, J.; Postma, D.S.; Brunekreef, B.; Hoekstra, M.O.; Aalberse, R.; Smit, H.A. Asthma at 8 years of age in children born by caesarean section. Thorax 2009, 64, 107–113. [Google Scholar] [CrossRef]
- Menezes, A.M.B.; Hallal, P.C.; Matijasevich, A.M.; Barros, A.J.D.; Horta, B.L.; Araujo, C.L.P.; Gigante, D.P.; Santos, I.S.; Minten, G.; Domingues, M.R.; et al. Caesarean sections and risk of wheezing in childhood and adolescence: Data from two birth cohort studies in Brazil. Clin. Exp. Allergy 2010, 41, 218–223. [Google Scholar] [CrossRef]
- Pyrhönen, K.; Näyhä, S.; Hiltunen, L.; Läärä, E. Caesarean section and allergic manifestations: Insufficient evidence of association found in population-based study of children aged 1 to 4 years. Acta Paediatr. 2013, 102, 982–989. [Google Scholar] [CrossRef]
- Lavin, T.; Franklin, P.; Preen, D.B. Association between Caesarean Delivery and Childhood Asthma in India and Vietnam. Paediatr. Périnat. Epidemiol. 2016, 31, 47–54. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group PRISMA 2009 Flow Diagram. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Lagercrantz, H. The good stress of being born. Acta Paediatr. 2016, 105, 1413–1416. [Google Scholar] [CrossRef]
- Tutdibi, E.; Gries, K.; Bücheler, M.; Misselwitz, B.; Schlosser, R.L.; Gortner, L. Impact of Labor on Outcomes in Transient Tachypnea of the Newborn: Population-Based Study. Pediatrics 2010, 125, e577–e583. [Google Scholar] [CrossRef]
- Ganchimeg, T.; Nagata, C.; Vogel, J.P.; Morisaki, N.; Pileggi-Castro, C.; Ortiz-Panozo, E.; Jayaratne, K.; Mittal, S.; Ota, E.; Souza, J.P.; et al. Optimal Timing of Delivery among Low-Risk Women with Prior Caesarean Section: A Secondary Analysis of the WHO Multicountry Survey on Maternal and Newborn Health. PLoS ONE 2016, 11, e0149091. [Google Scholar] [CrossRef]
- Wilmink, F.A.; Hukkelhoven, C.W.; Lunshof, S.; Mol, B.W.J.; Van Der Post, J.A.; Papatsonis, D.N. Neonatal outcome following elective cesarean section beyond 37 weeks of gestation: A 7-year retrospective analysis of a national registry. Am. J. Obstet. Gynecol. 2010, 202, 250.e1–250.e8. [Google Scholar] [CrossRef] [PubMed]
- Tita, A.T.; Landon, M.B.; Spong, C.Y.; Lai, Y.; Leveno, K.J.; Varner, M.W.; Moawad, A.H.; Caritis, S.N.; Meis, P.J.; Wapner, R.J.; et al. Eunice Kennedy Shriver NICHD Maternal-Fetal Medicine Units Network. Timing of elective repeat cesarean delivery at term and neonatal out- comes. N. Engl. J. Med. 2009, 360, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Hefny, S.M.; Hashem, A.M.T.; Abdel-Razek, A.-R.A.; Ayad, S.M. The neonatal respiratory outcome in relation to timing of elective cesarean section at 38 versus 39week gestation: A single center based study. Egypt. Pediatr. Assoc. Gaz. 2013, 61, 78–82. [Google Scholar] [CrossRef][Green Version]
- Nada, A.M.; Shafeek, M.; El Maraghy, M.; Nageeb, A.; El-Din, A.S.S.; Awad, M.; Salaheldine, A. Antenatal corticosteroid administration before elective caesarean section at term to prevent neonatal respiratory morbidity: A randomized controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 199, 88–91. [Google Scholar] [CrossRef]
- Sotiriadis, A.; Makrydimas, G.; Papatheodorou, S.; Ioannidis, J.P.; McGoldrick, E. Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term. Cochrane Database Syst. Rev. 2018, 8, CD006614. [Google Scholar] [CrossRef]
- Roberts, D.; Brown, J.; Medley, N.; Dalziel, S.R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2017, 3, CD004454. [Google Scholar] [CrossRef]
- Cho, C.E.; Norman, M. Cesarean section and development of the immune system in the offspring. Am. J. Obstet. Gynecol. 2013, 208, 249–254. [Google Scholar] [CrossRef]
- Cotten, C.M.; Klebanoff, M.A.; Signore, C. Delivery after Previous Cesarean: Long-Term Outcomes in the Child. Semin. Perinatol. 2010, 34, 281–292. [Google Scholar] [CrossRef]
- Mårild, K.; Stephansson, O.; Montgomery, S.M.; Murray, J.A.; Ludvigsson, J.F. Pregnancy Outcome and Risk of Celiac Disease in Offspring: A Nationwide Case-Control Study. Gastroenterology 2012, 142, 39–45.e3. [Google Scholar] [CrossRef]
- Li, H.-T.; Zhou, Y.-B.; Liu, J.-M. The impact of cesarean section on offspring overweight and obesity: A systematic review and meta-analysis. Int. J. Obes. 2012, 37, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Darabi, B.; Rahmati, S.; Hafeziahmadi, M.R.; Badfar, G.; Azami, M. The association between caesarean section and childhood asthma: An updated systematic review and meta-analysis. Allergy Asthma Clin. Immunol. 2019, 15, 62. [Google Scholar] [CrossRef]
- Chavarro, J.E.; Martín-Calvo, N.; Yuan, C.; Arvizu, M.; Rich-Edwards, J.W.; Michels, K.B.; Sun, Q. Association of Birth by Cesarean Delivery With Obesity and Type 2 Diabetes Among Adult Women. JAMA Netw. Open 2020, 3, e202605. [Google Scholar] [CrossRef]
- Boksa, P.; El-Khodor, B. Birth insult interacts with stress at adulthood to alter dopaminergic function in animal models: Possible implications for schizophrenia and other disorders. Neurosci. Biobehav. Rev. 2003, 27, 91–101. [Google Scholar] [CrossRef]
- Curran, E.A.; O’Neill, S.M.; Cryan, J.F.; Kenny, L.C.; Dinan, T.G.; Khashan, A.S.; Kearney, P.M. Research Review: Birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. J. Child Psychol. Psychiatry 2014, 56, 500–508. [Google Scholar] [CrossRef]
- Hasab, M.F. Effect of Mode of Delivery on Children Intelligence Quotient at Pre-School Age in El-Minia City. Assiut Sci. Nurs. J. 2013, 1, 153–164. [Google Scholar] [CrossRef][Green Version]
- Khadem, N.; Khadivzadeh, T. The intelligence quotient of school aged children delivered by cesarean section and vaginal delivery. Iran. J. Nurs. Midwifery Res. 2010, 15, 135–140. [Google Scholar] [CrossRef]
- Zhang, T.; Sidorchuk, A.; Sevilla-Cermeño, L.; Vilaplana-Pérez, A.; Chang, Z.; Larsson, H.; Mataix-Cols, D.; De La Cruz, L.F. Association of Cesarean Delivery With Risk of Neurodevelopmental and Psychiatric Disorders in the Offspring. JAMA Netw. Open 2019, 2, e1910236. [Google Scholar] [CrossRef] [PubMed]
- Deoni, S.C.L.; Adams, S.H.; Li, X.; Badger, T.; Pivik, R.; Glasier, C.; Ramakrishnaiah, R.; Rowell, A.; Ou, X. Cesarean Delivery Impacts Infant Brain Development. Am. J. Neuroradiol. 2018, 40, 169–177. [Google Scholar] [CrossRef]
- Macharey, G.; Toijonen, A.; Hinnenberg, P.; Gissler, M.; Heinonen, S.; Ziller, V. Term cesarean breech delivery in the first pregnancy is associated with an increased risk for maternal and neonatal morbidity in the subsequent delivery: A national cohort study. Arch. Gynecol. Obstet. 2020, 302, 85–91. [Google Scholar] [CrossRef]

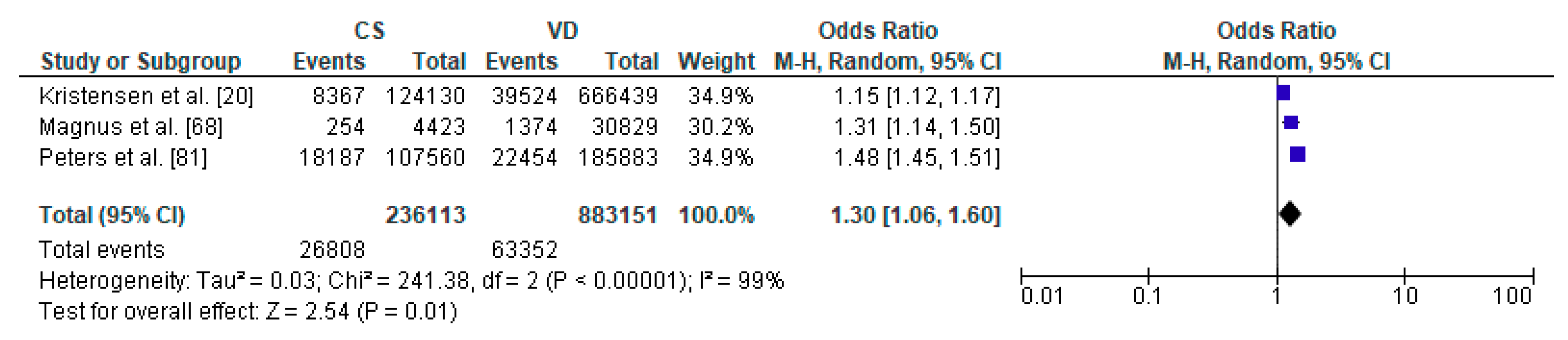
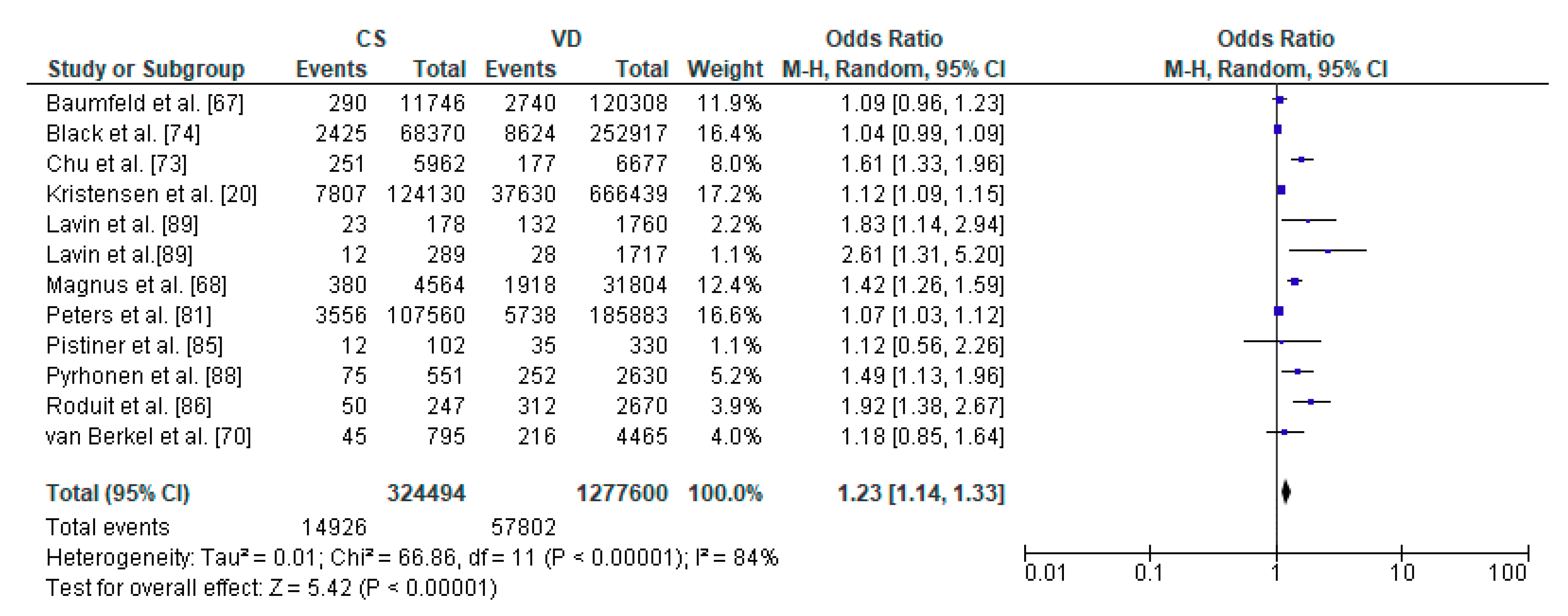
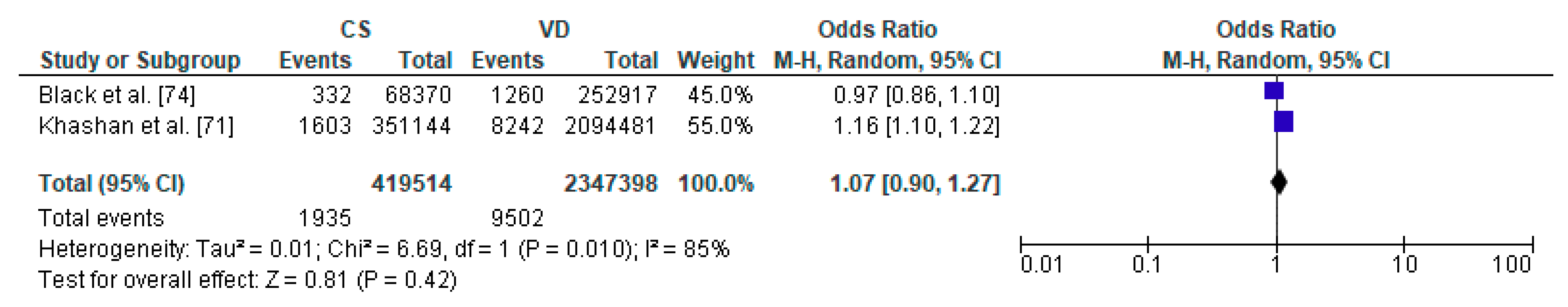
| Author | Country | Study Design | Sample Size | Age | Assessed Respiratory Morbidity |
|---|---|---|---|---|---|
| Kristensen et al. [20] | Denmark | Cohort Retrospective | 790,569 | from 0 to 14 y | lower respiratory tract infection |
| Magnus et al. [68] | Norway | Cohort Prospective (Norwegian Mother and Child Cohort Study) | 37,171 | 36 months | recurrent lower respiratory tract infections |
| Peters et al. [81] | Australia | Cohort Prospective | 491,590 | in the first 28 days and up to 5 y | respiratory infection |
| Author | Country | Study Design | Sample Size | Age | Assessed Asthma Morbidity |
|---|---|---|---|---|---|
| Kristensen et al. [20] | Denmark | Cohort Retrospective | 790,569 | from 0 to 14 y | Risk of asthma |
| Baumfeld et al. [67] | Israel | Cohort Retrospective | 132,054 | 18 y | Risk of asthma |
| Magnus et al. [68] | Norway | Prospective (Norwegian Mother and Child Cohort Study) | 37,171 | 3 y | Risk of asthma |
| van Berkel et al. [70] | Netherlands | Cohort Prospective | 6128 | 6 y | Risk of asthma |
| Chu et al. [73] | China | Case-Control Retrospective | 1385 | 5–12 y | Risk of asthma |
| Black et al. [74] | UK | Cohort Retrospective | 321,287 | 5 y | Asthma hospitalization |
| Peters et al. [81] | Australia | Cohort Prospective | 491,590 | in the first 28 days and up to 5 y | Risk of asthma |
| Pistiner et al. [85] | USA | Cohort Prospective | 498 | 9 y | Asthma symptoms |
| Roduit et al. [86] | Netherlands | Cohort Prospective | 2917 | 8 y | Risk of asthma |
| Pyrhonen et al. [88] | Finland | Cohort Retrospective | 4779 | 4 y | Risk of asthma |
| Lavin et al. [89] | Vietnam | Cohort Prospective | 2000 | 8 y | Risk of asthma |
| Lavin et al. [89] | India | Cohort Prospective | 2026 | 8 y | Risk of asthma |
| Author | Country | Study Design | Sample Size | Age | Assessed Metabolic Disorders |
|---|---|---|---|---|---|
| Black et al. [74] | UK | Cohort Retrospective | 321,287 | 5 y | diabetes mellitus type 1 |
| Khashan et al. [71] | Sweden | Cohort Prospective | 2,638,083 | 27 y | diabetes mellitus type 1 |
| Author | Country | Study Design | Sample Size | Age | Assessed Problems with Body Weight |
|---|---|---|---|---|---|
| Black et al. [74] | UK | Cohort Retrospective | 321,287 | 5 y | Obesity |
| Ajslev et al. [72] | Denmark | Cohort Prospective | 28,354 | 7 y | Obesity |
| Yuan et al. [75] | USA | Cohort Prospective | 22,068 | 20–28 y | Obesity |
| Mesquita et al. [76] | Brazil | Cohort Prospective | 2063 | 23–25 y | Risk of adiposity |
| Masukume et al. [77] | Ireland | Cohort Prospective (GUI study) | 11,134 | 3–5 y | Obesity |
| Alhqvist et al. [78] | Sweden | Cohort Prospective | 97,291 | 9–12 y | Obesity |
| Steur et al. [79] | Netherlands | Cohort Prospective (PIAMA study) | 1687 | 8 y | Overweight |
| Masukume et al. [80] | UK | Cohort Prospective (MCS) | 18,827 | 14 y | Body mas index and body fat |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Słabuszewska-Jóźwiak, A.; Szymański, J.K.; Ciebiera, M.; Sarecka-Hujar, B.; Jakiel, G. Pediatrics Consequences of Caesarean Section—A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 8031. https://doi.org/10.3390/ijerph17218031
Słabuszewska-Jóźwiak A, Szymański JK, Ciebiera M, Sarecka-Hujar B, Jakiel G. Pediatrics Consequences of Caesarean Section—A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2020; 17(21):8031. https://doi.org/10.3390/ijerph17218031
Chicago/Turabian StyleSłabuszewska-Jóźwiak, Aneta, Jacek Krzysztof Szymański, Michał Ciebiera, Beata Sarecka-Hujar, and Grzegorz Jakiel. 2020. "Pediatrics Consequences of Caesarean Section—A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 17, no. 21: 8031. https://doi.org/10.3390/ijerph17218031
APA StyleSłabuszewska-Jóźwiak, A., Szymański, J. K., Ciebiera, M., Sarecka-Hujar, B., & Jakiel, G. (2020). Pediatrics Consequences of Caesarean Section—A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 17(21), 8031. https://doi.org/10.3390/ijerph17218031






