Abstract
Overseas travel to regions where ticks are found can increase travellers’ exposure to ticks and pathogens that may be unfamiliar to medical professionals in their home countries. Previous studies have detailed non-native tick species removed from recently returned travellers, occasionally leading to travel-associated human cases of exotic tick-borne disease. There are 20 species of tick endemic to the UK, yet UK travellers can be exposed to many other non-native species whilst overseas. Here, we report ticks received by Public Health England’s Tick Surveillance Scheme from humans with recent travel history between January 2006 and December 2018. Altogether, 16 tick species were received from people who had recently travelled overseas. Confirmed imports (acquired outside of the UK) were received from people who recently travelled to 22 countries. Possible imports (acquired abroad or within the UK) were received from people who had recently travelled to eight European countries. Species-specific literature reviews highlighted nine of the sixteen tick species are known to vector at least one tick-borne pathogen to humans in the country of acquisition, suggesting travellers exposed to ticks may be at risk of being bitten by a species that is a known vector, with implications for novel tick-borne disease transmission to travellers.
1. Introduction
Since the 1950s, there has been year-on-year increases in worldwide international tourist arrivals [1]. In 2017 alone, there were 1,323 million worldwide tourist arrivals, increasing by 84 million international arrivals than 2016 [1]. Similar tourism increases have been seen in the United Kingdom (UK): overseas resident arrivals increased by 4% in 2017 compared with 2016, and 3% more overseas visits were made by UK residents in the same time period [2]. Such frequent movement could increase exposure of travellers to ticks and their pathogens. As ticks are the second most common vectors of disease-causing pathogens in humans [3], such exposure could present unique public health challenges, as ticks from one region may transmit pathogens that are unfamiliar to medical professionals in other parts of the world [4].
To date, several reports have detailed tick detection and removal on recently returned travellers, and ticks acquired in Africa, Asia, Australia, North and South America have been removed from residents in Asia, Europe, New Zealand and the USA [5,6,7,8,9,10,11,12,13]. In New Zealand, 50% of all tick importations that were intercepted at the border were associated with human travel [11]. There have also been cases of illness caused by tick-borne pathogens in recently returned travellers. More than 350 travel-associated cases of African tick bite fever (caused by Rickettsia africae) have been reported in Europe, USA, Australia, Argentina and Japan [14], and patients may not recall a tick bite, despite suffering from a tick-borne illness. For example, two USA residents developed skin lesions and flu-like symptoms within eight days of returning from Swaziland and were diagnosed with African tick bite fever, yet neither patient reported tick bites during the trips [15].
Whilst 20 tick species are considered endemic in the UK [16,17], UK residents can be exposed to non-native tick species whilst travelling abroad. As tick bites acquired overseas can present different health risks to those acquired in the UK, it is vital that existing public health guidance promotes the risks to both public health professionals and travellers. The following paper summarises imported ticks received by Public Health England’s passive Tick Surveillance Scheme (TSS) from humans with recent travel history and investigates the potential public health risk to UK residents bitten by ticks when travelling abroad.
2. Materials and Methods
Samples submitted to the TSS (see [18]) consisting of ticks likely acquired from outside of the UK between January 2006 and December 2018 were received from medical staff and members of the public. Upon arrival, specimens suspected to have been acquired overseas were frozen at −80 °C for 48 hours. Records were classed as imported if the recorder clearly stated that the tick had been acquired outside of the UK, the species was not endemic to the UK, or the tick could not have been acquired in the UK based on the level of engorgement and supplied travel information. All submissions of endemic species with a recent travel history that could not be confirmed as definitely acquired outside of the UK were classed as possible importation events, as local acquisition of the ticks could not be ruled out.
To identify species, keys for European ticks were initially used [19,20,21], with additional keys consulted for non-European species (e.g. [22]). Tick experts in the country of origin were contacted to verify specimens where necessary. The identification result was relayed to the person submitting the tick, as well as signposting to information about possible tick-borne diseases that are known to be transmitted by the tick species in the country of origin, and where necessary, follow-up questions were used to obtain more information about the tick encounter and the health of the person who had been bitten.
Following identification, a review of published literature was conducted using PubMed to further understand the ecology and potential public health risk following a bite from each tick species. First, searches were carried out for each tick species in the country of origin, for example ‘Ixodes ricinus AND France’, and then pathogens, for example ‘Ixodes ricinus AND France AND Borrelia burgdorferi’. The literature review of pathogens detected in ticks focused on articles that were published in English between January 2010 and March 2019, as well as the references cited therein. The relevance of articles identified by the database search (>900) were assessed first by their titles and abstracts, followed by an in-depth review of tick ecology and pathogen information.
3. Results
3.1. Tick Surveillance Scheme Imported Ticks
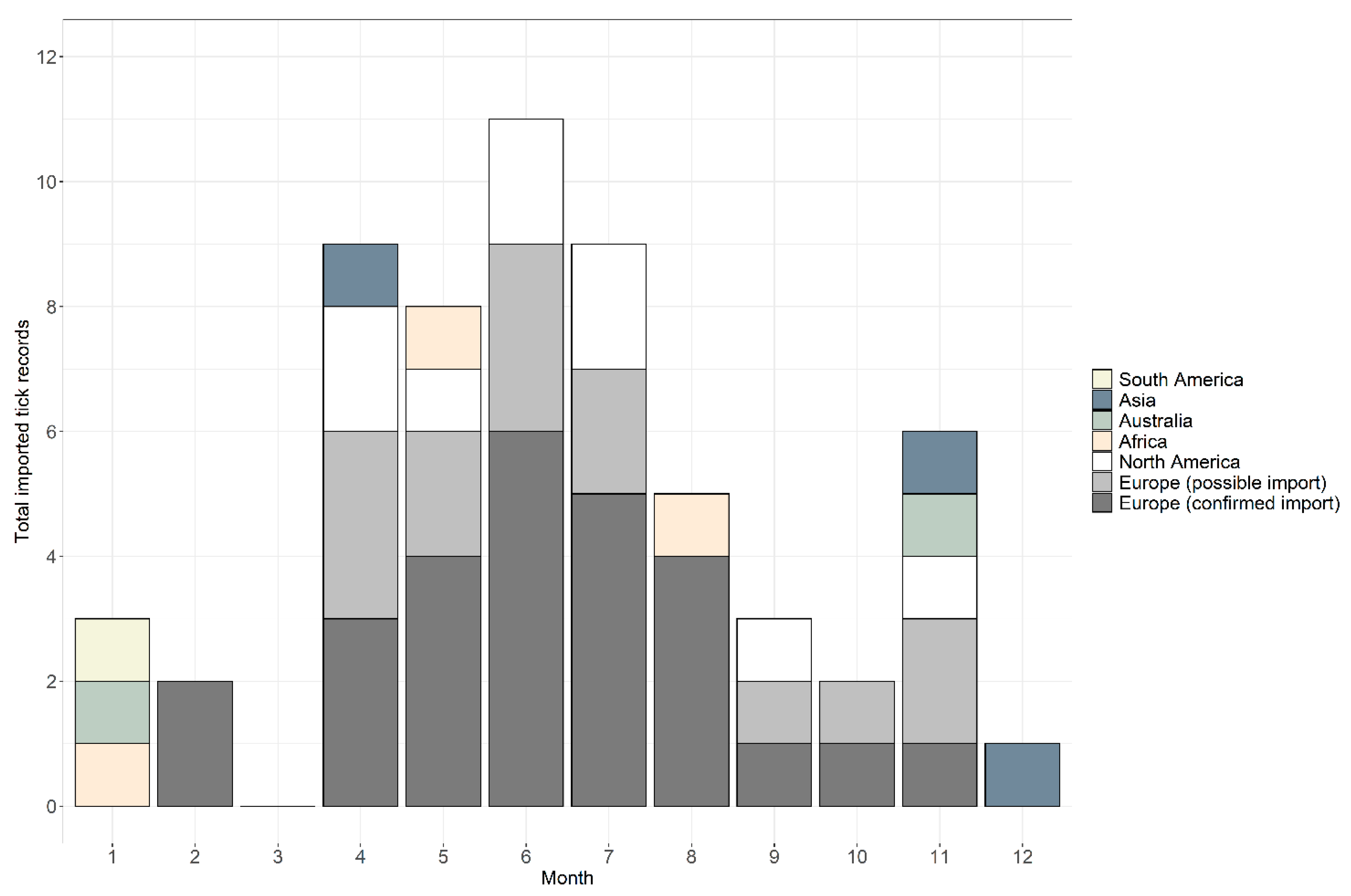
Between January 2006 and December 2018, 59 records were received from people with a recent travel history (Table 1). Records were comprised of 66 individual ticks belonging to 16 species, plus two damaged specimens that could only be identified at the genus level. In total, 76% of records (n = 45) were confirmed as imported and 24% (n = 14) were possible imports, where it was not possible to determine whether an endemic species received from someone with recent travel history was acquired abroad or in the UK. Considering only confirmed imported tick records, records were received in 2006 (n = 1), 2008 (n = 1), 2012 (n = 1), 2013 (n = 2), 2014 (n = 3), 2015 (n = 9), 2016 (n = 10) and 2017 (n = 10), and eight records in 2018. Possible imported records were received during 2013 (n = 2), 2015 (n = 2), 2016 (n = 2), 2017 (n = 3) and 2018 (n = 5). Across the years, ticks were removed from people with a history of travel during every month apart from March; the highest numbers were removed in June (n = 11), followed by July (n = 10), April (n = 9) and May (n = 8), totalling 63% of all records (see Figure 1 for a breakdown of confirmed and possible imported tick records). In addition, 20% of records (n = 12) were removed from travellers between November and February; half of these records originated from Southern Hemisphere countries (Figure 1).

Table 1.
Ticks submitted to Public Health England’s Tick Surveillance Scheme from people with recent travel history outside of the UK between 2006 and 2018. The tick species, history of travel, number of records associated with each tick species (including confirmed (C) and possible (P) imported ticks) and number of each life stage identified are shown (no larvae were submitted).
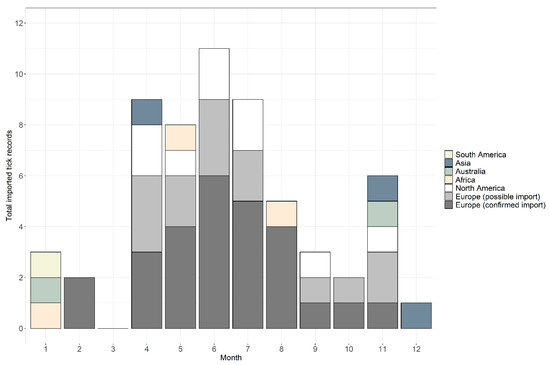
Figure 1.
Seasonality of tick records received by the Tick Surveillance Scheme from people with a history of travel that were acquired in South America (yellow), Asia (blue-grey), Australia (green), Africa (beige) and North America (white), and possible imports from Europe (light grey) and confirmed imports from Europe (dark grey).
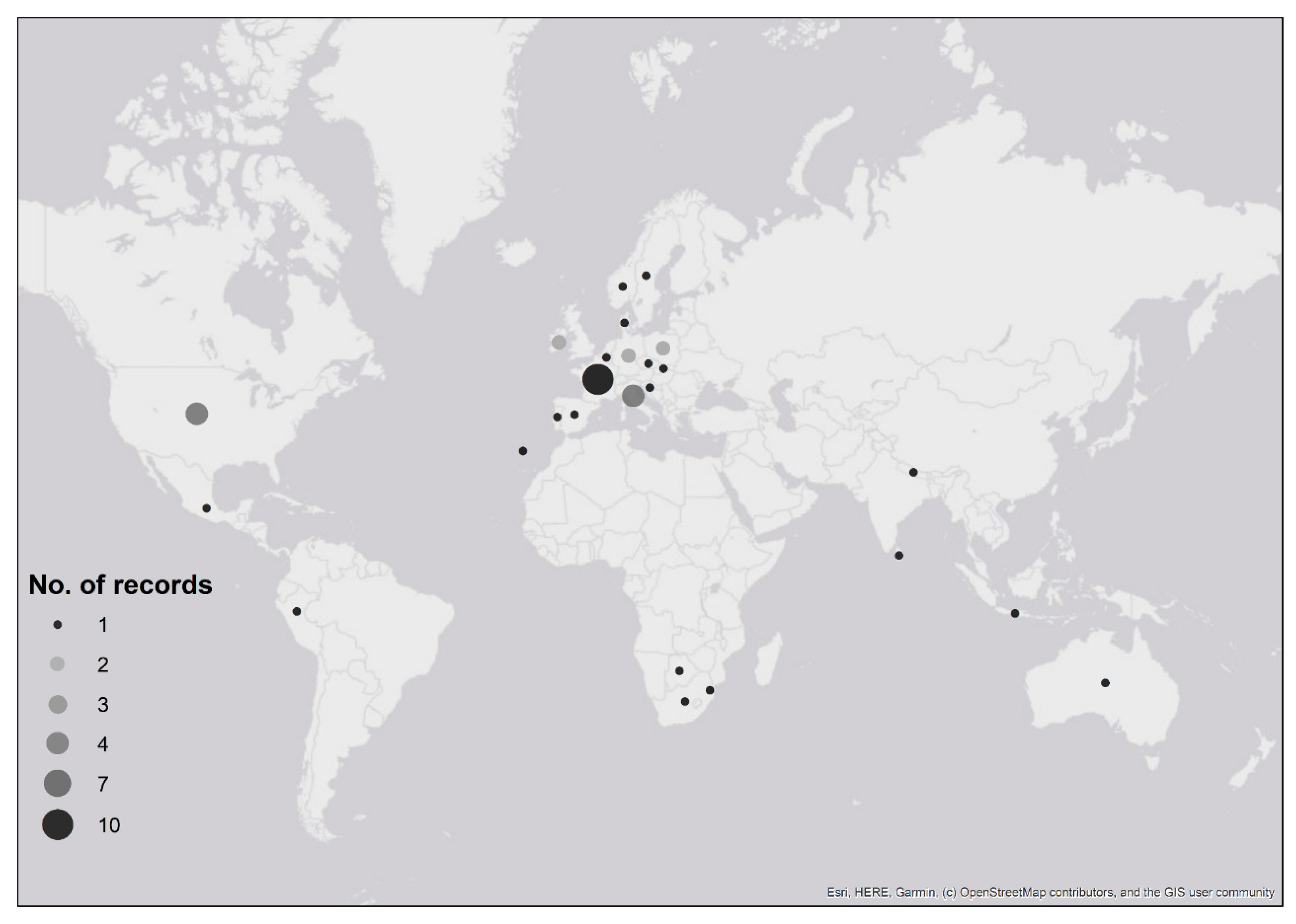
Ticks were received from people who had recently travelled to six continents: 70% of records were associated with travel in Europe, 15% from North America, 5% from both Africa and Asia, 3% from Australia and 2% from South America (Table 1; Figure 2). Confirmed imported ticks were removed from people that had recently travelled to 22 countries (Figure 2, Table 1), and possible imported ticks were removed from people with recent travel history to eight European countries (Figure 2, Table 1). There were two instances where the country of origin could not be determined as travel through multiple countries was reported (Table 1). One record, Dermacentor marginatus, was likely acquired either in Holland, Germany or France (although D. marginatus is not present in Holland (see [23])), whilst the second record, Dermacentor andersoni, came from either the Canadian Rockies or Washington State in north-western USA; despite the country of origin being unknown, both records were confirmed importation events as neither species is endemic to the UK. Exact locations where ticks were likely acquired were provided for 54% of records (n = 32), whilst regional/country information was provided for 46% (n = 27) of records.
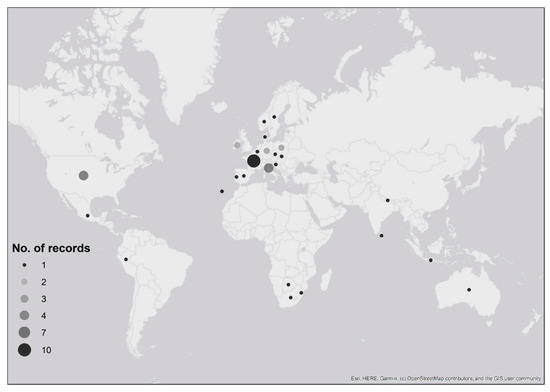
Figure 2.
Map showing recent travel history of people submitting confirmed and possible imported tick records to Public Health England’s Tick Surveillance Scheme between January 2006 and December 2018. The numbers of records received from each country are indicated by the size of the circle. Two records are not shown: one record that was acquired in either Holland, Germany or France; another record that was acquired in either the Canadian Rockies or north-western USA (see Table 1). Maps have been reproduced with permission from Ordnance Survey on behalf of Her Majesty’s Stationery Office, © Crown Copyright and database right. 2020. All rights reserved. Ordnance Survey License number 100016969/100022432.
In total, 16 tick species belonging to six genera were identified. Two species endemic to the UK were received: Ixodes hexagonus and Ixodes ricinus. In addition, fourteen non-native species were identified: Amblyomma americanum, Amblyomma cajennense, D. andersoni, Dermacentor auratus, D. marginatus, Dermacentor variablis, Haemaphysalis hystricis, Hyalomma lusitanicum, Hyalomma truncatum, Ixodes holocyclus, Ixodes pacificus, Rhipicephalus appendiculatus, Rhipicephalus gertrudae and Rhipicephalus sanguineus s.l. The most common tick species received was I. ricinus; there were 30 confirmed records and 12 possible records, representing 54% of all records, followed by A. americanum, which represented 8% (n = 5) of records. Nymphs were the most common (53% of all ticks) life stage received, followed by females (31%) and males (16%); no larvae were received. The ecology of each tick species, including distribution, habitat, hosts and seasonality, is presented in Table 2. Whilst human biting by the majority of the received species is commonly reported, human infestation of Hy. lusitanicum, Hy. truncatum and H. hystricis is considered rare or occasional (Table 2).

Table 2.
Review of distribution, habitat and climate requirements, hosts, seasonality and commonality of human biting of each confirmed and possible imported tick species, along with the prevalence of the most common human pathogens detected in the focal tick species in the country where the tick was confirmed or suspected to have been acquired. References are mostly focused on countries listed in the history of travel, but literature searches were extended if there was no information available for the tick species in the country.
All records involving the importation of species endemic to the UK (n = 35) were acquired in Europe (Figure 2): 21 records (60%) were definitively confirmed as imported, whilst 14 records (40%) were possible imports (Table 1). There was one confirmed import of I. hexagonus from Portugal and a possible import of I. hexagonus from Madeira. Six confirmed I. ricinus imports originated from France, three from Germany, two each from Italy, Norway and Sweden and one each from Czechia, Ireland, Poland, Slovenia and Spain. In addition, four possible imported records were received from France, two each from Ireland, Italy and Poland and one from Germany.
3.2. Literature Review of Pathogens Detected in Ticks in the Native Country
Literature reviews for pathogens infecting tick species in the country of origin highlighted nine of the received species (I. ricinus, D. marginatus, R. sanguineus, Hy. lusitanicum, A. americanum, D. variabilis, D. andersoni, I. pacificus, I. holocyclus), comprising 81% of all records, are known to transmit at least one pathogenic organism to humans; the most important pathogens in terms of human health in the country where the imported tick was likely acquired are shown in Table 2.
3.2.1. Europe—Ixodes ricinus
The most common pathogen detected in I. ricinus in Europe is the spirochete Borrelia burgdorferi sensu lato (s.l.), the causative agent of Lyme borreliosis (prevalence 0.06–46.6% [135]). Despite only being prevalent in the Northern Hemisphere, Lyme borreliosis is the most common tick-borne disease in the world [24]. It is transmitted by ticks belonging to the I. ricinus complex across Europe and North America, as well as in parts of northern Africa and Asia. The true incidence of infection in Europe is difficult to quantify, however, as Lyme borreliosis is not a notifiable disease. In Europe, the primary vectors of Lyme borreliosis are I. ricinus and I. persulcatus, but laboratory evidence suggests that I. hexagonus is also an efficient vector of B. burgdorferi s.l. [136,137].
There are multiple genospecies of B. burgdorferi s.l. circulating in Europe: Borrelia afzelii, Borrelia bissettiae, Borrelia bavariensis (formerly B. garinii OspA serotype 4), Borrelia burgdorferi sensu stricto (s.s.), Borrelia carolensis, Borrelia finlandensis, Borrelia garinii, Borrelia lusitaniae, Borrelia spielmanii, Borrelia turdi and Borrelia valaisiana [135,138]. Throughout Europe, the prevalence of genospecies varies geographically [138], which is likely driven by genospecies associated with different reservoir hosts. For example, whilst B. afzelii is associated with small mammals [139,140,141], ground-foraging birds are competent reservoirs of B. garinii and B. valaisiana, but are not able to maintain B. afzelii [142,143,144]. Not all genospecies are pathogenic, and in Europe at least five genospecies are well recognised as being pathogenic to humans—B. afzelii, B. bavariensis, B. burgdorferi s.s., B. garinii and B. spielmanii [145,146,147]. A meta-analysis investigating the prevalence of genospecies in questing I. ricinus across Europe found that B. afzelii (46.6%) and B. garinii (23.8%) were the most common genospecies, followed by B. valaisiana (11.4%), B. burgdorferi s.s. (10.2%), B. lusitaniae (7.0%), B. bavariensis (2.0%), B. spielmanii (1.7%), B. finlandensis (0.2%) and B. bissettiae (0.06%) [135]. Focusing on I. ricinus in the countries where recorders had a recent history of travel and subsequent tick bite, the most common genospecies reported in ticks, B. afzelii, B. burgdorferi s.s. and B. garinii, have been recorded from all focal European countries. In addition, other pathogenic species have been found: B. bavariensis in I. ricinus from Czechia and Germany, and B. spielmanii in Czechia, Denmark, France, Germany, Poland and Sweden.
Tick-borne encephalitis (TBE) is a severe neurological disease caused by a flavivirus transmitted by ticks in parts of Europe and Asia [148], although infection can be acquired to a lesser extent through consumption of raw milk from infected animals [149]. Tick-borne encephalitis virus (TBEV) is the most important tick-borne arboviral disease in Europe; it is endemic in 27 European countries and is expanding northwards and to higher altitude as a result of numerous factors including warming temperatures [150,151,152]. Adults were traditionally considered most at risk of TBE infection as the prognosis worsens with age, whereas children suffer milder symptoms [153,154,155,156,157]. A recent review of data from the European Centre for Disease Prevention and Control (ECDC), however, found that there is a greater risk of long-term cognitive impairment in children following TBE infection [158]. There are three subtypes of TBEV: European, Siberian and Far Eastern. The European subtype is transmitted by I. ricinus, whilst the other two are transmitted by I. persulcatus [155] (see Lindquist & Vapalahti, 2008). The virus is transmitted to a susceptible tick when it feeds adjacent to an infected tick, a process known as co-feeding [159], and key wildlife hosts are required for TBEV to persist in a population [160,161]. In southern Germany and Slovakia, most TBE cases occurred during June, July and August [162,163], which is likely linked to increased human activity during the summer period, despite reduced tick numbers [164]. Between 2012 and 2016, 12,500 cases of TBE were reported from 23 European countries, and of these, Czechia and Lithuania accounted for 38.6% of all reported cases [165]. Prevalence < 0.1–5% [166]. In the focal countries for the current study, TBEV has been found in I. ricinus in all countries apart from Ireland and Spain (Table 2), and the highest number of cases of TBEV have been reported from Czechia [165]. To date, five imported cases of TBE have been reported in the UK, although the country where the infection was acquired is unknown [165].
3.2.2. Europe: Dermacentor marginatus in France, Germany and Italy
Tick-borne lymphadenopathy (TIBOLA) or Dermacentor-borne necrosis erythema lymphadenopathy (DEBONEL) is caused by three species of Rickettsia: R. slovaca, R. raoultii and R. rioja [167,168,169,170,171,172]. Cases of infection have been reported from Bulgaria, France, Hungary, Italy, Portugal and Spain [168,173,174,175,176,177,178,179]. Compared to Lyme borreliosis, TIBOLA/DEBONEL is a relatively rare and mild infection, but awareness of symptoms is still important [180]. In Europe, D. marginatus is considered to be the main vector of TIBOLA/DEBONEL, and the peak in diagnosed cases coincides with the peak in adult activity [175]. The prevalence of R. slovaca infecting D. marginatus is between 15.7% and 50% [33,173,181,182,183,184,185], whilst the prevalence of R. raoultii is lower at 8.3–8.4% [33,184]. Of the focal countries in the current study, infection with R. slovaca has been reported in D. marginatus from France, Germany and Italy, and R. raoultii has been detected in D. marginatus from Italy.
3.2.3. Europe: Rhipicephalus sanguineus s.l. in Croatia and Italy
The most frequent rickettsiosis in Europe is Mediterranean spotted fever (MSF, also named fièvre boutonneuse), caused by Rickettsia conorii, and cases have been reported from much of Western and Southern Europe, as well as parts of Eastern Europe [175]. Mortality can occur, and a fatality rate of 32.3% of hospitalized patients with severe morbidity in southern Portugal has been reported [186]. As Rh. sanguineus is thought to act as both the vector and reservoir of the rickettsia, some mammals present in the Mediterranean may also be reservoir hosts [175]. Although Rh. sanguineus has a low probability of biting humans, most cases of infection are diagnosed in the spring and summer when Rh. sanguineus activity peaks [175]. In an MSF-endemic region in Spain, however, only one out of 4049 ticks was infected [187]. Very low infection prevalence (<1%) has been reported [188,189], but can be higher (see [190]). As other Rickettsia species were more prevalent, the authors suggested that many of the cases that had been attributed to MSF may have actually been caused by other Rickettsia species [187]. For example, Rickettsia conorii israelensis, which has also been detected in European Rh. sanguineus populations, results in a sudden fever, accompanied by headache, rash, and gastrointestinal symptoms [191]. Mortality has been reported in less than a third of patients [191], but is higher than that reported for MSF, suggesting R. conorii israelensis is more virulent than R. conorii [192]. Another Spotted Fever group rickettsia which has been detected in multiple species belonging to the Rhipicephalus genus [193], including Rh. sanguineus, is R. massiliae. Symptoms include fever, headache, muscular pain, rash and eschar [190,194,195,196], and in Italy and France, R. massiliae is considered the causative agent of a MSF-like illness in patients [190,194,195,196]. During the current study, two imported Rh. sanguineus were received; one from Italy and one from Croatia. Whilst to date, there is no information on pathogen prevalence in Rh. sanguineus in Croatia, R. conorii, R. conorii israelensis and R. massiliae have all been detected in Rh. sanguineus from Italy.
3.2.4. Europe: Hyalomma lusitanicum in Spain
Crimean Congo haemorrhagic fever virus (CCHFV) is caused by a member of the Nairovirus genus and is transmitted via bites from Hyalomma species (particularly Hy. marginatum marginatum but also Hy. lusitanicum), or following contact with infected body fluids or tissues [197]. To date, two studies have detected CCHFV in Hy. lusitanicum at a prevalence of 3.2% [45,198] feeding from red deer (Cervus elaphus) in Spain between 2010 and 2015, suggesting that CCHFV is circulating in southwestern Europe [45,198], but whether Hy. lusitanicum is a vector of the virus remains unclear. During 2016, two autochthonous cases of CCHFV were reported in Spain [199], followed by a subsequent fatal case reported in August 2018 [200]. Until the end of August 2020, there have been three autonomous cases of CCHF in Spain and Bulgaria, including a fatality [201,202,203]. To date, there have been three CCHFV cases reported in the UK. First, in 1997, a suspected case of CCHFV was diagnosed in a UK resident recently returned from Zimbabwe [204]. In 2012, there was a fatal CCHFV case in a UK national returning from Afghanistan [205]. Finally, in June 2014, there was a case in a UK national that had received a tick bite whilst in Bulgaria [206].
3.2.5. North America: Amblyomma americanum in USA
In the USA, Lyme borreliosis is caused by the genospecies B. burgdorferi s.s., and in the north-eastern USA where Lyme borreliosis is most prevalent, I. scapularis is the primary vector, whilst in the western states, the bacteria are vectored by I. pacificus. Infection of A. americanum with B. burgdorferi s.s. has been reported from a number of states [207,208,209,210], yet it is not considered a competent vector [211,212,213]. Furthermore, Lyme borreliosis-like symptoms have been associated with bites from A. americanum in some southern states when no trace of antibodies to B. burgdorferi s.l. have been detected in patients [214,215]. The spirochete responsible has been identified and termed ‘Borrelia lonestari’ [216], and has been detected at low prevalence (0.35–9.1% [216,217,218,219,220,221,222,223,224,225,226,227]) in questing A. americanum from multiple locations [216,217,218,219,220,221,222,223,224,225,226,227]). The resulting illness is named Master’s disease or southern-tick-associated rash-illness (STARI), and is clinically indistinguishable from the early stages of Lyme borreliosis [228]; however, the public health significance of B. lonestari remains unclear.
Ehrlichia chaffeensis and E. ewingii are gram-negative bacteria that are the causative agents of human ehrlichiosis [229,230]. Whilst ehrlichiosis caused by E. chaffeensis is considered to be underreported and probably as prevalent as Rocky Mountain spotted fever (see below), less than 20 cases of E. ewingii have been documented, and are mostly reported in immunosuppressed patients [52,231]. The prevalence of E. chaffeensis in ticks is higher (0.6–19%) [217,220,221,222,232,233,234,235,236,237,238,239] than E. ewingii (0.24–7.1%) [217,220,221,222,233,234,235,236,238]. It is thought that E. chaffeensis and E. ewingii are maintained in cycles involving a wide variety of competent vertebrate reservoir hosts, such as white-tailed deer, and A. americanum as the primary vector [52,235,240]. Laboratory experiments have demonstrated transmission of E. ewingii from A. americanum to dogs, highlighting the importance of A. americanum in maintaining and transmitting ehrlichiae to hosts [241]. In 2006, Panola Mountain Ehrlichia (PME), caused by a disease agent similar to Ehrlichia ruminantium, was discovered in a goat from Panola Mountain State Park in Georgia [242] and was later associated with a report of human illness following a bite from an Amblyomma nymph also acquired at the State Park [243]. Furthermore, A. americanum infected with PME have been collected from ten states on the east coast of the United States between 1998 and 2006, suggesting the infection has an extensive distribution throughout the A. americanum range and has not been recently introduced [244].
3.2.6. North America: Dermacentor variabilis in USA
Dermacentor variabilis is a primary vector USA [245,246]. Caused by the bacteria Rickettsia rickettsii, RMSF is the most common tick-borne rickettsial disease in the USA [247]. Severe illness can develop if symptoms remain untreated, and fatalities are possible [247]. Low prevalence rates of less than 2% in D. variabilis populations [248,249] have led some authors to question whether other tick species may play a more important vectoral role than D. variabilis [249]. For example, Rh. sanguineus was the cause of an outbreak of RMSF in eastern Arizona [250]. Furthermore, whilst A. americanum historically has not been considered to be a major vector of RMSF [251], much of the distribution overlaps with D. variabilis [252], and as both species feed on similar hosts, there is potential for A. americanum larvae to become infected with R. rickettsii either whilst feeding on an infected host or cofeeding next to an infected D. variabilis [252]. Under laboratory conditions, A. americanum has acquired the infection from guinea pigs, maintained infection throughout moulting and transmitted the bacteria to susceptible hosts during subsequent feeding, suggesting that under laboratory conditions, A. americanum is capable of acquiring, maintaining and transmitting R. rickettsii [252]. To date, however, there is no evidence to suggest that A. americanum is a competent vector of R. rickettsii in the USA. Rickettsia montanensis is another spotted fever group Rickettsia that has been detected in D. variabilis. Studies in the USA and Canada have found a mean infection prevalence of D. variabilis with R. montanensis of 1.2–10.5% [56,59,248,253,254,255,256,257,258,259]. In addition, a child from Georgia, USA developed an afebrile rash illness after being bitten by an individual D. variabilis infected with R. montanensis, suggesting the possibility that R. montanensis may be a human pathogen [260].
3.2.7. North America: Ixodes pacificus in USA
Ixodes pacificus is the primary vector of Lyme disease in western USA [110], and the highest regional incidence occurs in north-western California, where dense oak woodlands support high densities of I. pacificus [261]. In California, cases of Lyme borreliosis peak between May and July, following the nymph peak between April and June, suggesting nymphs are responsible for most cases in California [72].
Borrelia miyamotoi belongs to the relapsing fever group of Borrelia, and is distinctly related to B. burgdorferi s.l. It is transmitted by the same tick species as B. burgdorferi s.l., namely I. ricinus and I. persulcatus in Europe, and I. scapularis and I. pacificus in the USA. Three distinct genotypes of B. miyamotoi have been identified in the USA, Europe and Japan [262,263,264], and all three groups include strains that are pathogenic to humans [265]. Unlike the spirochetes that cause Lyme borreliosis, B. miyamotoi spirochetes are able to be maintained in ticks via both transstadial and transovarial transmission [266,267]. The first human cases of infection with B. miyamotoi were reported in Russia in 2011 [268], and further cases have been reported from elsewhere in Europe (Netherlands and France) and the USA [269,270,271,272]. A study of people bitten by ticks which tested positive for B. miyamotoi who went on to develop clinical disease estimated transmission efficiency in humans to be 8.3% (2/24 patients), compared with 4.4% (3/68) of humans bitten by ticks infected with B. burgdorferi s.l. who went on to develop symptoms [273,274]. In the USA, white-footed mice (P. leucopus) are important hosts of the bacteria [266], and a high prevalence of infection (58%) has been detected in wild turkeys (Maleagris gallopavo) [275]. Prevalence rates of infection in ticks are significantly lower (0.4–5% [276,277,278,279,280,281,282]) than B. burgdorferi s.l. (0.2–24.7% [64,261,276,277,279,281,283,284,285,286,287,288,289,290,291,292,293,294,295]), although a comparable prevalence of B. miyamotoi and B. burgdorferi in adult I. pacificus in California suggests that there is a similar risk of exposure to both species [276]. As well as I. pacificus from USA, B. miyamotoi infection has been reported in I. ricinus from Czechia, France, Germany, Norway, Poland, Spain and Sweden (Table 2). In Europe, bank voles (Myodes glareolus) and yellow-necked mice (Apodemus flavicollis) are reservoirs for B. miyamotoi [263,296,297], and whilst bacteria have been detected in engorged I. ricinus feeding on wild boar (Sus scrofa), roe deer (Capreolus capreolus) and a blackbird (Turdus merula), it is unknown whether these species are reservoir hosts of B. miyamotoi [298].
Anaplasma phagocytophilum is an obligate intracellular bacterium that is responsible for causing human granulocytic anaplasmosis (HGA), a moderate flu-like illness in humans. In the USA, HGA is the second most important tick-borne disease after Lyme borreliosis, and is a notifiable infection, although many infections may result in minimal or no clinical manifestations [299]. Small mammals, which feed immature tick stages, are the primary reservoirs for A. phagocytophilum [300,301,302]. In eastern USA, the main vector of HGA is I. scapularis, whilst I. pacificus is considered the main vector in western parts of the USA [303,304]. Further, the prevalence of A. phagocytophilum in I. pacificus is significantly lower (0–11%) than the prevalence in I. scapularis (0-51%) [277,283,285,288,295,300,305,306,307].
3.2.8. North America: Dermacentor andersoni in USA/Canada
Along with being a primary vector of RMSF [308], D. andersoni is also the principal vector of Colorado tick fever virus, which is found in western USA. The disease is considered under-reported, as non-specific symptoms may be misdiagnosed as other infections including RMSF [309]. Whilst producing a febrile illness in humans, the virus does not cause illness in its natural reservoir hosts. It was first detected in Montana, where adult D. andersoni were found to be infected with the virus, and infection was also present in ground squirrels, the preferred host for immature stages of D. andersoni [310,311]. A prevalence of 6.6% to 21% in D. andersoni has been reported [312,313]. Further, clinical cases of Colorado tick fever peak between May and July, concurrently with the peak of ticks [314].
Dermacentor andersoni has been associated with tick paralysis, with rapidly ascending paralysis occurring five to seven days after tick attachment. The tick often attaches to the head, and so can be hidden by hair; removal of the tick leads to an immediate improvement in symptoms [315]. In a review of 33 cases of tick paralysis that were reported to the Washington State Department of Health between 1946 and 1996, D. andersoni was identified as the culpable species [316].
3.2.9. North America: Amblyomma cajennense Sensu Lato in Mexico
There are no studies published to date reporting the association of A. cajennense with human disease in Mexico. In South America, however, A. cajennense is considered to be the most important vector of R. rickettsii (the causative bacteria of RMSF, also called Brazilian spotted fever (BSF) in Brazil [99]). Considered to be the deadliest of the spotted fevers in the world, BSF has a fatality rate of 31.5%, compared with 5–10% fatality for RMSF in the USA [317,318]. A laboratory experiment found that it was possible for transovarial transmission from a female A. cajennense to her offspring, but only some of the eggs became infected, suggesting that R. rickettsii is not able to be maintained by transovarial transmission only [319]. In addition, A. cajennense larvae that fed on guinea pigs experimentally infected with R. rickettsii were less susceptible to infection with R. rickettsii than other tick species [320].
3.2.10. Australia: Ixodes holocyclus
Ixodes holocyclus is responsible for the majority of (though not all) cases of tick paralysis in Australia [316]. Tick paralysis is a rare but potentially fatal condition. Between 1904 and 1945, there were 20 fatalities reported in Australia due to tick paralysis, with 70% of all deaths being children, but since 1945, there have been no further fatalities [321]. Ticks do not secrete detectable levels of the paralysis-inducing toxin until three days of feeding on a host [73]. As such, clinical signs of tick paralysis do not become apparent until three days following a tick bite [322,323]. Prompt tick removal is an important part of patient treatment [324], although, following removal of I. holocyclus, there can be a deterioration in the health of the patient [321]. Whilst nymphs may cause a mild paralysis, females are responsible for the most severe symptoms, and a bite from a single infected female tick is sufficient to cause paralysis [74,322,325]. Cases of paralysis occur year-round, but are most common in spring and summer [323]. It appears that children up to 5 years old are most affected [74]. Furthermore, I. holocyclus is usually found on the scalp and paralysis is less likely to occur if ticks are attached to other parts of the body [74].
3.2.11. Africa: Hyalomma truncatum in Botswana
Whilst there have been no published studies looking at the pathogen prevalence in H. truncatum collected in Botswana to date, horizontal transfer of CCHFV from larval H. truncatum to laboratory mice has been reported [326], and transovarial and sexual transmission of CCHFV in H. truncatum has been seen in laboratory studies [327,328]. In addition, whilst Rift Valley fever virus is predominantly associated with mosquito transmission or contact with infected blood/body fluids, it has been suggested that H. truncatum is a possible vector of the virus [329,330]. Horizontal and transstadial transmission has been demonstrated [329], and the geographical distribution of H. truncatum correlates with the incidence of Rift Valley fever virus [330]. Rickettsia aeschlimannii and R. africae have been detected in H. truncatum [331,332,333,334,335], although there does not appear to be confirmed evidence of transmission from laboratory experiments. The prevalence of R. aeschlimannii in H. truncatum was lower compared to other Hyalomma species, suggesting that H. truncatum is not the principal vector of the bacteria [331].
3.2.12. Africa: Rhipicephalus gertrudae in South Africa
To date, there is no information on whether Rh. gertrudae is involved in the transmission of any pathogens to humans in South Africa or further afield.
3.2.13. Africa: Rhipicephalus appendiculatus in Eswatini
Although Rh. appendiculatus is a known vector of diseases affecting cattle in Southern Africa, for example, East Coast fever [336], there have been no published studies to date to suggest it to vector any diseases which can be transmitted to humans in Eswatini or further afield.
3.2.14. Asia: Haemaphysalis hystricis in Java, Indonesia
Whilst to date there have been no studies published looking at the pathogen prevalence in H. hystricis collected specifically in Java or across Indonesia, the findings of studies that have been conducted in other Asian countries suggest that H. hystricis could act as a vector in Java. Haemaphysalis hystricis is considered to be one of the most important vectors of Rickettsia japonica, the bacteria responsible for Japanese spotted fever [120], and infected ticks have been found in Japan and China [337,338]. Following the diagnosis of a patient with Japanese spotted fever in Fukuoka Prefecture in Japan, an investigation of ticks found in the area where it was thought that the patient most likely acquired the tick bite found larvae were infected with R. japonica [339]. Similarly, in the Hubei province of China, R. japonica was detected in a single H. hystricis [338]. Focusing on other infections, Coxiella species have been detected in H. hystricis in Thailand, although infection prevalence in H. hystricis is thought to be lower than in other Haemaphysalis species also present in Thailand [340]. Following on from this, a study of H. hystricis from Malaysia suggested the possibility of infection with C. burnetii based on the phylogenetic clustering of the bacteria [341]. Finally, whilst Borrelia burgdorferi s.l. has not been detected in H. hystricis from China [115], Borrelia sp. closely related to the relapsing fever group (e.g. Borrelia miyamotoi) have been detected in H. hystricis from Malaysia [116], although it should be noted that the bacteria was only detected in a single tick.
3.2.15. Asia: Dermacentor auratus in Nepal and Sri Lanka
To date, there has been no investigation into pathogens found in D. auratus in either Nepal or Sri Lanka. Kyasanur forest disease and Lanjan virus have been isolated in questing D. auratus in India and Malaya, respectively [131,342], although it is unclear whether D. auratus also transmits the pathogens. A study of ticks removed from wild boar in Thailand found D. auratus to be infected with Rickettsia raoultii (3/11 = 27%) and Francisella-like endosymbionts (2/11 = 18%), whilst Coxiella bacteria were also investigated but not detected [132].
4. Discussion
Between January 2006 and December 2018, Public Health England’s Tick Surveillance Scheme (TSS) received 59 records from humans with recent travel history, comprised of 66 individual ticks belonging to 16 tick species that were associated with travel outside of the UK. Records were submitted by people with recent travel history to 25 different countries, with 70% of confirmed and possible imported records being associated with travel to Europe. Although less than five percent of total records received by the TSS are imported ticks [18], the findings suggest that UK travellers are exposed to a variety of tick species when abroad. Whilst it was not possible to test the ticks for pathogens in the current study, literature reviews suggest that just over half (9/16) of the received tick species are known to vector at least one tick-borne pathogen in the country of acquisition, suggesting that travellers exposed to ticks may be at risk of being bitten by a species that is a known vector, which could have implications for the transmission of novel tick-borne diseases to UK travellers.
Whilst there are several records of non-native ticks entering the UK previously on animal hosts, including dogs [44,343,344,345,346,347,348,349,350,351,352], horses [353], migratory birds [354,355] and reptiles [356,357,358], the current study is an extensive report of tick exposure to UK travellers submitted to the TSS. Reports of two records included in the current study have been published previously [6,359]. First, a female I. holocyclus was detected on a traveller recently returned from Australia who presented with swelling at the bite site and was prescribed antibiotics as an infected mole or wart was suspected [6]. In the second case, a female D. marginatus was received from a patient with no history of travel experiencing swelling, swollen glands and flu-like symptoms [359]. As D. marginatus is not endemic in the UK, further investigations revealed that the patient had had contact with several European visitors in the month preceding the bite, and a family member had driven her car through Holland, Germany and France the week before, so it was suggested that the tick was imported by one of the travellers [359]. Such an event highlights the importance for clinicians to obtain substantial information from patients presenting with tick bites. Investigations should include questions regarding extended travel history, including several weeks prior to tick removal, as several non-native tick records were received where the date of removal was several weeks after the person had returned from abroad. In addition, questions on contact with visitors who have recently been overseas should also be considered.
Some of the imported species received by the TSS have been reported as imported in the UK previously. Two days after returning from a two-month trip to Missouri, an 84-year-old male presented to his general practitioner in Northern Ireland, and an almost fully fed female A. americanum was detected [8]. Another study described 16 tick species removed from UK domestic and international travellers; six non-native species detected in the paper are reported in the current study (A. americanum, A. cajennense, D. variabilis, D. marginatus, I. scapularis and Rh. sanguineus), whilst six additional non-native species were also described (A. maculatum, A. hebraeum, A. variegatum, H. concinna, Hy. marginatum marginatum and Hy. marginatum rufipes), as well as four species acquired within the UK (D. reticulatus, H. punctata, I. ricinus and I. hexagonus) [13]. Whilst I. ricinus was the most common species detected, 25 of 28 records were acquired within the UK, whereas of tick records acquired from outside of the UK, the most common species detected was D. marginatus (four records), acquired in France, Italy, Greece and Romania [13]. Other published studies have described non-native tick species detected on imported dogs in the UK, including Hy. lusitanicum [346], D. variabilis [351], I. holocyclus [343] and Rh. sanguineus [351]. Four of the species reported in the current study, however, have been detected on UK travellers for the first time: H. hystricis, Hy. truncatum, Rh. appendiculatus and Rh. gertrudae. As imported tick species can transmit a range of pathogens, UK travellers may be exposed to novel pathogens which are unfamiliar to public health professionals in the UK. As such, issuing appropriate public health guidance on tick awareness and avoidance prior to travel could reduce the risk of acquiring tick bites and subsequent tick-borne infections whilst overseas.
Confirmed and possible imported ticks were removed from people with recent travel history in all months apart from March. Sixty-three percent of records detailed ticks removed between April and July, which may reflect travelling activity, as most travel abroad by UK residents occurs during these months [360]. Seasonality of adult and nymph I. ricinus in the UK and Europe is highest between April and June [361,362,363], however, so it is also possible that the peak in imported ticks between April and July may be a consequence of 54% of all records being I. ricinus. It should be noted, however, that 20% of imported records were received between November and February, when I. ricinus activity is at its lowest in the UK [18]. The results from the current study suggest that the risk of being bitten by a tick whilst travelling occurs throughout the year, but is likely to vary depending on travel destination, so advice on tick awareness, removal and tick-borne infections should be provided to travellers year-round, with special attention paid during late spring and early summer when tick bite risk is highest in the most common destinations for UK travellers.
Seventy percent of ticks received by the TSS from people with a recent travel history were associated with recent travel to Europe, and as Europe is a common destination for UK travellers, the risk of ticks and tick-borne diseases being imported from Europe is higher. The predominant species received from people recently returned from Europe was I. ricinus, which is also the most common tick species found throughout the UK [18]. In Europe, I. ricinus is the predominant vector of B. burgdorferi s.l., the causative agent of Lyme borreliosis, which is the most common tick-borne infection in Europe with approximately 65,000 cases reported annually between 1987–2006 [364,365,366]. Overall, the highest incidence of Lyme borreliosis has been reported in eastern and central Europe, and decreases from east to west, although local incidence can vary [135,367,368,369,370,371]. Lyme borreliosis should be considered in patients returning from Europe with a history of tick bites, yet whether there is an increased or reduced risk of UK travellers being bitten by B. burgdorferi-infected I. ricinus in European countries compared with the UK will be dependent upon the country of travel. As the incidence of Lyme borreliosis is higher in eastern and central Europe [135,367,368,369,370,371], travellers to these regions in particular should be made aware of the increased risk and symptoms of Lyme borreliosis.
Along with Lyme borreliosis, TBE should also be considered in patients presenting with symptoms and a history of tick bites following travel to Europe. It is the second most important tick-borne disease transmitted by I. ricinus in Europe, and to date, infected I. ricinus have been detected from nine of the eleven European countries where recorders had a recent history of travel and subsequent tick bite. Several cases of travellers contracting TBE whilst abroad have been previously reported. Five imported cases of TBE were reported in the USA between 2000 and 2009 from patients who had travelled to Czechia, Sweden, Russia and China, and four out of five of the patients described having multiple tick bites whilst abroad [372]. Between 2012 and 2016, five imported cases of TBE were described in the UK, although the country of origin was unknown [165]. In 2011, two Dutch travellers returning from Austria were diagnosed with TBE; and similarly to the previous study, both patients reported tick bites [373]. In parts of Austria, TBEV is endemic, and the risk of a tourist acquiring the infection after spending four weeks in an endemic region is estimated as 1 in 10,000, and following this, it is estimated that 60 travel-associated (i.e. exported) cases of TBE were likely to occur over the whole summer period [374]. As the popularity of outdoor activities such as hiking and biking increases, people may be more exposed to tick bites than in the past [375]. The risk of acquiring TBEV infection when abroad, therefore, should be made clear to travellers from non-endemic regions [375]. Furthermore, the incidence of TBE is expanding northwards to higher latitudes and altitudes [150,151,152], and could result in increased human exposure to infected ticks in the future.
Whilst Lyme borreliosis is the most important tick-borne infection in Europe and North America, it is important to remember this is not the case in other countries, so patients with travel history to other continents may present with different symptoms. It is also important to consider that the same organism may have a different pathogenicity in a different country. An example of this is the causative agent of human granulocytic anaplasmosis (HGA), Anaplasma phagocytophilum. In the USA, HGA is a notifiable infection, and the second most important tick-borne disease (after Lyme borreliosis), with increasing infection incidence from 1.4 cases per million persons in 2000 to 17.9 cases per million persons in 2017 [376]. In comparison, only 70 cases have ever been reported in Europe and are sporadic [377,378]. It is unclear whether the small number of confirmed cases in Europe is due to under-reporting, under-diagnosis or low pathogenicity of A. phagocytophilum strains circulating in Europe [299].
5. Conclusions
Since 2006, 16 tick species removed from humans with a recent travel history were submitted to the TSS. Furthermore, literature reviews suggested that nine of the received species are known to vector at least one organism that is pathogenic to humans in the country of travel. Such findings suggest that travellers exposed to ticks may be at risk of being bitten by species known to transmit at least one pathogen. As 70% of received ticks were confirmed or suspected to have been acquired in Europe, and European countries are common destinations for UK travellers, the risk of imported ticks and tick-borne diseases from Europe is higher, and UK travellers should be made aware of the risk preceding and following European travel.
Author Contributions
Conceptualization, E.L.G., B.C., J.M.M., K.H.; methodology, E.L.G., B.C., M.E.P., L.P.P., J.M.M., K.H.; investigation, E.L.G., B.C., M.E.P.; data curation (lit review), E.L.G.; writing—original draft preparation, E.L.G.; writing—review and editing, E.L.G., B.C., M.E.P., L.P.P., J.M.M., K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We gratefully thank the continued support from members of the public and health professionals who have submitted the ticks described here to the Scheme—without these contributions, the Scheme would not operate and we would miss vital information about ticks acquired by UK residents when overseas. We also gratefully acknowledge the assistance from overseas tick experts, who have assisted us with identification, including Dmitry Apanaskevich, Margarida Santos-Silva, Maxime Madder, Santiago Nava and the Tick Encounter team. We thank the Geographical Information Systems team at PHE (James Lewis, Francis Senyah, Peter Payne and Bryony Cook) and the Scientific Computing team (Anna Rance, Beth Mackenzie and Conor Newcombe) who maintain the software to run the Scheme.
Conflicts of Interest
There authors declare no conflicts of interest
References
- World Tourism Organization. UNWTO Annual Report 2017. UNWTO Madr. 2018, 6, 1–28. [Google Scholar]
- Office for National Statistics. Travel Trends: 2017. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/leisureandtourism/articles/traveltrends/2017 (accessed on 13 June 2019).
- Parola, P.; Raoult, D. Ticks and Tickborne Bacterial Diseases in Humans: An Emerging Infectious Threat. Clin. Infect. Dis. 2001, 32, 897–928. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D. Travel and tick-borne diseases: Lyme disease and beyond. Travel Med. Infect. Dis. 2018, 26, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Pek, C.H.; Cheong, C.S.J.; Yap, Y.L.; Doggett, S.; Lim, T.C.; Ong, W.C.; Lim, J. Rare Cause of Facial Palsy: Case Report of Tick Paralysis by Ixodes holocyclus Imported by a Patient Travelling into Singapore from Australia. J. Emerg. Med. 2016, 51, e109–e114. [Google Scholar] [CrossRef]
- Pietzsch, M.E.; Hansford, K.; Medlock, J.M.; Doggett, S.L. Australian paralysis tick imported on a traveller returning to the UK. Travel Med. Infect. Dis. 2014, 12, 196–197. [Google Scholar] [CrossRef]
- Mathison, B.A.; Gerth, W.J.; Pritt, B.S.; Baugh, S. Introduction of the exotic tick Hyalomma truncatum on a human with travel to Ethiopia: A case report. Ticks Tick-Borne Dis. 2015, 6, 152–154. [Google Scholar] [CrossRef]
- Alderdice, J.M.; Burgess, I.F. The travels of a lone star tick. J. Clin. Pathol. 1998, 51, 403. [Google Scholar] [CrossRef]
- Molaei, G.; Andreadis, T.G.; Anderson, J.F.; Iii, K.C.S. An Exotic Hitchhiker: A Case Report of Importation into Connecticut from Africa of the Human Parasitizing Tick, Hyalomma truncatum (Acari: Ixodidae). J. Parasitol. 2018, 104, 302–305. [Google Scholar] [CrossRef]
- Burridge, M.J.; Simmons, L.A.; Simbi, B.H.; Mahan, S.M.; Fournier, P.-E.; Raoult, D. Introduction of the Exotic Tick Amblyomma hebraeum into Florida on a Human Host. J. Parasitol. 2002, 88, 800. [Google Scholar] [CrossRef]
- Heath, A.; Hardwick, S. The role of humans in the importation of ticks to New Zealand: A threat to public health and biosecurity. N. Z. Med. J. 2011, 124, 67–82. [Google Scholar]
- Anderson, J.F.; Magnarelli, L.A.; Burgdorfer, W.; Casper, E.A.; Philip, R.N. Importation Into the United States from Africa of Rhipicephalus simus on a Boutonneuse Fever Patient. Am. J. Trop. Med. Hyg. 1981, 30, 897–899. [Google Scholar] [CrossRef] [PubMed]
- McGarry, J.W. Travel and disease vector ticks. Travel Med. Infect. Dis. 2011, 9, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, T.; Szymanek-Pasternak, A.; Mączka, I.; Fiecek, B.; Simon, K.; Tylewska-Wierzbanowska, S. Case report of African tick-bite fever from Poland. Advances in Dermatology and Allergology/Postȩpy Dermatol. Alergol. 2013, 30, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Neal, S.P. African tick- bite fever among international travelers—Oregon, 1998. Morb. Mortal. Wkly. Rep. 1998, 47, 950–952. [Google Scholar]
- Martyn, K.P. Provisional Atlas of the Ticks (Ixodoidea) of the British Isles; Biological Records Centre Institute of Terrestrial Ecology: Huntingdon, UK, 1988. [Google Scholar]
- Jameson, L.J.; Medlock, J.M. Tick Surveillance in Great Britain. Vector-Borne Zoonotic Dis. 2011, 11, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Cull, B.; Pietzsch, M.E.; Hansford, K.M.; Gillingham, E.L.; Medlock, J.M. Surveillance of British ticks: An overview of species records, host associations, and new records of Ixodes ricinus distribution. Ticks Tick-Borne Dis. 2018, 9, 605–614. [Google Scholar] [CrossRef]
- Clifford, C.M.; Arthur, D.R. British Ticks. J. Parasitol. 1964, 50, 285. [Google Scholar] [CrossRef]
- Hillyard, P.D. Ticks of North-West Europe (Synopses of the British Fauna); The Linnean Society of London: London, UK, 1996. [Google Scholar]
- Estrada-Peña, A.; Nava, S.; Petney, T. Description of all the stages of Ixodes inopinatus n. sp. (Acari: Ixodidae). Ticks Tick-Borne Dis. 2014, 5, 734–743. [Google Scholar] [CrossRef]
- Walker, A.; Keirans, J.E.; Horak, I.G. The Genus Rhipicephalus (Acari: Ixodidade): A Guide to the Brown Ticks of the World; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Rubel, F.; Brugger, K.; Pfeffer, M.; Chitimia-Dobler, L.; Didyk, Y.M.; Leverenz, S.; Dautel, H.; Kahl, O. Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick-Borne Dis. 2016, 7, 224–233. [Google Scholar] [CrossRef]
- Rizzoli, A.; Silaghi, C.; Obiegala, A.; Rudolf, I.; Hubálek, Z.; Földvári, G. Ixodes ricinus and Its Transmitted Pathogens in Urban and Peri-Urban Areas in Europe: New Hazards and Relevance for Public Health. Front. Public Health 2014, 2, 251. [Google Scholar] [CrossRef]
- Pato, F.J.; Panadero, R.; Vázquez, L.; López, C.M.; Díaz, P.; Vázquez, E.; Díez-Baños, P.; Morrondo, P.; Díaz, P. Seroprevalence of Borrelia burgdorferi sensu lato in roe deer (Capreolus capreolus) from northwestern Spain. J. Zoo Wildl. Med. 2013, 44, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Hoby, S.; Mathis, A.; Doherr, M.G.; Robert, N.; Ryser-Degiorgis, M.-P. Babesia capreoli infections in Alpine chamois (Rupicapra r. rupicapra), roe deer (Capreolus c. capreolus) and red deer (Cervus elaphus) from Switzerland. J. Wildl. Dis. 2009, 45, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, L.; Panadero, R.; DaCal, V.; Pato, F.J.; López, C.; Díaz, P.; Arias, M.S.; Fernández, G.; Díez-Baños, P.; Morrondo, P.; et al. Tick infestation (Acari: Ixodidae) in roe deer (Capreolus capreolus) from northwestern Spain: Population dynamics and risk stratification. Exp. Appl. Acarol. 2010, 53, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Pacilly, F.; Benning, M.; Jacobs, F.; Leidekker, J.; Sprong, H.; Van Wieren, S.; Takken, W. Blood feeding on large grazers affects the transmission of Borrelia burgdorferi sensu lato by Ixodes ricinus. Ticks Tick-Borne Dis. 2014, 5, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Cull, B.; Vaux, A.G.C.; Ottowell, L.J.; Gillingham, E.L.; Medlock, J.M. Tick infestation of small mammals in an English woodland. J. Vector Ecol. 2017, 42, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Hoodless, A.N.; Kurtenbach, K.; Peacey, M. The role of pheasants as hosts for ticks and Lyme disease spirochaetes in southern England. Game Wildl. 1998, 15, 477–490. [Google Scholar]
- Zdrazilova-Dubska, L.; Literak, I.; Kocianova, E.; Taragelova, V.; Sverakova, V.; Sychra, O.; Hromadko, M. Synanthropic Birds Influence the Distribution of Borrelia Species: Analysis of Ixodes ricinus Ticks Feeding on Passerine Birds. Appl. Environ. Microbiol. 2010, 77, 1115–1117. [Google Scholar] [CrossRef]
- Cayol, C.; Koskela, E.; Mappes, T.; Siukkola, A.; Kallio, E.R. Temporal dynamics of the tick Ixodes ricinus in northern Europe: Epidemiological implications. Parasites Vectors 2017, 10, 166. [Google Scholar] [CrossRef]
- Selmi, M.; Ballardini, M.; Salvato, L.; Ricci, E. Rickettsia spp. in Dermacentor marginatus ticks: Analysis of the host-vector-pathogen interactions in a northern Mediterranean area. Exp. Appl. Acarol. 2017, 72, 79–91. [Google Scholar] [CrossRef]
- Petney, T.N.; Pfäffle, M.; Skuballa, J.D. An annotated checklist of the ticks (Acari: Ixodida) of Germany. Syst. Appl. Acarol. 2012, 17, 115–170. [Google Scholar] [CrossRef]
- Walter, M.; Brugger, K.; Rubel, F. The ecological niche of Dermacentor marginatus in Germany. Parasitol. Res. 2016, 115, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Selmi, M.; Tomassone, L.; Ceballos, L.A.; Crisci, A.; Ragagli, C.; Pintore, M.D.; Mignone, W.; Pautasso, A.; Ballardini, M.; Casalone, C.; et al. Analysis of the environmental and host-related factors affecting the distribution of the tick Dermacentor marginatus. Exp. Appl. Acarol. 2018, 75, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Nosek, J. The ecology and public health importance of Dermacentor marginatus and D. reticulatus in Central Europe. Folia Parasitol. 1972, 19, 93–102. [Google Scholar]
- Hornok, S.; Farkas, R. Influence of biotope on the distribution and peak activity of questing ixodid ticks in Hungary. Med. Vet. Entomol. 2009, 23, 41–46. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Jongejan, F. Ticks Feeding on Humans: A Review of Records on Human-Biting Ixodoidea with Special Reference to Pathogen Transmission. Exp. Appl. Acarol. 1999, 23, 685–715. [Google Scholar] [CrossRef] [PubMed]
- Keirans, J.E.; Durden, L.A. Invasion: Exotic ticks (Acari: Argasidae, Ixodidae) imported into the United States. A review and new records. J. Med. Entomol. 2001, 38, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Figueredo, L.A.; Brandão-Filho, S.P. Rhipicephalus sanguineus (Acari: Ixodidae), the brown dog tick, parasitizing humans in Brazil. Rev. Soc. Bras. Med. Trop. 2006, 39, 64–67. [Google Scholar] [CrossRef]
- Dantas-Torres, F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): From taxonomy to control. Vet. Parasitol. 2008, 152, 173–185. [Google Scholar] [CrossRef]
- Renvoisé, A.; Delaunay, P.; Blanchouin, E.; Cannavo, I.; Cua, E.; Socolovschi, C.; Parola, P.; Raoult, D. Urban family cluster of spotted fever rickettsiosis linked to Rhipicephalus sanguineus infected with Rickettsia conorii subsp. caspia and Rickettsia massiliae. Ticks Tick-Borne Dis. 2012, 3, 389–392. [Google Scholar] [CrossRef]
- Hansford, K.M.; Phipps, L.P.; Cull, B.; Pietzsch, M.E.; Medlock, J.M. Rhipicephalus sanguineus importation into the UK: Surveillance, risk, public health awareness and One Health response. Vet. Rec. 2016, 180, 119. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Palomar, A.M.; Santibáñez, P.; Sánchez, N.; Habela, M.A.; Portillo, A.; Romero, L.; Oteo, J.A. Crimean-Congo Hemorrhagic Fever Virus in Ticks, Southwestern Europe, 2010. Emerg. Infect. Dis. 2012, 18, 179–180. [Google Scholar] [CrossRef]
- Apanaskevich, D.A.; Santos-Silva, M.M.; Horak, I.G. The genus Hyalomma Koch, 1844. IV. Redescription of all parasitic stages of H. (Euhyalomma) lusitanicum Koch, 1844 and the adults of H. (E.) franchinii Tonelli Rondelli, 1932 (Acari: Ixodidae) with a first description of its immature stages. Folia Parasitol. 2008, 55, 61–74. [Google Scholar] [CrossRef]
- Valcárcel, F.; González, J.; Sánchez, J.L.P.; Jaime, J.M.T.; Olmeda, A.S. Long-Term Ecological Study of Host-Seeking Adults of Hyalomma lusitanicum (Acari: Ixodidae) in a Meso-Mediterranean Climate. J. Med. Entomol. 2015, 53, 221–224. [Google Scholar] [CrossRef]
- Ruiz-Fons, F.; Fernández-De-Mera, I.G.; Acevedo, P.; Höfle, U.; Vicente, J.; De La Fuente, J.; Gortazár, C. Ixodid ticks parasitizing Iberian red deer (Cervus elaphus hispanicus) and European wild boar (Sus scrofa) from Spain: Geographical and temporal distribution. Vet. Parasitol. 2006, 140, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, M.M.; Beati, L.; Santos-Silva, M.M.; De Sousa, R.; Núncio, M.S.; Melo, P.; Santos-Reis, M.; Fonseca, C.M.M.S.; Formosinho, P.; Vilela, C.; et al. The hard-tick fauna of mainland Portugal (Acari: Ixodidae): An update on geographical distribution and known associations with hosts and pathogens. Exp. Appl. Acarol. 2011, 55, 85–121. [Google Scholar] [CrossRef] [PubMed]
- Barandika, J.F.; Olmeda, S.A.; Casado-Nistal, M.A.; Hurtado, A.; Juste, R.A.; Valcárcel, F.; Anda, P.; Garcia-Perez, A.L. Differences in Questing Tick Species Distribution Between Atlantic and Continental Climate Regions in Spain. J. Med. Entomol. 2011, 48, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Requena-García, F.; Cabrero-Sanudo, F.J.; Olmeda-García, S.; González, J.; Valcárcel, F. Influence of environmental temperature and humidity on questing ticks in central Spain. Exp. Appl. Acarol. 2017, 71, 277–290. [Google Scholar] [CrossRef]
- Childs, J.E.; Paddock, C.D. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu. Rev. Entomol. 2003, 48, 307–337. [Google Scholar] [CrossRef] [PubMed]
- Springer, Y.P.; Eisen, L.; Beati, L.; James, A.M.; Eisen, R.J. Spatial Distribution of Counties in the Continental United States With Records of Occurrence of Amblyomma americanum (Ixodida: Ixodidae). J. Med. Entomol. 2014, 51, 342–351. [Google Scholar] [CrossRef]
- A Merten, H.; Durden, L. A state-by-state survey of ticks recorded from humans in the United States. J. Vector Ecol. 2000, 25, 102–113. [Google Scholar]
- Stromdahl, E.Y.; Hickling, G.J. Beyond Lyme: Aetiology of Tick-borne Human Diseases with Emphasis on the South-Eastern United States. Zoonoses Public Health 2012, 59, 48–64. [Google Scholar] [CrossRef]
- Nadolny, R.M.; Wright, C.L.; Sonenshine, D.E.; Hynes, W.L.; Gaff, H.D. Ticks and spotted fever group rickettsiae of southeastern Virginia. Ticks Tick-Borne Dis. 2014, 5, 53–57. [Google Scholar] [CrossRef]
- Minigan, J.N.; Hager, H.A.; Peregrine, A.S.; Newman, J.A. Current and potential future distribution of the American dog tick (Dermacentor variabilis, Say) in North America. Ticks Tick-Borne Dis. 2018, 9, 354–362. [Google Scholar] [CrossRef]
- Burg, J.G. Seasonal activity and spatial distribution of host-seeking adults of the tick Dermacentor variabilis. Med. Vet. Entomol. 2001, 15, 413–421. [Google Scholar] [CrossRef]
- Stromdahl, E.Y.; Jiang, J.; Vince, M.; Richards, A.L. Infrequency of Rickettsia rickettsii in Dermacentor variabilis removed from humans, with comments on the role of other human-biting ticks associated with spotted fever group rickettsiae in the United States. Vector-Borne Zoonotic Dis. 2011, 11, 969–977. [Google Scholar] [CrossRef]
- James, A.M. Distribution, seasonality, and hosts of the Rocky Mountain wood tick in the United States. J. Med. Entomol. 2006, 43, 17–24. [Google Scholar] [CrossRef]
- Lane, R.S. Ecology of tick-borne agents in California. II. Further observations on rickettsiae. In Rickettsiae and Rickettsial Diseases; Burgdorfer, W., Ed.; Academic Press: Cambridge, MA, USA, 1981; pp. 575–584. [Google Scholar]
- Eads, R.B.; Smith, G.C. Seasonal activity and Colorado tick fever virus infection rates in Rocky Mountain wood ticks, Dermacentor andersoni (Acari: Ixodidae), in north-central Colorado, USA. J. Med. Entomol. 1983, 20, 49–55. [Google Scholar] [CrossRef]
- Eisen, R.J.; Eisen, L.; Beard, C.B. County-Scale Distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Continental United States. J. Med. Entomol. 2016, 53, 349–386. [Google Scholar] [CrossRef]
- Eisen, R.J.; Eisen, L.; Castro, M.B.; Lane, R.S. Environmentally Related Variability in Risk of Exposure to Lyme Disease Spirochetes in Northern California: Effect of Climatic Conditions and Habitat Type. Environ. Entomol. 2003, 32, 1010–1018. [Google Scholar] [CrossRef]
- Padgett, K.; Lane, R.S. Life Cycle of Ixodes pacificus (Acari: Ixodidae): Timing of Developmental Processes Under Field and Laboratory Conditions. J. Med. Entomol. 2001, 38, 684–693. [Google Scholar] [CrossRef]
- Dingler, R.J.; Wright, S.A.; Donohue, A.M.; Macedo, P.A.; Foley, J. Surveillance for Ixodes pacificus and the tick-borne pathogens Anaplasma phagocytophilum and Borrelia burgdorferi in birds from California’s Inner Coast Range. Ticks Tick-Borne Dis. 2014, 5, 436–445. [Google Scholar] [CrossRef]
- Castro, M.B.; Wright, S.A. Vertebrate hosts of Ixodes pacificus (Acari: Ixodidae) in California. J. Vector Ecol. 2007, 32, 140–149. [Google Scholar] [CrossRef]
- Eisen, L.; Eisen, R.J.; Lane, R.S. The roles of birds, lizards, and rodents as hosts for the western black-legged tick Ixodes pacificus. J. Vector Ecol. 2004, 29, 295–308. [Google Scholar]
- Eisen, R.J. Prevalence and abundance of Ixodes pacificus immatures (Acari: Ixodidae) infesting western fence lizards (Sceloporus occidentalis) in northern California: Temporal trends and environmental correlates. J. Parasitol. 2001, 87, 1301–1307. [Google Scholar] [CrossRef]
- Eisen, L.; Eisen, R.J.; Lane, R.S. Seasonal activity patterns of Ixodes pacificus nymphs in relation to climatic conditions. Med. Vet. Entomol. 2002, 16, 235–244. [Google Scholar] [CrossRef]
- Clover, J.R.; Lane, R.S. Evidence Implicating Nymphal Ixodes pacificus (Acari: Ixodidae) in the Epidemiology of Lyme Disease in California. Am. J. Trop. Med. Hyg. 1995, 53, 237–240. [Google Scholar] [CrossRef]
- Salkeld, D.J.; Castro, M.B.; Bonilla, D.; Kjemtrup, A.; Kramer, V.L.; Lane, R.S.; Padgett, K.A. Seasonal activity patterns of the western black-legged tick, Ixodes pacificus, in relation to onset of human Lyme disease in northwestern California. Ticks Tick-Borne Dis. 2014, 5, 790–796. [Google Scholar] [CrossRef]
- Masina, S.; Broady, K. Tick paralysis: Development of a vaccine. Int. J. Parasitol. 1999, 29, 535–541. [Google Scholar] [CrossRef]
- Barker, S.C.; Walker, A.R. Ticks of domestic animals and humans in Australia. Zootaxa 2014, 3816, 1–144. [Google Scholar] [CrossRef]
- Hall-Mendelin, S.; Craig, S.B.; Hall, R.; O’Donoghue, P.; Tulsiani, S.M.; Graham, G.C. Tick paralysis in Australia caused by Ixodes holocyclus Neumann. Ann. Trop. Med. Parasitol. 2011, 105, 95–106. [Google Scholar] [CrossRef]
- Jahfari, S.; Ruyts, S.C.; Frazer-Mendelewska, E.; Jaarsma, R.I.; Verheyen, K.; Sprong, H. Melting pot of tick-borne zoonoses: The European hedgehog contributes to the maintenance of various tick-borne diseases in natural cycles urban and suburban areas. Parasites Vectors 2017, 10, 134. [Google Scholar] [CrossRef]
- Sándor, A.D.; D’Amico, G.; Gherman, C.M.; Dumitrache, M.O.; Domșa, C.; Mihalca, A.D. Mesocarnivores and macroparasites: Altitude and land use predict the ticks occurring on red foxes (Vulpes vulpes). Parasites Vectors 2017, 10, 173. [Google Scholar] [CrossRef][Green Version]
- Estrada-Peña, A.; Roura, X.; Sainz, A.; Miró, G.; Solano-Gallego, L. Species of ticks and carried pathogens in owned dogs in Spain: Results of a one-year national survey. Ticks Tick-Borne Dis. 2017, 8, 443–452. [Google Scholar] [CrossRef]
- Pfäffle, M.; Petney, T.; Skuballa, J.; Taraschewski, H. Comparative population dynamics of a generalist (Ixodes ricinus) and specialist tick (I. hexagonus) species from European hedgehogs. Exp. Appl. Acarol. 2011, 54, 151–164. [Google Scholar] [CrossRef]
- Beichel, E.; Petney, T.N.; Hassler, D.; Brückner, M.; Maiwald, M. Tick infestation patterns and prevalence of Borrelia burgdorferi in ticks collected at a veterinary clinic in Germany. Vet. Parasitol. 1996, 65, 147–155. [Google Scholar] [CrossRef]
- Meyer-Kayser, E.; Hoffmann, L.; Silaghi, C.; Pfister, K.; Mahling, M.; Passos, L.M.F. Dynamics of tick infestations in foxes in Thuringia, Germany. Ticks Tick-Borne Dis. 2012, 3, 232–239. [Google Scholar] [CrossRef]
- Faulde, M.K.; Rutenfranz, M.; Hepke, J.; Rogge, M.; Görner, A.; Keth, A. Human tick infestation pattern, tick-bite rate, and associated Borrelia burgdorferi s.l. infection risk during occupational tick exposure at the Seedorf military training area, northwestern Germany. Ticks Tick-Borne Dis. 2014, 5, 594–599. [Google Scholar] [CrossRef]
- Nijhof, A.M.; Bodaan, C.; Postigo, M.; Nieuwenhuijs, H.; Opsteegh, M.; Franssen, L.; Jebbink, F.; Jongejan, F. Ticks and Associated Pathogens Collected from Domestic Animals in the Netherlands. Vector-Borne Zoonotic Dis. 2007, 7, 585–596. [Google Scholar] [CrossRef]
- Sanogo, Y.O.; Parola, P.; Shpynov, S.; Camicas, J.L.; Brouqui, P.; Caruso, G.; Raoult, D. Genetic Diversity of Bacterial Agents Detected in Ticks Removed from Asymptomatic Patients in Northeastern Italy. Ann. N. Y. Acad. Sci. 2003, 990, 182–190. [Google Scholar] [CrossRef]
- Hubbard, M.J.; Baker, A.S.; Cann, K.J. Distribution of Borrelia burgdorferi s.l. spirochaete DNA in British ticks (Argasidae and Ixodidae) since the 19th Century, assessed by PCR. Med. Vet. Entomol. 1998, 12, 89–97. [Google Scholar] [CrossRef]
- Carter, W.I. A case of human parasitization by Ixodes hexagonus, Leach (hedgehog tick). Br. Med. J. 1955, 22, 1012. [Google Scholar] [CrossRef][Green Version]
- Estrada-Peña, A.; Guglielmone, A.; Mangold, A.J. The distribution and ecological ’preferences’ of the tick Amblyomma cajennense (Acari: Ixodidae), an ectoparasite of humans and other mammals in the Americas. Ann. Trop. Med. Parasitol. 2004, 98, 283–292. [Google Scholar] [CrossRef]
- Pires, M.S.; Dos Santos, T.M.; Santos, H.A.; Vilela, J.A.R.; Peixoto, M.P.; Roier, E.C.R.; Da Silva, C.B.; Barreira, J.D.; De Lemos, E.R.S.; Massard, C.L. Amblyomma cajennense infestation on horses in two microregions of the state of Rio de Janeiro, Brazil. Rev. Bras. Parasitol. Vet. 2013, 22, 235–242. [Google Scholar] [CrossRef]
- Oliveira, P.R.; Borges, L.M.F.; Leite, R.C.; Freitas, C.M.V. Seasonal dynamics of the Cayenne tick, Amblyomma cajennense on horses in Brazil. Med. Vet. Entomol. 2003, 17, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Labruna, M.B.; Kasai, N.; Ferreira, F.; Faccini, J.L.; Gennari, S.M. Seasonal dynamics of ticks (Acari: Ixodidae) on horses in the state of São Paulo, Brazil. Vet. Parasitol. 2002, 105, 65–77. [Google Scholar] [CrossRef]
- Ramos, V.D.N.; Piovezan, U.; Franco, A.H.A.; Osava, C.F.; Herrera, G.P.; Szabó, M.P.J. Feral pigs as hosts for Amblyomma sculptum (Acari: Ixodidae) populations in the Pantanal, Mato Grosso do Sul, Brazil. Exp. Appl. Acarol. 2014, 64, 393–406. [Google Scholar] [CrossRef]
- Martins, T.F.; Barbieri, A.R.M.; Costa, F.B.; Terassini, F.A.; Camargo, L.M.A.; Peterka, C.R.L.; Pacheco, R.D.C.; Dias, R.A.; Nunes, P.H.; Marcili, A.; et al. Geographical distribution of Amblyomma cajennense (sensu lato) ticks (Parasitiformes: Ixodidae) in Brazil, with description of the nymph of A. cajennense (sensu stricto). Parasites Vectors 2016, 9, 1–14. [Google Scholar] [CrossRef]
- Saraiva, D.G.; Fournier, G.F.S.R.; Martins, T.F.; Leal, K.P.G.; Vieira, F.N.; Câmara, E.M.V.C.; Costa, C.G.; Onofrio, V.C.; Barros-Battesti, D.M.; Guglielmone, A.A.; et al. Ticks (Acari: Ixodidae) associated with small terrestrial mammals in the state of Minas Gerais, southeastern Brazil. Exp. Appl. Acarol. 2012, 58, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Labruna, M.B.; Terassini, F.A.; Camargo, L.M.A. Notes on Population Dynamics of Amblyomma Ticks (Acari: Ixodidae) in Brazil. J. Parasitol. 2009, 95, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.R.; Borges, L.; Lopes, C.; Leite, R.C. Population dynamics of the free-living stages of Amblyomma cajennense (Fabricius, 1787) (Acari: Ixodidae) on pastures of Pedro Leopoldo, Minas Gerais State, Brazil. Vet. Parasitol. 2000, 92, 295–301. [Google Scholar] [CrossRef]
- Brites-Neto, J.; Nieri-Bastos, F.A.; Brasil, J.; Duarte, K.M.R.; Martins, T.F.; Veríssimo, C.J.; Barbieri, A.R.M.; Labruna, M.B. Environmental infestation and rickettsial infection in ticks in an area endemic for Brazilian spotted fever. Rev. Bras. Parasitol. Vet. 2013, 22, 367–372. [Google Scholar] [CrossRef]
- Beck, D.L.; Zavala, J.; Montalvo, E.O.; Quintana, F.G. Meteorological indicators for Amblyomma cajennense and population dynamics in the Tamaulipan Biotic Province in Texas. J. Vector Ecol. 2011, 36, 135–146. [Google Scholar] [CrossRef]
- Guglielmone, A.A.; Beati, L.; Barros-Battesti, D.M.; Labruna, M.B.; Nava, S.; Venzal, J.M.; Mangold, A.J.; Szabó, M.P.J.; Martins, J.R.; González-Acuña, D.; et al. Ticks (Ixodidae) on humans in South America. Exp. Appl. Acarol. 2006, 40, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Labruna, M.B. Ecology of Rickettsia in South America. Ann. N. Y. Acad. Sci. 2009, 1166, 156–166. [Google Scholar] [CrossRef]
- Apanaskevich, D.A.; Horak, I.G. The genus Hyalomma. VI. Systematics of H. (Euhyalomma) truncatum and the closely related species, H. (E.) albiparmatum and H. (E.) nitidum (Acari: Ixodidae). Exp. Appl. Acarol. 2008, 44, 115–136. [Google Scholar] [CrossRef]
- Magano, S.R.; Els, D.; Chown, S. Feeding patterns of immature stages of Hyalomma truncatum and Hyalomma marginatum rufipes on different hosts. Exp. Appl. Acarol. 2000, 24, 301–313. [Google Scholar] [CrossRef]
- Apanaskevich, D.A.; Schuster, A.L.; Horak, I.G. The genus Hyalomma: VII. Redescription of all parasitic stages of Hy. (Euhyalomma) dromedarii and H. (E.) schulzei (Acari: Ixodidae). Morpho. System. Evo. 2008, 45, 817–831. [Google Scholar] [CrossRef]
- Dreyer, K.; Fourie, L.; Kok, D.J. Tick diversity, abundance and seasonal dynamics in a resource-poor urban environment in the Free State Province. Onderstepoort J. Vet. Res. 1998, 65, 305–316. [Google Scholar]
- Horak, I.G.; Fourie, L.; Braack, L. Small mammals as hosts of immature ixodid ticks. Onderstepoort J. Vet. Res. 2005, 72, 255–261. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Horak, I.; Golezardy, H.; Uys, A. Ticks associated with the three largest wild ruminant species in Southern Africa. Onderstepoort J. Vet. Res. 2007, 74, 231–242. [Google Scholar] [CrossRef][Green Version]
- Walker, J.B. A review of the Ixodid ticks (Acari, Ixodidae) occurring in southern Africa. Onderstepoort J. Vet. Res. 1991, 58, 81–105. [Google Scholar]
- Fourie, L.; Kok, D.J.; Heyne, H. Adult ixodid ticks on two cattle breeds in the south-western Free State, and their seasonal dynamics. Onderstepoort J. Vet. Res. 1996, 63, 19–23. [Google Scholar]
- Horak, I.G.; Matthee, S. Parasites of domestic and wild animals in South Africa. XLIII. Ixodid ticks of domestic dogs and cats in the Western Cape Province. Onderstepoort J. Vet. Res. 2003, 70, 187–195. [Google Scholar]
- Brain, C.; Bohrmann, R. Tick infestation of baboons (Papio ursinus) in the Namib Desert. J. Wildl. Dis. 1992, 28, 188–191. [Google Scholar] [CrossRef]
- Horak, I.; Fourie, L. Parasites of domestic and wild animals in South Africa. XXXI. Adult ixodid ticks on sheep in the Cape Province and in the Orange Free State. Onderstepoort J. Vet. Res. 1992, 59, 275–283. [Google Scholar]
- Horak, I.; Fourie, L.; Heyne, H.; Walker, J.B.; Needham, G. Ixodid ticks feeding on humans in South Africa: With notes on preferred hosts, geographic distribution, seasonal occurrence and transmission of pathogens. Exp. Appl. Acarol. 2002, 27, 113–136. [Google Scholar] [CrossRef]
- Lessard, P.; L’Eplattenier, R.; Norval, R.; Kundert, K.; Dolan, T.T.; Croze, H.; Walker, J.B.; Irvin, A.D.; Perry, B.D. Geographical information systems for studying the epidemiology of cattle diseases caused by Theileria parva. Vet. Rec. 1990, 126, 255–262. [Google Scholar]
- Walker, A.R. Ticks of Domestic Animals in Africa: A Guide to Identification of Species; Bioscience Reports: Edinburgh, UK, 2003. [Google Scholar]
- Hoogstraal, H. Studies on southeast Asian Haemaphysalis ticks (Ixodoidea, Ixodidae). The identity, distribution, and hosts of H. (Kaiseriana) hystricis Supino. J. Parasitol. 1965, 51, 467–480. [Google Scholar] [CrossRef]
- Hou, J.; Ling, F.; Chai, C.; Lu, Y.; Yu, X.; Lin, J.; Sun, J.; Chang, Y.; Ye, X.; Gu, S.; et al. Prevalence of Borrelia burgdorferi sensu lato in ticks from eastern China. Am. J. Trop. Med. Hyg. 2014, 92, 262–266. [Google Scholar] [CrossRef]
- Khoo, J.J.; Lim, F.S.; Tan, K.-K.; Chen, F.S.; Phoon, W.H.; Khor, C.S.; Pike, B.L.; Chang, L.Y.; Abubakar, S. Detection in Malaysia of a Borrelia sp. From Haemaphysalis hystricis (Ixodida: Ixodidae). J. Med. Èntomol. 2017, 54, 1444–1448. [Google Scholar] [CrossRef]
- Shimada, Y.; Beppu, T.; Inokuma, H.; Okuda, M.; Onishi, T. Ixodid tick species recovered from domestic dogs in Japan. Med. Vet. Entomol. 2003, 17, 38–45. [Google Scholar] [CrossRef]
- Durden, L.A.; Merker, S.; Beati, L. The tick fauna of Sulawesi, Indonesia (Acari: Ixodoidea: Argasidae and Ixodidae). Exp. Appl. Acarol. 2008, 45, 85–110. [Google Scholar] [CrossRef]
- Grassman, L.I.; Sarataphan, N.; Tewes, M.E.; Silvy, N.J.; Nakanakrat, T. Ticks (Acari: Ixodidae) Parasitizing Wild Carnivores in Phu Khieo Wildlife Sanctuary, Thailand. J. Parasitol. 2004, 90, 657–659. [Google Scholar] [CrossRef]
- Yamauchi, T.; Yano, S.; Yamamoto, T.; Yamamoto, E.; Miyamoto, T. Ticks (Acari: Ixodidae) from medium-sized to large mammals in Ehime Prefecture, Japan. Exp. Appl. Acarol. 2012, 60, 263–270. [Google Scholar] [CrossRef]
- Tateno, M.; Sunahara, A.; Nakanishi, N.; Izawa, M.; Matsuo, T.; Setoguchi, A.; Endo, Y. Molecular survey of arthropod-borne pathogens in ticks obtained from Japanese wildcats. Ticks Tick-Borne Dis. 2015, 6, 281–289. [Google Scholar] [CrossRef]
- Ajithkumar, K.; Ravindran, R.; Ghosh, S. Dermacentor auratus Supino, 1897 (Acarina, Ixodidae) reported from Wayanad, Kerala. Indian J. Med. Res. 2012, 135, 435–436. [Google Scholar]
- Mariana, A.; Zuraidawati, Z.; Ho, T.M.; Kulaimi, B.M.; Saleh, I.; Shukor, M.N.; Shahrul-Anuar, M.S. Ticks (Ixodidae) and other ectoparasites in Ulu Muda Forest Reserve, Kedah, Malaysia. Southeast Asian J. Trop. Med. Public Health 2008, 39, 496–506. [Google Scholar]
- Vongphayloth, K.; Hertz, J.C.; Lakeomany, K.; Apanaskevich, D.; Robbins, R.G.; Sutherland, I.W.; Brey, P.T. The Genus Dermacentor (Acari: Ixodidae) in Laos: A Review and Update of Species Records. J. Med. Entomol. 2018, 55, 1047–1050. [Google Scholar] [CrossRef]
- Parola, P.; Cornet, J.-P.; Sanogo, Y.O.; Miller, R.S.; Van Thien, H.; Gonzalez, J.-P.; Raoult, D.; Telford, S.R., III; Wongsrichanalai, C. Detection of Ehrlichia spp., Anaplasma spp., Rickettsia spp., and Other Eubacteria in Ticks from the Thai-Myanmar Border and Vietnam. J. Clin. Microbiol. 2003, 41, 1600–1608. [Google Scholar] [CrossRef]
- Ariyarathne, S.; Dilrukshi, P.R.M.P.; Amarasinghe, P.H.; Rajakaruna, R.S. Intra-aural ecdysis of Dermacentor auratus Supino, 1897, in a human host. Ceylon Med. J. 2011, 56, 133. [Google Scholar] [CrossRef]
- Sumrandee, C.; Baimai, V.; Trinachartvanit, W.; Ahantarig, A. Hepatozoon and Theileria species detected in ticks collected from mammals and snakes in Thailand. Ticks Tick-Borne Dis. 2015, 6, 309–315. [Google Scholar] [CrossRef]
- Kirwan, E.O. A tick on the upper eye-lid. Br. J. Ophthalmol. 1935, 19, 659–661. [Google Scholar] [CrossRef][Green Version]
- Liyanaarachchi, D.; Rajakaruna, R.; Dikkumbura, A.; Rajapakse, R. Ticks infesting wild and domestic animals and humans of Sri Lanka with new host records. Acta Trop. 2015, 142, 64–70. [Google Scholar] [CrossRef]
- Ariyarathne, S.; Apanaskevich, D.A.; Amarasinghe, P.H.; Rajakaruna, R.S. Diversity and distribution of tick species (Acari: Ixodidae) associated with human otoacariasis and socio-ecological risk factors of tick infestations in Sri Lanka. Exp. Appl. Acarol. 2016, 70, 99–123. [Google Scholar] [CrossRef]
- Hoogstraal, H.; Wassef, H.Y. Dermacentor (Indocentor) auratus (Acari: Ixodoidea: Ixodidae): Hosts, Distribution, and Medical Importance in Tropical Asia. J. Med. Entomol. 1985, 22, 170–177. [Google Scholar] [CrossRef]
- Sumrandee, C.; Baimai, V.; Trinachartvanit, W.; Ahantarig, A. Molecular detection of Rickettsia, Anaplasma, Coxiella and Francisella bacteria in ticks collected from Artiodactyla in Thailand. Ticks Tick-Borne Dis. 2016, 7, 678–689. [Google Scholar] [CrossRef]
- Ghosh, S.; Bansal, G.C.; Gupta, S.C.; Ray, D.; Khan, M.Q.; Irshad, H.; Shahiduzzaman, M.; Seitzer, U.; Ahmed, J.S. Status of tick distribution in Bangladesh, India and Pakistan. Parasitol. Res. 2007, 101, 207–216. [Google Scholar] [CrossRef]
- Edussuriya, B.D.; Weilgama, D. Case reports: Intra-aural tick infestations in humans in Sri Lanka. Trans. R. Soc. Trop. Med. Hyg. 2004, 97, 412–413. [Google Scholar] [CrossRef]
- Strnad, M.; Hönig, V.; Růžek, D.; Grubhoffer, L.; Rego, R.O.M. Europe-Wide Meta-Analysis of Borrelia burgdorferi Sensu Lato Prevalence in Questing Ixodes ricinus Ticks. Appl. Environ. Microbiol. 2017, 83, e00609–e00617. [Google Scholar] [CrossRef]
- Gern, L.; Toutoungi, L.N.; Hu, C.M.; Aeschlimann, A. Ixodes (Pholeoixodes) hexagonus, an efficient vector of Borrelia burgdorferi in the laboratory. Med. Vet. Entomol. 1991, 5, 431–435. [Google Scholar] [CrossRef]
- Toutoungi, L.N.; Gern, L. Ability of transovarially and subsequent transstadially infected Ixodes hexagonus ticks to maintain and transmit Borrelia burgdorferi in the laboratory. Exp. Appl. Acarol. 1993, 17, 581–586. [Google Scholar] [CrossRef]
- Franke, J.; Hildebrandt, A.; Dorn, W. Exploring gaps in our knowledge on Lyme borreliosis spirochaetes – Updates on complex heterogeneity, ecology, and pathogenicity. Ticks Tick-Borne Dis. 2013, 4, 11–25. [Google Scholar] [CrossRef]
- Humair, P.-F.; Rais, O.; Gern, L. Transmission of Borrelia afzelii from Apodemus mice and Clethrionomys voles to Ixodes ricinus ticks: Differential transmission pattern and overwintering maintenance. Parasitology 1999, 118, 33–42. [Google Scholar] [CrossRef]
- Kurtenbach, K.; Peacey, M.; Rijpkema, S.G.T.; Hoodless, A.N.; Nuttall, P.A.; Randolph, S.E. Differential Transmission of the Genospecies of Borrelia burgdorferi Sensu Lato by Game Birds and Small Rodents in England. Appl. Environ. Microbiol. 1998, 64, 1169–1174. [Google Scholar] [CrossRef]
- Humair, P.-F.; Peter, O.; Wallich, R.; Gern, L. Strain Variation of Lyme Disease Spirochetes Isolated from Ixodes ricinus Ticks and Rodents Collected in Two Endemic Areas in Switzerland. J. Med. Entomol. 1995, 32, 433–438. [Google Scholar] [CrossRef]
- Kurtenbach, K.; Carey, D.; Hoodless, A.N.; Nuttall, P.A.; Randolph, S.E. Competence of Pheasants as Reservoirs for Lyme Disease Spirochetes. J. Med. Entomol. 1998, 35, 77–81. [Google Scholar] [CrossRef]
- Humair, P.-F.; Postic, D.; Wallich, R.; Gern, L. An Avian Reservoir (Turdus merula) of the Lyme Borreliosis Spirochetes. Zentralblatt Fur Bakteriol. 1998, 287, 521–538. [Google Scholar] [CrossRef]
- Kurtenbach, K.; De Michelis, S.; Etti, S.; Schäfer, S.M.; Sewell, H.-S.; Brade, V.; Kraiczy, P. Host association of Borrelia burgdorferi sensu lato—The key role of host complement. Trends Microbiol. 2002, 10, 74–79. [Google Scholar] [CrossRef]
- Baranton, G.; Postic, D.; Girons, I.S.; Boerlin, P.; Piffaretti, J.-C.; Assous, M.; Grimont, P.A.D. Delineation of Borrelia burgdorferi Sensu Stricto, Borrelia garinii sp. nov., and Group VS461 Associated with Lyme borreliosis. Int. J. Syst. Bacteriol. 1992, 42, 378–383. [Google Scholar] [CrossRef]
- Richter, D.; Schlee, D.B.; Matuschka, F.-R. Relationships of a Novel Lyme Disease Spirochete, Borrelia spielmani sp. nov., with Its Hosts in Central Europe. Appl. Environ. Microbiol. 2004, 70, 6414–6419. [Google Scholar] [CrossRef]
- Stanek, G.; Reiter, M. The expanding Lyme Borrelia complex—Clinical significance of genomic species? Clin. Microbiol. Infect. 2011, 17, 487–493. [Google Scholar] [CrossRef]
- Gritsun, T.S.; Lashkevich, V.A.; Gould, E.A. Tick-borne encephalitis. Antivir. Res. 2003, 57, 129–146. [Google Scholar] [CrossRef]
- Holzmann, H.; Aberle, S.W.; Stiasny, K.; Werner, P.; Mischak, A.; Zainer, B.; Netzer, M.; Koppi, S.; Bechter, E.; Heinz, F.X. Tick-borne Encephalitis from Eating Goat Cheese in a Mountain Region of Austria. Emerg. Infect. Dis. 2009, 15, 1671–1673. [Google Scholar] [CrossRef]
- Dobler, G.; Gniel, D.; Petermann, R.; Pfeffer, M. Epidemiology and distribution of tick-borne encephalitis. Wien. Med. Wochenschr. 2012, 162, 230–238. [Google Scholar] [CrossRef]
- Amicizia, D.; Domnich, A.; Panatto, D.; Lai, P.L.; Cristina, M.; Avio, U.; Gasparini, R. Epidemiology of tick-borne encephalitis (TBE) in Europe and its prevention by available vaccines. Hum. Vaccines Immunother. 2013, 9, 1163–1171. [Google Scholar] [CrossRef]
- Danielová, V.; Daniel, M.; Schwarzová, L.; Materna, J.; Rudenko, N.; Golovchenko, M.; Holubová, J.; Grubhoffer, L.; Kilian, P. Integration of a Tick-Borne Encephalitis Virus and Borrelia burgdorferi sensu lato into Mountain Ecosystems, Following a Shift in the Altitudinal Limit of Distribution of Their Vector, Ixodes ricinus (Krkonoše Mountains, Czech Republic). Vector-Borne Zoonotic Dis. 2010, 10, 223–230. [Google Scholar] [CrossRef]
- Arnez, M.; Avšič-Županc, T. Tick-borne encephalitis in children: An update on epidemiology and diagnosis. Expert Rev. Anti Infect. Ther. 2009, 7, 1251–1260. [Google Scholar] [CrossRef]
- Haglund, M.; Günther, G. Tick-borne encephalitis—pathogenesis, clinical course and long-term follow-up. Vaccine 2003, 21, S11–S18. [Google Scholar] [CrossRef]
- Lindquist, L.; Vapalahti, O. Tick-borne encephalitis. Lancet 2008, 371, 1861–1871. [Google Scholar] [CrossRef]
- Logar, M.; Bogovič, P.; Cerar, D.; Avšič-Županc, T.; Strle, F. Tick-borne encephalitis in Slovenia from 2000 to 2004: Comparison of the course in adult and elderly patients. Wien. Klin. Wochenschr. 2006, 118, 702–707. [Google Scholar] [CrossRef]
- Logar, M.; Arnez, M.; Kolbl, J.; Avšič-Županc, T.; Strle, F. Comparison of the epidemiological and clinical features of tick-borne encephalitis in children and adults. Infection 2000, 28, 74–77. [Google Scholar] [CrossRef]
- Steffen, R. Tick-borne encephalitis (TBE) in children in Europe: Epidemiology, clinical outcome and comparison of vaccination recommendations. Ticks Tick-Borne Dis. 2018, 10, 100–110. [Google Scholar] [CrossRef]
- Labuda, M.; Jones, L.D.; Williams, T.; Danielová, V.; Nuttall, P.A. Efficient Transmission of Tick-Borne Encephalitis Virus Between Cofeeding Ticks. J. Med. Entomol. 1993, 30, 295–299. [Google Scholar] [CrossRef]
- Labuda, M.; Kozuch, O.; Zuffová, E.; Elecková, E.; Hails, R.S.; Nuttall, P.A. Tick-Borne Encephalitis Virus Transmission between Ticks Cofeeding on Specific Immune Natural Rodent Hosts. Virology 1997, 235, 138–143. [Google Scholar] [CrossRef]
- Perkins, S.E.; Cattadori, I.M.; Tagliapietra, V.; Rizzoli, A.; Hudson, P.J. Empirical evidence for key hosts in persistence of a tick-borne disease. Int. J. Parasitol. 2003, 33, 909–917. [Google Scholar] [CrossRef]
- Kaiser, R. The clinical and epidemiological profile of tick-borne encephalitis in southern Germany 1994-98. A prospective study of 656 patients. Brain 1999, 122, 2067–2078. [Google Scholar] [CrossRef]
- Lesnicar, G.; Poljak, M.; Seme, K.; Lesnicar, J. Pediatric tick-borne encephalitis in 371 cases from an endemic region in Slovenia, 1959 to 2000. Pediatr. Infect. Dis. J. 2003, 22, 612–617. [Google Scholar] [CrossRef]
- Randolph, S.; Asokliene, L.; Avsic-Zupanc, T.; Bormane, A.; Burri, C.; Gern, L.; Golovljova, I.; Hubalek, Z.; Knap, N.; Kondrusik, M.; et al. Variable spikes in tick-borne encephalitis incidence in 2006 independent of variable tick abundance but related to weather. Parasites Vectors 2008, 1, 44. [Google Scholar] [CrossRef]
- Beauté, J.; Spiteri, G.; Warns-Petit, E.; Zeller, H. Tick-borne encephalitis in Europe, 2012 to 2016. Eurosurveillance 2018, 23, 1800201. [Google Scholar] [CrossRef]
- Süss, J. Epidemiology and ecology of TBE relevant to the production of effective vaccines. Vaccine 2003, 21, S19–S35. [Google Scholar] [CrossRef]
- Raoult, D.; Berbis, P.; Roux, V.; Xu, W.; Maurin, M. A new tick-transmitted disease due to Rickettsia slovaca. Lancet 1997, 350, 112–113. [Google Scholar] [CrossRef]
- Oteo, J.; Ibarra, V.; Blanco, J.; De Artola, V.M.; Márquez, F.J.; Portillo, A.; Raoult, D.; Anda, P. Dermacentor-borne necrosis erythema and lymphadenopathy: Clinical and epidemiological features of a new tick-borne disease. Clin. Microbiol. Infect. 2004, 10, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, V.; Portillo, A.; Santibáñez, S.; Blanco, J.; Pérez-Martínez, L.; Márquez, F.J.; Oteo, J.A. DEBONEL/TIBOLA: Is Rickettsia slovaca the Only Etiological Agent? Ann. N. Y. Acad. Sci. 2005, 1063, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Portillo, A.; Ibarra, V.; Santibáñez, S.; Pérez-Martínez, L.; Blanco, J.; Oteo, J.A. Genetic characterisation of ompA, ompB and gltA genes from Candidatus Rickettsia rioja. Clin. Microbiol. Infect. 2009, 15, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Mediannikov, O.; Matsumoto, K.; Samoylenko, I.; Drancourt, M.; Roux, V.; Rydkina, E.; Davoust, B.; Tarasevich, I.; Brouqui, P.; Fournier, P.-E. Rickettsia raoultii sp. nov., a spotted fever group rickettsia associated with Dermacentor ticks in Europe and Russia. Int. J. Syst. Evol. Microbiol. 2008, 58, 1635–1639. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, L.; Portillo, A.; Allegue, F.; Zulaica, A.; Oteo, J.; Caeiro, J.; Fabeiro, J. Dermacentor-borne Necrosis Erythema and Lymphadenopathy (DEBONEL): A Case Associated with Rickettsia rioja. Acta Derm. Venereol. 2010, 90, 214–215. [Google Scholar] [CrossRef]
- Selmi, M.; Bertolotti, L.; Tomassone, L.; Mannelli, A. Rickettsia slovaca in Dermacentor marginatus and Tick-borne Lymphadenopathy, Tuscany, Italy. Emerg. Infect. Dis. 2008, 14, 817–820. [Google Scholar] [CrossRef]
- Lakos, A. Tick-borne lymphadenopathy–a new rickettsial disease? Lancet 1997, 350, 1006. [Google Scholar] [CrossRef]
- Oteo, J.A.; Portillo, A. Tick-borne rickettsioses in Europe. Ticks Tick-Borne Dis. 2012, 3, 271–278. [Google Scholar] [CrossRef]
- Komitova, R.; Lakos, A.; Aleksandrov, A.; Christova, I.; Murdjeva, M. A case of tick-transmitted lymphadenopathy in Bulgaria associated with Rickettsia slovaca. Scand. J. Infect. Dis. 2003, 35, 213. [Google Scholar] [CrossRef]
- Raoult, D.; Lakos, A.; Fenollar, F.; Beytout, J.; Brouqui, P.; Fournier, P.-E. Spotless Rickettsiosis Caused by Rickettsia slovaca and Associated with Dermacentor Ticks. Clin. Infect. Dis. 2002, 34, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Rovery, C.; Rolain, J.M.; Brouqui, P.; Davoust, B.; Raoult, D. Rickettsia slovaca and R. raoultii in Tick-borne Rickettsioses. Emerg. Infect. Dis. 2009, 15, 1105–1108. [Google Scholar] [CrossRef]
- Ibarra, V.; Oteo, J.A.; Portillo, A.; Santibáñez, S.; Blanco, J.; Metola, L.; Eiros, J.; Pérez-Martínez, L.; Sanz, M. Rickettsia slovaca Infection: DEBONEL/TIBOLA. Ann. N. Y. Acad. Sci. 2006, 1078, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Földvári, G.; Rigó, K.; Lakos, A. Transmission of Rickettsia slovaca and Rickettsia raoultii by male Dermacentor marginatus and Dermacentor reticulatus ticks to humans. Diagn. Microbiol. Infect. Dis. 2013, 76, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Sanogo, Y.; Davoust, B.; Parola, P.; Camicas, J.L.; Brouqui, P.; Raoult, D. Prevalence of Rickettsia spp. in Dermacentor marginatus ticks removed from game pigs (Sus scrofa) in southern France. Ann. N. Y. Acad. Sci. 2003, 990, 191–195. [Google Scholar] [CrossRef]
- Márquez, F.J.; Rojas, A.; Ibarra, V.; Cantero, A.; Rojas, J.; Oteo, J.A.; Muniain, M. Prevalence Data of Rickettsia slovaca and Other SFG Rickettsiae Species in Dermacentor marginatus in the Southeastern Iberian Peninsula. Ann. N. Y. Acad. Sci. 2006, 1078, 328–330. [Google Scholar] [CrossRef]
- Masala, G.; Chisu, V.; Satta, G.; Socolovschi, C.; Raoult, D.; Parola, P. Rickettsia slovaca from Dermacentor marginatus ticks in Sardinia, Italy. Ticks Tick-Borne Dis. 2012, 3, 393–395. [Google Scholar] [CrossRef]
- Špitalská, E.; Štefanidesová, K.; Kocianová, E.; Boldiš, V. Rickettsia slovaca and Rickettsia raoultii in Dermacentor marginatus and Dermacentor reticulatus ticks from Slovak Republic. Exp. Appl. Acarol. 2012, 57, 189–197. [Google Scholar] [CrossRef]
- Maioli, G.; Pistone, D.; Bonilauri, P.; Pajoro, M.; Barbieri, I.; Patrizia, M.; Vicari, N.; Dottori, M. Ethiological agents of rickettsiosis and anaplasmosis in ticks collected in Emilia-Romagna region (Italy) during 2008 and 2009. Exp. Appl. Acarol. 2012, 57, 199–208. [Google Scholar] [CrossRef]
- De Sousa, R.; Nóbrega, S.D.; Bacellar, F.; Torgal, J. Mediterranean spotted fever in Portugal: Risk factors for fatal outcome in 105 hospitalized patients. Ann. N. Y. Acad. Sci. 2003, 990, 285–294. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Pérez-Sánchez, R.; Álamo-Sanz, R.; Encinas-Grandes, A. Spotted Fever Group Rickettsiae in Ticks Feeding on Humans in Northwestern Spain. Ann. N. Y. Acad. Sci. 2006, 1078, 331–333. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Raoult, D. Tick-Borne Rickettsioses around the World: Emerging Diseases Challenging Old Concepts. Clin. Microbiol. Rev. 2005, 18, 719–756. [Google Scholar] [CrossRef] [PubMed]
- Márquez, F.J.; Rodríguez-Liébana, J.J.; Soriguer, R.C.; Muniáin, M.A.; Bernabeu-Wittel, M.; Caruz, A.; Contreras-Chova, F. Spotted fever group Rickettsia in brown dog ticks Rhipicephalus sanguineus in southwestern Spain. Parasitol. Res. 2008, 103, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Socolovschi, C.; Jeanjean, L.; Bitam, I.; Fournier, P.-E.; Sotto, A.; Labauge, P.; Raoult, D. Warmer Weather Linked to Tick Attack and Emergence of Severe Rickettsioses. PLoS Negl. Trop. Dis. 2008, 2, e338. [Google Scholar] [CrossRef] [PubMed]
- Colomba, C.; Trizzino, M.; Giammanco, A.; Bonura, C.; Di Bona, D.; Tolomeo, M.; Cascio, A. Israeli Spotted Fever in Sicily. Description of two cases and minireview. Int. J. Infect. Dis. 2017, 61, 7–12. [Google Scholar] [CrossRef]
- de Sousa, R. Host and microbial risk factors and pathophysiology of fatal Rickettsia conorii infection in Portuguese patients. J. Infect. Dis. 2008, 198, 576–585. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.-E.; et al. Update on Tick-Borne Rickettsioses around the World: A Geographic Approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef]
- Vitale, G.; Mansueto, S.; Rolain, J.-M.; Raoult, D. Rickettsia massiliae Human Isolation. Emerg. Infect. Dis. 2006, 12, 174–175. [Google Scholar] [CrossRef]
- García-García, J.C. Case report: A patient from Argentina infected with Rickettsia massiliae. Am. J. Trop. Med. Hyg. 2010, 82, 691–692. [Google Scholar] [CrossRef]
- Cascio, A.; Torina, A.; Valenzise, M.; Blanda, V.; Camarda, N.; Bombaci, S.; Iaria, C.; De Luca, F.; Wasniewska, M. Scalp Eschar and Neck Lymphadenopathy Caused by Rickettsia massiliae. Emerg. Infect. Dis. 2013, 19, 836–837. [Google Scholar] [CrossRef]
- Önder, E. Crimean-Congo haemorrhagic fever. Lancet Infect. Dis. 2006, 6, 203–214. [Google Scholar] [CrossRef]
- Negredo, A.; Habela, M.Á.; De Arellano, E.R.; Diez, F.; LaSala, F.; López, P.; Sarriá, A.; Labiod, N.; Calero-Bernal, R.; Arenas, M.; et al. Survey of Crimean-Congo Hemorrhagic Fever Enzootic Focus, Spain, 2011-2015. Emerg. Infect. Dis. 2019, 25, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Rapid Risk Assessment: Crimean-Congo Haemorrhagic Fever in Spain; European Centre for Disease Prevention and Control: Solna stad, Sweden, 9 September 2016.
- Communicable Disease Threats Report, Week 33; European Centre for Disease Prevention and Control: Solna stad, Sweden, 12–18 August 2018.
- Communicable Disease Threats Report, Week 33; European Centre for Disease Prevention and Control: Solna stad, Sweden, 9–15 August 2020.
- Communicable Disease Threats Report, Week 33; European Centre for Disease Prevention and Control: Solna stad, Sweden, 28 June–4 July 2020.
- Communicable Disease Threats Report, Week 33; European Centre for Disease Prevention and Control: Solna stad, Sweden, 7–13 June 2020.
- Stuart, J. Suspected case of Crimean-Congo haemorrhagic fever in British traveller returning from Zimbabwe. Eurosurveillance 1998, 2, 1256. [Google Scholar] [CrossRef]
- Atkinson, B.; Latham, J.; Chamberlain, J.; Logue, C.; O’Donoghue, L.; Osborne, J.; Carson, G.; Brooks, T.; Carroll, M.; Jacobs, M.; et al. Sequencing and phylogenetic characterisation of a fatal Crimean-Congo haemorrhagic fever case imported into the United Kingdom, October 2012. Eurosurveillance 2012, 17, 20327. [Google Scholar] [PubMed]
- Lumley, S.; Atkinson, B.; Dowall, S.D.; Pitman, J.K.; Staplehurst, S.; Busuttil, J.; Simpson, A.J.; Aarons, E.J.; Petridou, C.; Nijjar, M.; et al. Non-fatal case of Crimean-Congo haemorrhagic fever imported into the United Kingdom (ex Bulgaria), June 2014. Eurosurveillance 2014, 19, 20864. [Google Scholar] [CrossRef] [PubMed]
- Teltow, G.J.; Rawlings, J.A.; Fournier, P.V. Isolation of Borrelia burgdorferi from arthropods Collected in Texas. Am. J. Trop. Med. Hyg. 1991, 44, 469–474. [Google Scholar] [CrossRef]
- Feir, D.; Masters, E.; Weil, G.; Santanello, C.R.; Xie, C.-S.; Li, B.-W.; Marconi, R. Evidence Supporting the Presence of Borrelia burgdorferi in Missouri. Am. J. Trop. Med. Hyg. 1994, 51, 475–482. [Google Scholar] [CrossRef]
- Schulze, T.L.; Bowen, G.S.; Bosler, E.M.; Lakat, M.F.; E Parkin, W.; Altman, R.; Ormiston, B.G.; Shisler, J.K. Amblyomma americanum: A potential vector of Lyme disease in New Jersey. Science 1984, 224, 601–603. [Google Scholar] [CrossRef]
- Rudenko, N.; Golovchenko, M.; Clark, K.; Oliver, J.H.; Grubhoffer, L. Detection of Borrelia burgdorferi sensu stricto in Amblyomma americanum ticks in the southeastern United States: The case of selective compatibility. Emerg. Microbes Infect. 2016, 5, e48–e53. [Google Scholar] [CrossRef][Green Version]
- Oliver, J.H.; Chandler, F.W.; Luttrell, M.P.; James, A.M.; Stallknecht, D.E.; McGuire, B.S.; Hutcheson, H.J.; Cummins, G.A.; Lane, R.S. Isolation and transmission of the Lyme disease spirochete from the southeastern United States. Proc. Natl. Acad. Sci. USA 1993, 90, 7371–7375. [Google Scholar] [CrossRef]
- Piesman, J.; Sinsky, R.J. Ability of Ixodes scapularis, Dermacentor variabilis, and Amblyomma americanum (Acari: Ixodidae) to Acquire, Maintain, and Transmit Lyme Disease Spirochetes (Borrelia burgdorferi). J. Med. Entomol. 1988, 25, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Sanders, F.H.; Oliver, H. Evaluation of Ixodes scapularis, Amblyomma americanum and Dermacentor variabilis (Acari: Ixodidae) from Georgia as vectors of a Florida strain of the Lyme disease spirochete, Borrelia burgdorferi. J. Med. Entomol. 1995, 32, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.M.; Brunet, L.R.; Spielman, A.; Telford, S.R. Risk of Lyme disease: Perceptions of residents of a Lone Star tick-infested community. Bull. World Health Organ. 2001, 79, 916–925. [Google Scholar]
- Kirkland, K.B. Erythema migrans-like rash illness at a camp in North Carolina: A new tick-borne disease? Arch. Intern. Med. 1997, 157, 2635–2641. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.G.; Maupin, G.O.; Teltow, G.J.; Carter, C.J.; Piesman, J. Identification of an Uncultivable Borrelia Species in the Hard Tick Amblyomma americanum: Possible Agent of a Lyme Disease-like Illness. J. Infect. Dis. 1996, 173, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Killmaster, L.F.; Loftis, A.D.; Zemtsova, G.E.; Levin, M.L. Detection of Bacterial Agents in Amblyomma americanum (Acari: Ixodidae) From Georgia, USA, and the Use of a Multiplex Assay to Differentiate Ehrlichia chaffeensis and Ehrlichia ewingii. J. Med. Entomol. 2014, 51, 868–872. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mixson, T.R. Prevalence of Ehrlichia, Borrelia and Rickettsial agents in Amblyomma americanum (Acari: Ixodidae) collected from nine states. J. Med. Entomol. 2006, 43, 1261–1268. [Google Scholar] [CrossRef]
- Stegall-Faulk, T.; Clark, D.C.; Wright, S.M. Detection of Borrelia lonestari in Amblyomma americanum (Acari: Ixodidae) from Tennessee. J. Med. Entomol. 2003, 40, 100–102. [Google Scholar] [CrossRef]
- Hudman, D.; Sargentini, N. Detection of Borrelia, Ehrlichia, and Rickettsia spp. in ticks in northeast Missouri. Ticks Tick-Borne Dis. 2016, 7, 915–921. [Google Scholar] [CrossRef]
- A Hudman, D.; Sargentini, N.J. Prevalence of Tick-Borne Pathogens in Northeast Missouri. Mo. Med. 2018, 115, 162–168. [Google Scholar]
- Fritzen, C.M.; Huang, J.; Dunlap, B.; Moncayo, A.C.; Westby, K.; Dunn, J.R.; Yabsley, M.J.; Jones, T.F.; Schardein, M.; Freye, J.D. Infection Prevalences of Common Tick-borne Pathogens in Adult Lone Star Ticks (Amblyomma americanum) and American Dog Ticks (Dermacentor variabilis) in Kentucky. Am. J. Trop. Med. Hyg. 2011, 85, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Schulze, T.L.; Jordan, R.A.; Schulze, C.J.; Mixson, T.; Papero, M. Relative Encounter Frequencies and Prevalence of Selected Borrelia, Ehrlichia, and Anaplasma Infections in Amblyomma americanum and Ixodes scapularis (Acari: Ixodidae) Ticks from Central New Jersey. J. Med. Entomol. 2005, 42, 450–456. [Google Scholar] [CrossRef]
- Schulze, T.L.; Jordan, R.A.; Healy, S.P.; Roegner, V.E.; Meddis, M.; Jahn, M.B.; Guthrie, D.L. Relative abundance and prevalence of selected Borrelia infections in Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) from publicly owned lands in Monmouth County, New Jersey. J. Med. Entomol. 2006, 43, 1269–1275. [Google Scholar] [CrossRef]
- Williamson, P.C.; Billingsley, P.M.; Teltow, G.J.; Seals, J.P.; Turnbough, M.A.; Atkinson, S.F. Borrelia, Ehrlichia, and Rickettsia spp. in Ticks Removed from Persons, Texas, USA. Emerg. Infect. Dis. 2010, 16, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.A.; Williamson, P.C.; Billingsley, P.M.; Seals, J.P.; Ferguson, E.E.; Allen, M.S. Frequency and Distribution of Rickettsiae, Borreliae, and Ehrlichiae Detected in Human-Parasitizing Ticks, Texas, USA. Emerg. Infect. Dis. 2016, 22, 312–315. [Google Scholar] [CrossRef]
- A Sayler, K.; Loftis, A.; Beatty, S.S.K.; Boyce, C.L.; Garrison, E.; Clemons, B.; Cunningham, M.; Alleman, A.R.; Barbet, A.F. Prevalence of Tick-Borne Pathogens in Host-Seeking Amblyomma americanum (Acari: Ixodidae) and Odocoileus virginianus (Artiodactyla: Cervidae) in Florida. J. Med. Entomol. 2016, 53, 949–956. [Google Scholar] [CrossRef]
- James, A.M.; Liveris, D.; Wormser, G.P.; Schwartz, I.; Montecalvo, M.A.; Johnson, B.J.B. Borrelia lonestari Infection after a Bite by an Amblyomma americanum Tick. J. Infect. Dis. 2001, 183, 1810–1814. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.; E Dawson, J.; Jones, D.C.; Wilson, K.H. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J. Clin. Microbiol. 1991, 29, 2838–2842. [Google Scholar] [CrossRef] [PubMed]
- Buller, R.S. Ehrlichia ewingii, a newly recognised agent of human ehrlichiosis. N. Engl. J. Med. 1999, 341, 148–155. [Google Scholar] [CrossRef]
- Ismail, N.; Bloch, K.C.; McBride, J.W. Human Ehrlichiosis and Anaplasmosis. Clin. Lab. Med. 2010, 30, 261–292. [Google Scholar] [CrossRef]
- Little, S.E.; Barrett, A.W.; Nagamori, Y.; Herrin, B.H.; Normile, D.; Heaney, K.; Armstrong, R. Ticks from cats in the United States: Patterns of infestation and infection with pathogens. Vet. Parasitol. 2018, 257, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kakumanu, M.L.; Ponnusamy, L.; Vaughn, M.F.; Funkhouser, S.W.; Thornton, H.; Meshnick, S.R.; Apperson, C.S. Prevalence of Rickettsiales in ticks removed from the skin of outdoor workers in North Carolina. Parasites Vectors 2014, 7, 1–14. [Google Scholar] [CrossRef]
- Maegli, A.; Loy, J.D.; Cortinas, R. Note on Ehrlichia chaffeensis, Ehrlichia ewingii, and “Borrelia lonestari” infection in lone star ticks (Acari: Ixodidae), Nebraska, USA. Ticks Tick-Borne Dis. 2016, 7, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.L.; Gaff, H.D.; Hynes, W.L. Prevalence of Ehrlichia chaffeensis and Ehrlichia ewingii in Amblyomma americanum and Dermacentor variabilis collected from southeastern Virginia, 2010–2011. Ticks Tick-Borne Dis. 2014, 5, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Harmon, J.R.; Scott, M.C.; Baker, E.M.; Jones, C.J.; Hickling, G.J. Molecular identification of Ehrlichia species and host bloodmeal source in Amblyomma americanum L. from two locations in Tennessee, United States. Ticks Tick-Borne Dis. 2015, 6, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Ijdo, J.W.; Wu, C.; Magnarelli, L.A.; Stafford, K.C.; Anderson, J.F.; Fikrig, E. Detection of Ehrlichia chaffeensis DNA in Amblyomma americanum Ticks in Connecticut and Rhode Island. J. Clin. Microbiol. 2000, 38, 4655–4656. [Google Scholar] [CrossRef] [PubMed]
- Steiert, J.G.; Gilfoy, F. Infection rates of Amblyomma americanum and Dermacentor variabilis by Ehrlichia chaffeensis and Ehrlichia ewingii in southwest Missouri. Vector-Borne Zoonotic Dis. 2002, 2, 53–60. [Google Scholar] [CrossRef]
- Simpson, D.T.; Teague, M.; Weeks, J.K.; Lewis, A.D.; D’Addio, P.M.; Moore, J.D.; Thompson, J.; Harris, A.C.; Cannella, R.T.; Kaup, B.Z.; et al. Broad, Multi-Year Sampling Effort Highlights Complex Dynamics of the Tick-Borne Pathogen Ehrlichia chaffeensis (Rickettsiales: Anaplasmatacae). J. Med. Entomol. 2018, 56, 162–168. [Google Scholar] [CrossRef]
- Yabsley, M.J. Natural History of Ehrlichia chaffeensis: Vertebrate hosts and tick vectors from the United States and evidence for endemic transmission in other countries. Vet. Parasitol. 2010, 167, 136–148. [Google Scholar] [CrossRef]
- Anziani, O.S.; A Ewing, S.; Barker, R.W. Experimental transmission of a granulocytic form of the tribe Ehrlichieae by Dermacentor variabilis and Amblyomma americanum to dogs. Am. J. Vet. Res. 1990, 51, 929–931. [Google Scholar]
- Loftis, A.D.; Reeves, W.K.; Spurlock, J.P.; Mahan, S.M.; Troughton, D.R.; A Dasch, G.; Levin, M.L. Infection of a goat with a tick-transmitted Ehrlichia from Georgia, USA., that is closely related to Ehrlichia ruminantium. J. Vector Ecol. 2006, 31, 213–223. [Google Scholar] [CrossRef]
- Reeves, W.K.; Loftis, A.; Nicholson, W.L.; Czarkowski, A.G. The first report of human illness associated with the Panola Mountain Ehrlichia species: A case report. J. Med. Case Rep. 2008, 2, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Loftis, A.; Mixson, T.R.; Stromdahl, E.Y.; Yabsley, M.J.; E Garrison, L.; Williamson, P.C.; Fitak, R.R.; Fuerst, P.; Kelly, D.J.; Blount, K.W. Geographic distribution and genetic diversity of the Ehrlichia sp. from Panola Mountain in Amblyomma americanum. BMC Infect. Dis. 2008, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Burgdorfer, W. Review Article: A Review of Rocky Mountain Spotted Fever (Tick-Borne Typhus), its Agent, and its Tick Vectors in the United States. J. Med. Entomol. 1975, 12, 269–278. [Google Scholar] [CrossRef]
- McDade, J.E.; Newhouse, M.J. Natural history of Rickettsia rickettsii. Annu. Rev. Microbiol. 1986, 40, 287–309. [Google Scholar] [CrossRef]
- Dantas-Torres, F. Rocky Mountain spotted fever. Lancet Infect. Dis. 2007, 7, 724–732. [Google Scholar] [CrossRef]
- Kakumanu, M.L.; Ponnusamy, L.; Sutton, H.; Meshnick, S.R.; Nicholson, W.L.; Apperson, C.S. Prevalence of Rickettsia Species (Rickettsiales: Rickettsiaceae) in Dermacentor variabilis Ticks (Acari: Ixodidae) in North Carolina. J. Med. Entomol. 2018, 55, 1284–1291. [Google Scholar] [CrossRef]
- Hecht, J.A.; Allerdice, M.E.; Dykstra, E.A.; Mastel, L.; Eisen, R.J.; Johnson, T.L.; Gaff, H.D.; Varela-Stokes, A.S.; Goddard, J.; Pagac, B.B.; et al. Multistate Survey of American Dog Ticks (Dermacentor variabilis) for Rickettsia Species. Vector-Borne Zoonotic Dis. 2019, 19, 652–657. [Google Scholar] [CrossRef]
- Demma, L.J.; Traeger, M.S.; Nicholson, W.L.; Paddock, C.D.; Blau, D.M.; Eremeeva, M.E.; Dasch, G.A.; Levin, M.L.; Singleton, J.; Zaki, S.R.; et al. Rocky Mountain Spotted Fever from an Unexpected Tick Vector in Arizona. N. Engl. J. Med. 2005, 353, 587–594. [Google Scholar] [CrossRef]
- Goddard, J.; Norment, B.R. Spotted Fever Group Rickettsiae in the Lone Star Tick, Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 1986, 23, 465–472. [Google Scholar] [CrossRef]
- Levin, M.L.; Zemtsova, G.E.; Killmaster, L.F.; Snellgrove, A.; Schumacher, L.B. Vector competence of Amblyomma americanum (Acari: Ixodidae) for Rickettsia rickettsii. Ticks Tick-Borne Dis. 2017, 8, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Wood, H.; Dillon, L.; Patel, S.N.; Ralevski, F. Prevalence of Rickettsia species in Dermacentor variabilis ticks from Ontario, Canada. Ticks Tick-Borne Dis. 2016, 7, 1044–1046. [Google Scholar] [CrossRef]
- Dergousoff, S.J.; Gajadhar, A.J.A.; Chilton, N.B. Prevalence of Rickettsia Species in Canadian Populations of Dermacentor andersoni and D. variabilis. Appl. Environ. Microbiol. 2009, 75, 1786–1789. [Google Scholar] [CrossRef] [PubMed]
- Yunik, M.E.; Galloway, T.D.; Lindsay, L.R. Assessment of Prevalence and Distribution of Spotted Fever Group Rickettsiae in Manitoba, Canada, in the American Dog Tick, Dermacentor variabilis (Acari: Ixodidae). Vector-Borne Zoonotic Dis. 2015, 15, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Ammerman, N.C. Spotted-fever group Rickettsia in Dermacentor variabilis, Maryland. Emerg. Infect. Dis. 2004, 10, 1478–1481. [Google Scholar] [CrossRef] [PubMed]
- Pagac, B.B.; Miller, M.K.; Mazzei, M.C.; Nielsen, D.H.; Jiang, J.; Richards, A.L. Rickettsia parkeri and Rickettsia montanensis, Kentucky and Tennessee, USA. Emerg. Infect. Dis. 2014, 20, 1750–1752. [Google Scholar] [CrossRef]
- Smith, M.P.; Ponnusamy, L.; Jiang, J.; Abu Ayyash, L.; Richards, A.L.; Apperson, C.S. Bacterial Pathogens in Ixodid Ticks from a Piedmont County in North Carolina: Prevalence of Rickettsial Organisms. Vector-Borne Zoonotic Dis. 2010, 10, 939–952. [Google Scholar] [CrossRef]
- Fryxell, R.T.T.; Hendricks, B.M.; Pompo, K.; Mays, S.E.; Paulsen, D.J.; Operario, D.J.; Houston, A.E. Investigating the Adult Ixodid Tick Populations and Their Associated Anaplasma, Ehrlichia, and Rickettsia Bacteria at a Rocky Mountain Spotted Fever Hotspot in Western Tennessee. Vector-Borne Zoonotic Dis. 2017, 17, 527–538. [Google Scholar] [CrossRef]
- McQuiston, J.H.; Zemtsova, G.; Perniciaro, J.; Hutson, M.; Singleton, J.; Nicholson, W.L.; Levin, M.L. Afebrile Spotted Fever Group Rickettsia Infection After a Bite from a Dermacentor variabilis Tick Infected with Rickettsia montanensis. Vector-Borne Zoonotic Dis. 2012, 12, 1059–1061. [Google Scholar] [CrossRef]
- Macdonald, A.J.; Hyon, D.W.; Brewington, J.B.; O’Connor, K.E.; Swei, A.; Briggs, C.J. Lyme disease risk in southern California: Abiotic and environmental drivers of Ixodes pacificus (Acari: Ixodidae) density and infection prevalence with Borrelia burgdorferi. Parasites Vectors 2017, 10, 7. [Google Scholar] [CrossRef]
- Crowder, C.D.; Carolan, H.E.; Rounds, M.A.; Hönig, V.; Mothes, B.; Haag, H.; Nolte, O.; Luft, B.J.; Grubhoffer, L.; Ecker, D.J.; et al. Prevalence of Borrelia miyamotoi in Ixodes Ticks in Europe and the United States. Emerg. Infect. Dis. 2014, 20, 1678–1682. [Google Scholar] [CrossRef] [PubMed]
- Cosson, J.-F.; Michelet, L.; Chotte, J.; Le Naour, E.; Cote, M.; Devillers, E.; Poulle, M.-L.; Huet, D.; Galan, M.; Geller, J.; et al. Genetic characterization of the human relapsing fever spirochete Borrelia miyamotoi in vectors and animal reservoirs of Lyme disease spirochetes in France. Parasites Vectors 2014, 7, 233. [Google Scholar] [CrossRef] [PubMed]
- Mukhacheva, T.; Salikhova, I.I.; Kovalev, S.Y. Multilocus spacer analysis revealed highly homogeneous genetic background of Asian type of Borrelia miyamotoi. Infect. Genet. Evol. 2015, 31, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Siński, E.; Welc-Falęciak, R.; Zajkowska, J. Borrelia miyamotoi: A human tick-borne relapsing fever spirochete in Europe and its potential impact on public health. Adv. Med. Sci. 2016, 61, 255–260. [Google Scholar] [CrossRef]
- Scoles, G.A.; Papero, M.; Beati, L.; Fish, D. A Relapsing Fever Group Spirochete Transmitted by Ixodes scapularis Ticks. Vector-Borne Zoonotic Dis. 2001, 1, 21–34. [Google Scholar] [CrossRef]
- Barbour, A.G.; Fish, D.; Hoen, A.G.; Tsao, J.I.; Diuk-Wasser, M.A.; Bunikis, J.; Travinsky, B. Niche Partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the Same Tick Vector and Mammalian Reservoir Species. Am. J. Trop. Med. Hyg. 2009, 81, 1120–1131. [Google Scholar] [CrossRef]
- Platonov, A.E.; Karan, L.S.; Kolyasnikova, N.M.; Makhneva, N.A.; Toporkova, M.G.; Maleev, V.V.; Fish, D.; Krause, P.J. Humans Infected with Relapsing Fever Spirochete Borrelia miyamotoi, Russia. Emerg. Infect. Dis. 2011, 17, 1816–1823. [Google Scholar] [CrossRef]
- Krause, P.J.; Narasimhan, S.; Wormser, G.P.; Rollend, L.; Fikrig, E.; Lepore, T.; Barbour, A.; Fish, D. Human Borrelia miyamotoi Infection in the United States. N. Engl. J. Med. 2013, 368, 291–293. [Google Scholar] [CrossRef]
- Gugliotta, J.L.; Goethert, H.K.; Berardi, V.P.; Telford, S.R. Meningoencephalitis from Borrelia miyamotoi in an Immunocompromised Patient. N. Engl. J. Med. 2013, 368, 240–245. [Google Scholar] [CrossRef]
- Hovius, J.W.R. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet 2013, 382, 658. [Google Scholar] [CrossRef]
- Aubry, C.; Socolovschi, C.; Raoult, D.; Parola, P. Bacterial agents in 248 ticks removed from people from 2002 to 2013. Ticks Tick-Borne Dis. 2016, 7, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Sarksyan, D.S.; Platonov, A.E.; Karan, L.S.; Shipulin, G.; Sprong, H.; Hovius, J.W. Probability of Spirochete Borrelia miyamotoi Transmission from Ticks to Humans. Emerg. Infect. Dis. 2015, 21, 2273–2274. [Google Scholar] [CrossRef]
- Hofhuis, A.; Herremans, T.; Notermans, D.W.; Sprong, H.; Fonville, M.; van der Giessen, J.W.B.; Van Pelt, W. A Prospective Study among Patients Presenting at the General Practitioner with a Tick Bite or Erythema Migrans in the Netherlands. PLoS ONE 2013, 8, e64361. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.C.; Rosen, M.E.; Hamer, S.A.; Baker, E.; Edwards, H.; Crowder, C.; Tsao, J.I.; Hickling, G.J. High-Prevalence Borrelia miyamotoi Infection Among Wild Turkeys (Meleagris gallopavo) in Tennessee. J. Med. Entomol. 2010, 47, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- Padgett, K.; Bonilla, D.; Kjemtrup, A.; Vilcins, I.-M.; Yoshimizu, M.H.; Hui, L.; Sola, M.; Quintana, M.; Kramer, V. Large Scale Spatial Risk and Comparative Prevalence of Borrelia miyamotoi and Borrelia burgdorferi Sensu Lato in Ixodes pacificus. PLoS ONE 2014, 9, e110853. [Google Scholar] [CrossRef]
- Eshoo, M.W.; Carolan, H.E.; Massire, C.; Chou, D.M.; Crowder, C.D.; Rounds, M.A.; Phillipson, C.A.; Schutzer, S.E.; Ecker, D.J. Survey of Ixodes pacificus Ticks in California Reveals a Diversity of Microorganisms and a Novel and Widespread Anaplasmataceae Species. PLoS ONE 2015, 10, e0135828. [Google Scholar] [CrossRef]
- Lynn, G.E.; Graham, C.B.; Horiuchi, K.; Eisen, L.; Johnson, T.L.; Lane, R.S.; Eisen, R.J. Prevalence and Geographic Distribution of Borrelia miyamotoi in Host-Seeking Ixodes pacificus (Acari: Ixodidae) Nymphs in Mendocino County, California. J. Med. Entomol. 2018, 55, 711–716. [Google Scholar] [CrossRef]
- Salkeld, D.J.; Cinkovich, S.; Nieto, N.C. Tick-borne Pathogens in Northwestern California, USA. Emerg. Infect. Dis. 2014, 20, 493–494. [Google Scholar] [CrossRef]
- Mun, J.; Eisen, R.J.; Eisen, L.; Lane, R.S. Detection of a Borrelia miyamotoi Sensu Lato Relapsing-Fever Group Spirochete from Ixodes pacificus in California. J. Med. Entomol. 2006, 43, 120–123. [Google Scholar] [CrossRef]
- Fedorova, N.; Kleinjan, J.E.; James, D.; Hui, L.T.; Peeters, H.; Lane, R.S. Remarkable diversity of tick or mammalian-associated Borreliae in the metropolitan San Francisco Bay Area, California. Ticks Tick-Borne Dis. 2014, 5, 951–961. [Google Scholar] [CrossRef]
- Cutler, S.J.; Vayssier-Taussat, M.; Estrada-Peña, A.; Potkonjak, A.; Mihalca, A.D.; Zeller, H. A new Borrelia on the block: Borrelia miyamotoi–A human health risk? Eurosurveillance 2019, 24, 1800170. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.S.; Steinlein, D.B.; Mun, J. Human Behaviors Elevating Exposure to Ixodes pacificus (Acari: Ixodidae) Nymphs and Their Associated Bacterial Zoonotic Agents in a Hardwood Forest. J. Med. Entomol. 2004, 41, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Newman, E.A.; Eisen, L.; Eisen, R.J.; Fedorova, N.; Hasty, J.M.; Vaughn, C.; Lane, R.S. Borrelia burgdorferi Sensu Lato Spirochetes in Wild Birds in Northwestern California: Associations with Ecological Factors, Bird Behavior and Tick Infestation. PLoS ONE 2015, 10, e0118146. [Google Scholar] [CrossRef] [PubMed]
- Holden, K.; Boothby, J.T.; Kasten, R.W.; Chomel, B.B. Co-detection of Bartonella henselae, Borrelia burgdorferi, and Anaplasma phagocytophilum in Ixodes pacificus Ticks from California, USA. Vector-Borne Zoonotic Dis. 2006, 6, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Rose, I.; Yoshimizu, M.H.; Bonilla, D.L.; Fedorova, N.; Lane, R.S.; Padgett, K. Phylogeography of Borrelia spirochetes in Ixodes pacificus and Ixodes spinipalpis ticks highlights differential acarological risk of tick-borne disease transmission in northern versus southern California. PLoS ONE 2019, 14, e0214726. [Google Scholar] [CrossRef]
- Eisen, R.J.; Mun, J.; Eisen, L.; Lane, R.S. Life Stage-Related Differences in Density of Questing Ticks and Infection with Borrelia burgdorferi sensu lato Within a Single Cohort of Ixodes pacificus(Acari: Ixodidae). J. Med. Entomol. 2004, 41, 768–773. [Google Scholar] [CrossRef]
- Lane, R.S. Acarologic risk of exposure to emerging tick-borne bacterial pathogens in a semi-rural community in northern California. Vector-Borne Zoonotic Dis. 2001, 1, 197–210. [Google Scholar] [CrossRef]
- Wright, S.A.; Thompson, M.A.; Miller, M.J.; Knerl, K.M.; Elms, S.L.; Karpowicz, J.C.; Young, J.F.; Kramer, V.L. Ecology of Borrelia burgdorferi in Ticks (Acari: Ixodidae), Rodents, and Birds in the Sierra Nevada Foothills, Placer County, California. J. Med. Entomol. 2000, 37, 909–918. [Google Scholar] [CrossRef]
- Lane, R.S.; Fedorova, N.; Kleinjan, J.E.; Maxwell, M. Eco-epidemiological factors contributing to the low risk of human exposure to ixodid tick-borne borreliae in southern California, USA. Ticks Tick-Borne Dis. 2013, 4, 377–385. [Google Scholar] [CrossRef]
- Salkeld, D.J.; Nieto, N.C.; Carbajales-Dale, P.; Dale, M.; Cinkovich, S.S.; Lambin, E.F. Disease Risk & Landscape Attributes of Tick-Borne Borrelia Pathogens in the San Francisco Bay Area, California. PLoS ONE 2015, 10, e0134812. [Google Scholar] [CrossRef]
- Davis, R.S.; Ramirez, R.A.; Anderson, J.L.; Bernhardt, S. Distribution and Habitat of Ixodes pacificus (Acari: Ixodidae) and Prevalence of Borrelia burgdorferi in Utah. J. Med. Entomol. 2015, 52, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.J.; Eisen, L.; Girard, Y.A.; Fedorova, N.; Mun, J.; Slikas, B.; Leonhard, S.; Kitron, U.; Lane, R.S. A spatially-explicit model of acarological risk of exposure to Borrelia burgdorferi-infected Ixodes pacificus nymphs in northwestern California based on woodland type, temperature, and water vapor. Ticks Tick-Borne Dis. 2010, 1, 35–43. [Google Scholar] [CrossRef]
- Swei, A.; Meentemeyer, R.; Briggs, C.J. Influence of abiotic and environmental factors on the density and infection prevalence of Ixodes pacificus (Acari:Ixodidae) with Borrelia burgdorferi. J. Med. Entomol. 2011, 48, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Pearson, P.; Dykstra, E.; Andrews, E.S.; Rich, S.M. Human-Biting Ixodes Ticks and Pathogen Prevalence from California, Oregon, and Washington. Vector-Borne Zoonotic Dis. 2019, 19, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Burri, C.; Schumann, O.; Gern, L.; Schümann, C. Are Apodemus spp. mice and Myodes glareolus reservoirs for Borrelia miyamotoi, Candidatus Neoehrlichia mikurensis, Rickettsia helvetica, R. monacensis and Anaplasma phagocytophilum? Ticks Tick-Borne Dis. 2014, 5, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, S.; Coipan, E.C.; Rigó, K.; Majoros, G.; Jahfari, S.; Sprong, H.; Földvári, G. Eco-epidemiology of Borrelia miyamotoi and Lyme borreliosis spirochetes in a popular hunting and recreational forest area in Hungary. Parasites Vectors 2015, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Wodecka, B.; Rymaszewska, A.; Skotarczak, B. Host and pathogen DNA identification in blood meals of nymphal Ixodes ricinus ticks from forest parks and rural forests of Poland. Exp. Appl. Acarol. 2013, 62, 543–555. [Google Scholar] [CrossRef]
- Stuen, S.; Granquist, E.G.; Silaghi, C. Anaplasma phagocytophilum—A widespread multi-host pathogen with highly adaptive strategies. Front. Cell. Infect. Microbiol. 2013, 3, 31. [Google Scholar] [CrossRef]
- Foley, J.; Piovia-Scott, J. Vector biodiversity did not associate with tick-borne pathogen prevalence in small mammal communities in northern and central California. Ticks Tick-Borne Dis. 2014, 5, 299–304. [Google Scholar] [CrossRef]
- Telford, S.R.; Dawson, J.E.; Katavolos, P.; Warner, C.K.; Kolbert, C.P.; Persing, D.H. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc. Natl. Acad. Sci. USA 1996, 93, 6209–6214. [Google Scholar] [CrossRef]
- Walls, J.J.; Greig, B.; Neitzel, D.F.; Dumler, J.S. Natural infection of small mammal species in Minnesota with the agent of human granulocytic ehrlichiosis. J. Clin. Microbiol. 1997, 35, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.; Foley, P.; Brown, R.N.; Lane, R.S.; Dumlers, J.S.; E Madigan, J. Ecology of Anaplasma phagocytophilum and Borrelia burgdorferi in the western United States. J. Vector Ecol. 2004, 29, 41–50. [Google Scholar] [PubMed]
- Woldehiwet, Z. Anaplasma phagocytophilum in Ruminants in Europe. Ann. N. Y. Acad. Sci. 2006, 1078, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.J.; Falco, R.C.; Schwartz, I.; Varde, S.; Robbins, R.G. Deer ticks (Ixodes scapularis) and the agents of Lyme disease and human granulocytic ehrlichiosis in a New York City park. Emerg. Infect. Dis. 1997, 3, 353–355. [Google Scholar] [CrossRef]
- A Magnarelli, L.; Stafford, K.C.; Mather, T.N.; Yeh, M.T.; Horn, K.D.; Dumler, J.S. Hemocytic rickettsia-like organisms in ticks: Serologic reactivity with antisera to Ehrlichiae and detection of DNA of agent of human granulocytic ehrlichiosis by PCR. J. Clin. Microbiol. 1995, 33, 2710–2714. [Google Scholar] [CrossRef]
- Fritz, C.L.; Bronson, L.R.; Smith, C.R.; Crawford-Miksza, L.; Yeh, E.; Schnurr, D. Clinical, epidemiologic, and environmental surveillance for ehrlichiosis and anaplasmosis in an endemic area of northern California. J. Vector Ecol. 2005, 30, 4–10. [Google Scholar]
- Burgdorfer, W.; Lackman, D. Identification of Rickettsia rckettsii in the Wood Tick, Dermacentor andersoni, by Means of Fluorescent Antibody. J. Infect. Dis. 1960, 107, 241–244. [Google Scholar] [CrossRef]
- Johnson, A.J.; Karabatsos, N.; Lanciotti, R.S. Detection of Colorado tick fever virus by using reverse transcriptase PCR and application of the technique in laboratory diagnosis. J. Clin. Microbiol. 1997, 35, 1203–1208. [Google Scholar] [CrossRef]
- Burgdorfer, W.; Eklund, C.M. Studies on the ecology of Colorado tick fever virus in Western Montana. Am. J. Epidemiol. 1959, 69, 127–137. [Google Scholar] [CrossRef]
- Burgdorfer, W.; Eklund, C.M. Colorado Tick Fever I. Further Ecological Studies in Western Montana. J. Infect. Dis. 1960, 107, 379–383. [Google Scholar] [CrossRef]
- Geissler, A.L.; Thorp, E.; Van Houten, C.; Lanciotti, R.S.; Panella, N.; Cadwell, B.L.; Murphy, T.; Staples, J.E. Infection with Colorado Tick Fever Virus Among Humans and Ticks in a National Park and Forest, Wyoming. Vector-Borne Zoonotic Dis. 2014, 14, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Williamson, B.N.; Fischer, R.J.; Lopez, J.E.; Ebihara, H.; Schwan, T.G. Prevalence and Strains of Colorado Tick Fever Virus in Rocky Mountain Wood Ticks in the Bitterroot Valley, Montana. Vector-Borne Zoonotic Dis. 2019, 19, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.E.; Miller, B.R.; McLean, R.G.; Knudson, D.L. Co-Circulation of Multiple Colorado Tick Fever Virus Genotypes. Am. J. Trop. Med. Hyg. 1989, 40, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Elston, D.M.; Hivnor, C. What’s eating you? Dermacentor andersoni. Cutis 2001, 67, 113–115. [Google Scholar]
- Dworkin, M.S.; Shoemaker, P.C.; Anderson, J.D.E. Tick Paralysis: 33 Human Cases in Washington State, 1946–1996. Clin. Infect. Dis. 1999, 29, 1435–1439. [Google Scholar] [CrossRef]
- Krawczak, F.S.; Nieri-Bastos, F.A.; Nunes, F.P.; Soares, J.F.; Moraes-Filho, J.; Labruna, M.B. Rickettsial infection in Amblyomma cajennense ticks and capybaras (Hydrochoerus hydrochaeris) in a Brazilian spotted fever-endemic area. Parasites Vectors 2014, 7, 7. [Google Scholar] [CrossRef]
- Biggs, H.M.; Behravesh, C.B.; Bradley, K.K.; Dahlgren, F.S.; Drexler, N.A.; Dumler, J.S.; Folk, S.M.; Kato, C.Y.; Lash, R.R.; Levin, M.L.; et al. Diagnosis and Management of Tickborne Rickettsial Diseases: Rocky Mountain Spotted Fever and Other Spotted Fever Group Rickettsioses, Ehrlichioses, and Anaplasmosis—United States. MMWR Recomm. Rep. 2016, 65, 1–44. [Google Scholar] [CrossRef]
- Soares, J.F.; Soares, H.S.; Barbieri, A.M.; Labruna, M.B. Experimental infection of the tick Amblyomma cajennense, Cayenne tick, with Rickettsia rickettsii, the agent of Rocky Mountain spotted fever. Med. Vet. Entomol. 2011, 26, 139–151. [Google Scholar] [CrossRef]
- Labruna, M.B. Comparative susceptibility of larval stages of Amblyomma aureolatum, Amblyomma cajennense, and Rhipicephalus sanguineus to infection by Rickettsia rickettsii. J. Med. Entomol. 2008, 45, 1156–1159. [Google Scholar] [CrossRef]
- Grattan-Smith, P.J.; Morris, J.G.; Johnston, H.M.; Yiannikas, C.; Malik, R.; Russell, R.; Ouvrier, R. Clinical and neurophysiological features of tick paralysis. Brain 1997, 120, 1975–1987. [Google Scholar] [CrossRef]
- Goodrich, B.; Murray, M. Factors influencing the toxicity of salivary gland extracts of Ixodes holocyclus Neumann. Int. J. Parasitol. 1978, 8, 313–320. [Google Scholar] [CrossRef]
- Eppleston, K.; Kelman, M.; Ward, M.P. Distribution, seasonality and risk factors for tick paralysis in Australian dogs and cats. Vet. Parasitol. 2013, 196, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Edlow, J.A.; McGillicuddy, D.C. Tick Paralysis. Infect. Dis. Clin. North. Am. 2008, 22, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.C.; Walker, A.R.; Campelo, D. A list of the 70 species of Australian ticks; diagnostic guides to and species accounts of Ixodes holocyclus (paralysis tick), Ixodes cornuatus (southern paralysis tick) and Rhipicephalus australis (Australian cattle tick); and consideration of the place of Australia in the evolution of ticks with comments on four controversial ideas. Int. J. Parasitol. 2014, 44, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Logan, T.M.; Watts, D.M.; Linthicum, K.J.; Moulton, J.R.; Bailey, C.L. Experimental Transmission of Crimean-Congo Hemorrhagic Fever Virus by Hyalomma truncatum Koch. Am. J. Trop. Med. Hyg. 1989, 40, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.; Camicas, J.; Cornet, J.; Faye, O.; Wilson, M. Sexual and transovarian transmission of Crimean-Congo haemorrhagic fever virus in Hyalomma truncatum ticks. Res. Virol. 1992, 143, 23–28. [Google Scholar] [CrossRef]
- Wilson, M.; Gonzalez, J.-P.; Cornet, J.-P.; Camicas, J.-L. Transmission of Crimean-Congo haemorrhagic fever virus from experimentally infected sheep to Hyalomma truncatum ticks. Res. Virol. 1991, 142, 395–404. [Google Scholar] [CrossRef]
- Linthicum, K.J.; Logan, T.M.; Bailey, C.L.; Dohm, D.J.; Moulton, J.R. Transstadial and Horizontal Transmission of Rift Valley Fever Virus in Hyalomma truncatum. Am. J. Trop. Med. Hyg. 1989, 41, 491–496. [Google Scholar] [CrossRef]
- Nchu, F.; Rand, A. Rift Valley fever outbreaks: Possible implication of Hyalomma truncatum (Acari: Ixodidae). Afr. J. Microbiol. Res. 2013, 7, 3891–3894. [Google Scholar]
- Kumsa, B.; Socolovschi, C.; Raoult, D.; Parola, P. Spotted fever group rickettsiae in ixodid ticks in Oromia, Ethiopia. Ticks Tick-Borne Dis. 2015, 6, 8–15. [Google Scholar] [CrossRef]
- Mediannikov, O.; Diatta, G.; Fenollar, F.; Sokhna, C.; Trape, J.-F.; Raoult, D. Tick-Borne Rickettsioses, Neglected Emerging Diseases in Rural Senegal. PLoS Neglected Trop. Dis. 2010, 4, e821. [Google Scholar] [CrossRef] [PubMed]
- Morita, C.; El Hussein, A.R.M.; Matsuda, E.; Gabbar, K.M.A.A.; Muramatsu, Y.; Rahman, M.B.A.; Eleragi, A.M.H.; Hassan, S.M.; Chitambo, A.M.; Ueno, H. Spotted fever group rickettsiae from ticks captured in Sudan. Jpn. J. Infect. Dis. 2004, 57, 107–109. [Google Scholar]
- Mutai, B.K.; Wainaina, J.M.; Magiri, C.G.; Nganga, J.K.; Ithondeka, P.M.; Njagi, O.N.; Jiang, J.; Richards, A.L.; Waitumbi, J.N. Zoonotic Surveillance for Rickettsiae in Domestic Animals in Kenya. Vector-Borne Zoonotic Dis. 2013, 13, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Tomassone, L.; De Meneghi, D.; Adakal, H.; Rodighiero, P.; Pressi, G.; Grego, E. Detection of Rickettsia aeschlimannii and Rickettsia africae in ixodid ticks from Burkina Faso and Somali Region of Ethiopia by new real-time PCR assays. Ticks Tick-Borne Dis. 2016, 7, 1082–1088. [Google Scholar] [CrossRef]
- Perry, B.; Kruska, R.; Lessard, P.; Norval, R.; Kundert, K. Estimating the distribution and abundance of Rhipicephalus appendiculatus in Africa. Prev. Vet. Med. 1991, 11, 261–268. [Google Scholar] [CrossRef]
- Mahara, F. Japanese Spotted Fever: Report of 31 Cases and Review of the Literature. Emerg. Infect. Dis. 1997, 3, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Tian, J.-H.; Yu, B.; Guo, W.-P.; Holmes, E.C.; Zhang, Y.-Z. Extensive diversity of rickettsiales bacteria in ticks from Wuhan, China. Ticks Tick-Borne Dis. 2017, 8, 574–580. [Google Scholar] [CrossRef]
- Seki, M.; Ikari, N.; Yamamoto, S.; Yamagata, Y.; Kosai, K.; Yanagihara, K.; Kakugawa, T.; Kurihara, S.; Izumikawa, K.; Miyazaki, Y.; et al. Severe Japanese Spotted Fever Successfully Treated with Fluoroquinolone. Intern. Med. 2006, 45, 1323–1326. [Google Scholar] [CrossRef][Green Version]
- Arthan, W.; Sumrandee, C.; Hirunkanokpun, S.; Kitthawee, S.; Baimai, V.; Trinachartvanit, W.; Ahantarig, A. Detection of Coxiella-like endosymbiont in Haemaphysalis tick in Thailand. Ticks Tick-Borne Dis. 2015, 6, 63–68. [Google Scholar] [CrossRef]
- Khoo, J.-J.; Lim, F.-S.; Chen, F.; Phoon, W.-H.; Khor, C.-S.; Pike, B.L.; Chang, L.-Y.; Abubakar, S. Coxiella Detection in Ticks from Wildlife and Livestock in Malaysia. Vector-Borne Zoonotic Dis. 2016, 16, 744–751. [Google Scholar] [CrossRef]
- Tan, D.S.K.; Smith, C.E.G.; McMahon, D.A.; Bowen, E.T.W. Lanjan Virus, a New Agent isolated from Dermacentor auratus in Malaya. Nat. Cell Biol. 1967, 214, 1154–1155. [Google Scholar] [CrossRef]
- Adamantos, S. Australian tick paralysis in a dog imported into the UK. Vet. Rec. 2005, 83, 327. [Google Scholar] [CrossRef]
- Attipa, C.; Maguire, D.; Solano-Gallego, L.; Szladovits, B.; Barker, E.N.; Farr, A.; Baneth, G.; Tasker, S. Hepatozoon canis in three imported dogs: A new tickborne disease reaching the United Kingdom. Vet. Rec. 2018, 183, 716. [Google Scholar] [CrossRef] [PubMed]
- Hansford, K.M.; Pietzsch, M.; Cull, B.; Medlock, J.M.; Wall, R. Overwintering of the brown dog tick in residential properties in England–raising awareness. Vet. Rec. 2015, 177, 156. [Google Scholar] [CrossRef]
- Hansford, K.M.; Medlock, J.M.; Atkinson, B.; Santos-Silva, M.M. Importation of a Hyalomma lusitanicum tick into the UK on a dog. Vet. Rec. 2016, 179, 415. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.; Helps, C.; Tasker, S.; Newbury, H.; Wall, R. Ticks infesting domestic dogs in the UK: A large-scale surveillance programme. Parasites Vectors 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Hansford, K.M.; Pietzsch, M.; Cull, B.; Medlock, J.M. Brown dog tick infestation of a home in England. Vet. Rec. 2015, 176, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Hansford, K.M.; E Pietzsch, M.; Cull, B.; Medlock, J.M. Importation of R sanguineus into the UK via dogs: Tickborne diseases. Vet. Rec. 2014, 175, 385–386. [Google Scholar] [CrossRef]
- Featherstone, C.; Phipps, P.; Pietzsch, M.; Hansford, K.; Medlock, J. Tick surveillance in the UK. Vet. Rec. 2012, 171. [Google Scholar] [CrossRef]
- Hansford, K.M.; E Pietzsch, M.; Cull, B.; Gillingham, E.L.; Medlock, J.M. Potential risk posed by the importation of ticks into the UK on animals: Records from the Tick Surveillance Scheme. Vet. Rec. 2017, 182, 107. [Google Scholar] [CrossRef]
- Bates, P.; Rankin, M.; Shickle, L. Importation of the brown dog or kennel tick (Rhipicephalus sanguineus) into the UK. Vet. Rec. 2002, 150, 224. [Google Scholar]
- Hansford, K.M.; Carter, D.; Gillingham, E.L.; Hernandez-Triana, L.M.; Chamberlain, J.; Cull, B.; McGinley, L.; Phipps, L.P.; Medlock, J.M. Hyalomma rufipes on an untraveled horse: Is this the first evidence of Hyalomma nymphs successfully moulting in the United Kingdom? Ticks Tick-Borne Dis. 2019, 10, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Jameson, L.J.; Morgan, P.J.; Medlock, J.M.; Watola, G.; Vaux, A.G. Importation of Hyalomma marginatum, vector of Crimean-Congo haemorrhagic fever virus, into the United Kingdom by migratory birds. Ticks Tick-Borne Dis. 2012, 3, 95–99. [Google Scholar] [CrossRef]
- Pietzsch, M.; Mitchell, R.; Jameson, L.J.; Morgan, C.; Medlock, J.; Collins, D.; Chamberlain, J.; Gould, E.; Hewson, R.; Taylor, M.; et al. Preliminary evaluation of exotic tick species and exotic pathogens imported on migratory birds into the British Isles. Vet. Parasitol. 2008, 155, 328–332. [Google Scholar] [CrossRef]
- Mihalca, A.D. Ticks imported to Europe with exotic reptiles. Vet. Parasitol. 2015, 213, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Pietzsch, M.; Quest, R.; Hillyard, P.D.; Medlock, J.M.; Leach, S. Importation of Exotic Ticks into the United Kingdom via the International Trade in Reptiles. Exp. Appl. Acarol. 2006, 38, 59–65. [Google Scholar] [CrossRef]
- Kenny, M.J.; Shaw, S.E.; Hillyard, P.D.; Forbes, A.B. Ectoparasite and haemoparasite risks associated with imported exotic reptiles. Vet. Rec. 2004, 154, 434–435. [Google Scholar] [CrossRef] [PubMed]
- Pietzsch, M.E. Detection of Dermacentor marginatus and a possible Rickettsia slovaca case in the United Kingdom—The risk of the visiting traveller. Travel Med. Infect. Dis. 2015, 13, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Office for National Statistics. Overseas Travel and Tourism, Monthly. 2019. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/leisureandtourism/datasets/monthlyoverseastravelandtourismreferencetables (accessed on 1 September 2020).
- Dobson, A.D.M.; Taylor, J.L.; Randolph, S.E. Tick (Ixodes ricinus) abundance and seasonality at recreational sites in the UK: Hazards in relation to fine-scale habitat types revealed by complementary sampling methods. Ticks Tick-Borne Dis. 2011, 2, 67–74. [Google Scholar] [CrossRef]
- Pérez, D.; Kneubühler, Y.; Rais, O.; Gern, L. Seasonality of Ixodes ricinus Ticks on Vegetation and on Rodents and Borrelia burgdorferi sensu lato Genospecies Diversity in Two Lyme Borreliosis–Endemic Areas in Switzerland. Vector-Borne Zoonotic Dis. 2012, 12, 633–644. [Google Scholar] [CrossRef]
- Schulz, M.; Mahling, M.; Pfister, K. Abundance and seasonal activity of questing Ixodes ricinus ticks in their natural habitats in southern Germany in 2011. J. Vector Ecol. 2014, 39, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Hubálek, Z. Epidemiology of Lyme Borreliosis. Curr. Probl. Dermatol. 2009, 37, 31–50. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, A.; Hauffe, H.; Carpi, G.; Neteler, M.; Rosa, R. Lyme borreliosis in Europe. Eurosurveillance 2011, 16, 1–8. [Google Scholar]
- Stanek, G. Lyme borreliosis. Lancet 2012, 379, 461–473. [Google Scholar] [CrossRef]
- Hofhuis, A. Lyme borreliosis in the Netherlands: Strong increase in GP consultations and hospital admissions in the past ten years. Eurosurveillance 2006, 11, e060622.2. [Google Scholar] [CrossRef]
- Vanthomme, K.; Bossuyt, N.; Boffin, N.; van Casteren, V. Incidence and management of presumption of Lyme borreliosis in Belgium: Recent data from the sentinel network of general practitioners. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2385–2390. [Google Scholar] [CrossRef]
- Letrilliart, L.; Ragon, B.; Hanslik, T.; Flahault, A. Lyme disease in France: A primary care-based prospective study. Epidemiol. Infect. 2005, 133, 935–942. [Google Scholar] [CrossRef]
- Smith, R.; Takkinen, J.; Collective Editorial Team. Lyme borreliosis: Europe-wide coordinated surveillance and action needed? Eurosurveillance 2006, 11, 2977. [Google Scholar] [CrossRef]
- Sykes, R.A.; Makiello, P. An estimate of Lyme borreliosis incidence in Western Europe. J. Public Health 2016, 39, 76–87. [Google Scholar] [CrossRef]
- Granger, D.M. Tick-borne encephalitis among US travelers to Europe and Asia 2000–2009. Morb. Mortal. Wkly. Rep. 2010, 59, 335–338. [Google Scholar]
- Reusken, C.B.; Reimerink, J.; Verduin, C.; Sabbe, L.; Cleton, N.; Koopmans, M. Case report: Tick-borne encephalitis in two Dutch travellers returning from Austria, Netherlands, July and August 2011. Eurosurveillance 2011, 16, 20003. [Google Scholar] [PubMed]
- Rendi-Wagner, P. Risk and Prevention of Tick-borne Encephalitis in Travelers. J. Travel Med. 2006, 11, 307–312. [Google Scholar] [CrossRef]
- Haditsch, M.; Kunze, U. Tick-borne encephalitis: A disease neglected by travel medicine. Travel Med. Infect. Dis. 2013, 11, 295–300. [Google Scholar] [CrossRef]
- CDC. Anaplasmosis. 2019. Available online: https://www.cdc.gov/anaplasmosis/stats/index.html (accessed on 8 August 2019).
- Dumler, J.S.; Choi, K.-S.; Garcia-Garcia, J.C.; Barat, N.S.; Scorpio, D.G.; Garyu, J.W.; Grab, D.J.; Bakken, J.S. Human Granulocytic Anaplasmosis and Anaplasma phagocytophilum. Emerg. Infect. Dis. 2005, 11, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Oteo, J.A. Human granulocytic ehrlichiosis in Europe. Clin. Microbiol. Infect. 2002, 8, 763–772. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).