A Comparative Study on the Effect of Task Specific Training on Right Versus Left Chronic Stroke Patients
Abstract
1. Introduction
2. Materials and Methods
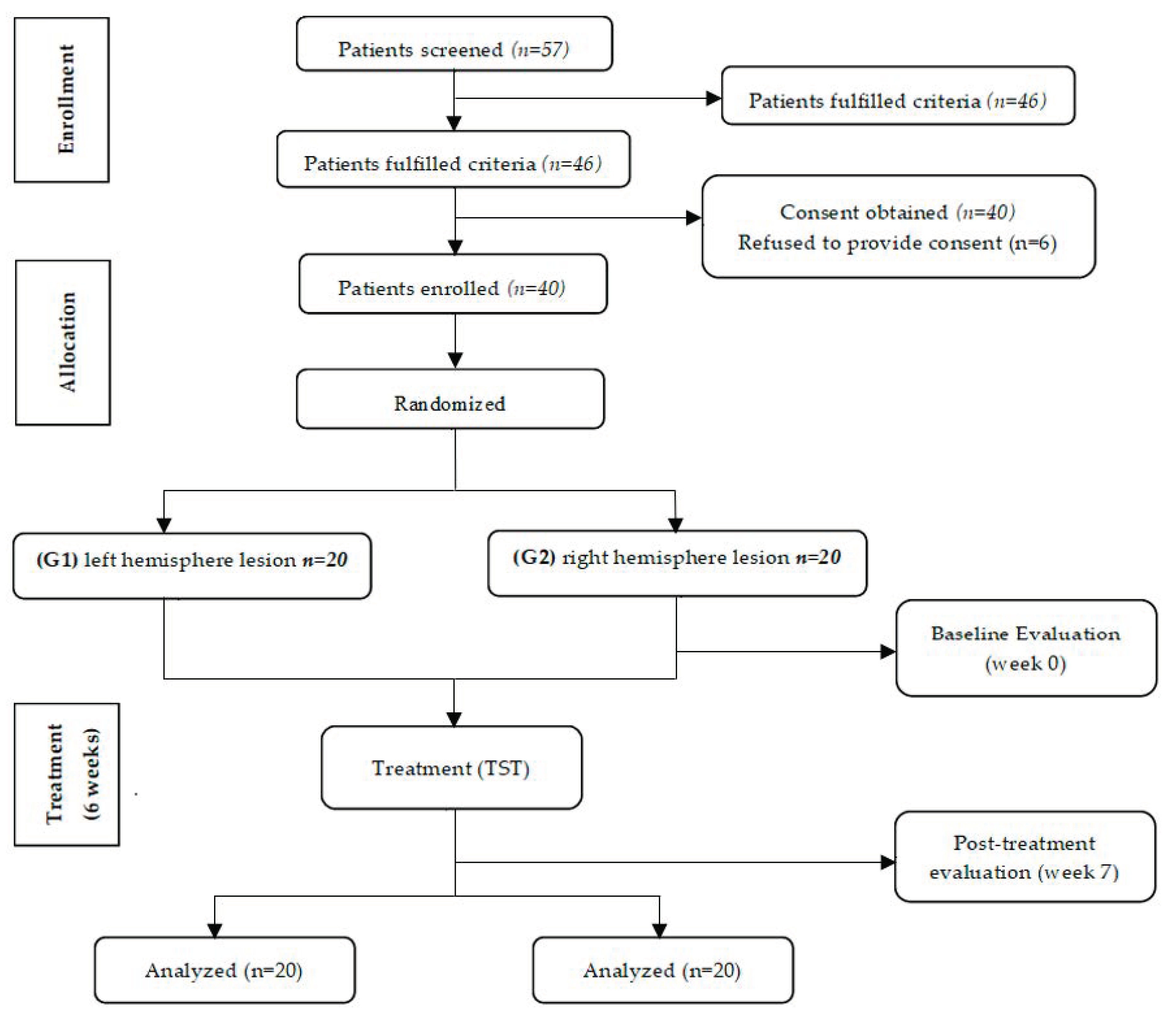
2.1. Participants
2.2. Randomization
2.3. Sample Size
2.4. Clinical Examination
2.4.1. Wolf Motor Function Test (WMFT)
2.4.2. Box and Block Test (BBT)
2.4.3. Quantitative Electroencephalogram (QEEG)
2.5. Therapeutic Interventions
- (a)
- simulating drinking water from a glass;
- (b)
- lifting a glass of water to a level of 90° shoulder flexion with an extended elbow;
- (c)
- moving 5 tennis balls from the table to a box;
- (d)
- wiping the table with a towel with the elbow extended;
- (e)
- moving a cone from a table to a shelf; and
- (f)
- combing hair.
2.6. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Clinical Scales
3.3. Quantitative Electroencephalogram (QEEG)
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Park, H.; Kim, S.; Winstein, C.J.; Gordon, J.; Schweighofer, N. Short-duration and intensive training improves long-term reaching performance in individuals with chronic stroke. Neurorehabil. Neural Repair 2016, 30, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.E.; Bland, M.D.; Bailey, R.R.; Schaefer, S.Y.; Birkenmeier, R.L. Assessment of upper extremity impairment, function, and activity after stroke: Foundations for clinical decision making. J. Hand Ther. 2013, 26, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, Y.-Z.; Zhang, N.; Wang, S.; Ungvari, G.S.; Ng, C.H.; Wang, Y.-L.; Zhao, X.-Q.; Wang, Y.-J.; Wang, C.-X.; et al. The disability rate of 5-year post-stroke and its correlation factors: A national survey in China. PLoS ONE 2016, 11, e0165341. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.H.; Shepherd, R.B. Neurological Rehabilitation: Optimizing Motor Performance; Churchill Livingstone: London, UK, 2010. [Google Scholar]
- Lund, C.; Dalgas, U.; Grønborg, T.K.; Andersen, H.; Severinsen, K.; Riemenschneider, M.; Overgaard, K. Balance and walking performance are improved after resistance and aerobic training in persons with chronic stroke. Disabil. Rehabil. 2018, 40, 2408–2415. [Google Scholar] [CrossRef]
- McCombe Waller, S.; Whitall, J. Bilateral arm training: Why and who benefits? NeuroRehabilitation 2008, 23, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, I.J.; Parsons, M.W.; Neilson, C.; Carey, L.M. Task-specific training: Evidence for and translation to clinical practice. Occup. Ther. Int. 2009, 16, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Bayona, N.A.; Bitensky, J.; Salter, K.; Teasell, R. The role of task-specific training in rehabilitation therapies. Top. Stroke Rehabil. 2005, 12, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Khandare, S.S.; Singaravelan, R.M.; Khatri, S.M. Comparison of task specific exercises and mirror therapy to improve upper limb function in subacute stroke patients. J. Diagn. Med Sonogr. 2013, 7, 5–14. [Google Scholar] [CrossRef]
- French, B.; Thomas, L.H.; Coupe, J.; McMahon, N.E.; Connell, L.; Harrison, J.; Sutton, C.J.; Tishkovskaya, S.; Watkins, C.L. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst. Rev. 2016, 11, CD006073. [Google Scholar] [CrossRef]
- Shepherd, R.B. Exercise and training to optimize functional motor performance in stroke: Driving neural reorganization? Neural Plast. 2001, 8, 121–129. [Google Scholar] [CrossRef]
- Yang, B.I.; Song, B.K.; Joung, S.M. Effects of two-handed task training on upper limb function of chronic hemiplegic patients after stroke. J. Phys. Ther. Sci. 2017, 29, 102–105. [Google Scholar] [CrossRef]
- Carr, J.; Shepherd, R. The changing face of neurological rehabilitation. Rev. Bras. Fisioter. 2006, 10, 147–156. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, J.U. The effect of self-directed exercise using a task board on pain and function in the upper extremities of stroke patients. J. Phys. Ther. Sci. 2013, 25, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Kim, Y.-H.; Cho, S.-H.; Lee, J.-H.; Park, J.-W.; Kwon, Y.-H. Cortical reorganization induced by task-oriented training in chronic hemiplegic stroke patients. NeuroReport 2003, 14, 137–141. [Google Scholar] [CrossRef]
- Richards, L.G.; Stewart, K.C.; Woodbury, M.L.; Senesac, C.; Cauraugh, J.H. Movement-dependent stroke recovery: A systematic review and meta-analysis of TMS and fMRI evidence. Neuropsychologia 2008, 46, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.F.; Kleim, J.A.; Wolf, S.L. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabilit. Neural Repair 2009, 23, 313–319. [Google Scholar] [CrossRef]
- Finnigan, S.; van Putten, M.J.A.M. EEG in ischaemic stroke: Quantitative EEG can uniquely inform (sub-)acute prognoses and clinical management. Clin. Neurophysiol. 2013, 124, 10–19. [Google Scholar] [CrossRef]
- Schleiger, E.; Sheikh, N.; Rowland, T.; Wong, A.; Read, S.; Finnigan, S. Frontal EEG delta/alpha ratio and screening for post-stroke cognitive deficits: The power of four electrodes. Int. J. Psychophysiol. 2014, 94, 19–24. [Google Scholar] [CrossRef]
- Trujillo, P.; Mastropietro, A.; Scano, A.; Chiavenna, A.; Mrakic-Sposta, S.; Caimmi, M.; Molteni, F.; Rizzo, G. Quantitative EEG for predicting upper limb motor recovery in chronic stroke robot-assisted rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1058–1067. [Google Scholar] [CrossRef]
- Hodics, T.M.; Nakatsuka, K.; Upreti, B.; Alex, A.; Smith, P.S.; Pezzullo, J.C. Wolf Motor Function Test for characterizing moderate to severe hemiparesis in stroke patients. Arch. Phys. Med. Rehabil. 2012, 93, 1963–1967. [Google Scholar] [CrossRef]
- Edwards, D.F.; Lang, C.E.; Wagner, J.M.; Birkenmeier, R.; Dromerick, A.W. An evaluation of the Wolf Motor Function Test in motor trials early after stroke. Arch. Phys. Med. Rehabil. 2012, 93, 660–668. [Google Scholar] [CrossRef]
- Wolf, S.L.; Catlin, P.A.; Ellis, M.; Archer, A.L.; Morgan, B.; Piacentino, A. Assessing Wolf Motor Function Test as outcome measure for research in patients after stroke. Stroke 2001, 32, 1635–1639. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-H.; Hsu, M.J.; Sheu, C.F.; Wu, T.-S.; Lin, R.-T.; Chen, C.-H.; Hsieh, C.-L. Psychometric comparisons of 4 measures for assessing upper-extremity function in people with stroke. Phys. Ther. 2009, 89, 840–850. [Google Scholar] [CrossRef]
- Whitall, J.; Savin, D.N., Jr.; Harris-Love, M.; Waller, S.M. Psychometric properties of a modified Wolf Motor Function Test for people with mild and moderate upper-extremity hemiparesis. Arch. Phys. Med. Rehabil. 2006, 87, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.M.; Uswatte, G.; Crago, J.E.; Cook, E.W.; Taub, E. The reliability of the Wolf Motor Function Test for assessing upper extremity function after stroke. Arch. Phys. Med. Rehabil. 2001, 82, 750–755. [Google Scholar] [CrossRef]
- Kontson, K.; Marcus, I.; Myklebust, B.; Civillico, E. Targeted box and blocks test: Normative data and comparison to standard tests. PLoS ONE 2017, 12, e0177965. [Google Scholar] [CrossRef]
- Chen, H.-M.; Chen, C.C.; Hsueh, I.-P.; Huang, S.-L.; Hsieh, C.-L. Test-retest reproducibility and smallest real difference of 5 hand function tests in patients with stroke. Neurorehabilit. Neural Repair 2009, 23, 435–440. [Google Scholar] [CrossRef]
- Santisteban, L.; Térémetz, M.; Bleton, J.-P.; Baron, J.-C.; Maier, M.A.; Lindberg, P.G. Upper limb outcome measures used in stroke rehabilitation studies: A systematic literature review. PLoS ONE 2016, 11, e0154792. [Google Scholar] [CrossRef] [PubMed]
- Mathiowetz, V.; Volland, G.; Kashman, N.; Weber, K. Adult norms for the Box and Block Test of manual dexterity. Am. J. Occup. Ther. 1985, 39, 386–391. [Google Scholar] [CrossRef]
- Hammond, D.C. The Need for individualization in neurofeedback: Heterogeneity in QEEG patterns associated with diagnoses and symptoms. Appl. Psychophysiol. Biofeedback 2010, 35, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Kleim, J.A.; Jones, T.A. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J. Speech Lang. Hear. Res. 2008, 51, S225–S239. [Google Scholar] [CrossRef]
- Thant, A.A.; Wanpen, S.; Nualnetr, N.; Puntumetakul, R.; Chatchawan, U.; Hla, K.M.; Khin, M.T. Effects of task-oriented training on upper extremity functional performance in patients with sub-acute stroke: A randomized controlled trial. J. Phys. Ther. Sci. 2019, 31, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Riès, S.K.; Dronkers, N.F.; Knight, R.T. Choosing words: Left hemisphere, right hemisphere, or both? Perspective on the lateralization of word retrieval. Ann. N. Y. Acad. Sci. 2016, 1369, 111–131. [Google Scholar] [CrossRef]
- El Amki, M.; Baumgartner, P.; Bracko, O.; Luft, A.R.; Wegener, S. Task-specific motor rehabilitation therapy after stroke improves performance in a different motor task: Translational evidence. Transl. Stroke Res. 2017, 8, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.C.; Aguiar, L.T.; Nadeau, S.; Scianni, A.S.; Teixeira-Salmela, L.F.; Faria, C.D.C.M. Efficacy of task-specific training on physical activity levels of people with stroke: Protocol for a randomized controlled trial. Phys. Ther. 2017, 97, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Ocklenburg, S.; Friedrich, P.; Güntürkün, O.; Genç, E. Intrahemispheric white matter asymmetries: The missing link between brain structure and functional lateralization? Rev. Neurosci. 2016, 27, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.A.; Chevidikunnan, M.F.; Khan, F.R.; Gaowgzeh, R.A. Effectiveness of knowledge of result and knowledge of performance in the learning of a skilled motor activity by healthy young adults. J. Phys. Ther. Sci. 2016, 28, 1482–1486. [Google Scholar] [CrossRef]
- Fritsch, B.; Reis, J.; Martinowich, K.; Schambra, H.M.; Ji, Y.; Cohen, L.G.; Lu, B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: Potential implications for motor learning. Neuron 2010, 66, 198–204. [Google Scholar] [CrossRef]
- Timmermans, A.A.; Seelen, H.A.; Willmann, R.D.; Kingma, H. Technology-assisted training of arm-hand skills in stroke: Concepts on reacquisition of motor control and therapist guidelines for rehabilitation technology design. J. Neuroeng. Rehabil. 2009, 6, 1. [Google Scholar] [CrossRef]
- Mutha, P.K.; Haaland, K.Y.; Sainburg, R.L. The effects of brain lateralization on motor control and adaptation. J. Mot. Behav. 2012, 44, 455–469. [Google Scholar] [CrossRef]
- MacNeilage, P.F.; Rogers, L.J.; Vallortigara, G. Origins of the left & right brain. Sci. Am. 2009, 301, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, A. Effects of number of repetitions and number of hours of shaping practice during constraint-induced movement therapy: A randomized controlled trial. Neurol. Res. Int. 2018, 2018, 5496408. [Google Scholar] [CrossRef]
- Timmermans, A.A.; Spooren, A.I.; Kingma, H.; Seelen, H.A. Influence of task-oriented training content on skilled arm-hand performance in stroke: A systematic review. Neurorehabilit. Neural Repair 2010, 24, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Veerbeek, J.M.; van Wegen, E.; van Peppen, R.; van der Wees, P.J.; Hendriks, E.; Rietberg, M.; Kwakkel, G. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS ONE 2014, 9, e87987. [Google Scholar] [CrossRef]
| Demographic Data | G1 (n = 20) | G2 (n = 20) | p Value |
|---|---|---|---|
| Age (years) | 55.8 ± 5.7 | 56.6 ± 4.9 | 0.584 a |
| Type (Infarction/Hemorrhage) | 12/8 | 13/7 | 0.652 b |
| Height (cm) | 166.1 ±7.21 | 164.7 ± 8.34 | 0.784 a |
| Weight (kg) | 65.2 ± 9.42 | 67.3 ± 11.31 | 0.651 a |
| Duration of stroke (month) | 23.22 ± 2.09 | 22.11± 3.07 | 0.949 a |
| WMFT | G1 | G2 | p Value |
|---|---|---|---|
| Pre Test | 24.29 ± 9.1 | 21.86 ± 8.3 | 0.45 b |
| Post Test | 55.86 ± 9.87 | 46.14 ± 12.8 | 0.04b * |
| p Value | 0.0001 a,* | 0.0001 a,* | - |
| BBT | G1 | G2 | p Value |
|---|---|---|---|
| Pre Test | 22.9 ± 12.1 | 23.75 ± 14.4 | 0.62 b |
| Post Test | 31.45 ± 10.47 | 29.96 ± 10.17 | 0.005 b,* |
| p Value | 0.001 a,* | 0.002 a,* | - |
| QEEG (C3/C4) | G1 | G2 | p Value |
|---|---|---|---|
| Pre Test | 8.501 ± 0.1 | 8.326± 0.13 | 0.394 b |
| Post Test | 9.443 ± 0.4 | 8.298 ± 0.03 | 0.001 b,* |
| p Value | 0.0005 a,* | 0.886 a | - |
| QEEG (P3/P4) | G1 | G2 | p Value |
|---|---|---|---|
| Pre Test | 8.96 ± 0.63 | 8.876 ± 0.4 | 0.832 b |
| Post Test | 9.97 ± 0.86 | 8.652 ± 0.5 | 0.011 b,* |
| p Value | 0.005 a,* | 0.665 a | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alwhaibi, R.M.; Mahmoud, N.F.; Zakaria, H.M.; Badawy, W.M.; Elzanaty, M.Y.; Ragab, W.M.; Benjadid, M.S.; Al Awaji, N.N.; Elserougy, H.R. A Comparative Study on the Effect of Task Specific Training on Right Versus Left Chronic Stroke Patients. Int. J. Environ. Res. Public Health 2020, 17, 7950. https://doi.org/10.3390/ijerph17217950
Alwhaibi RM, Mahmoud NF, Zakaria HM, Badawy WM, Elzanaty MY, Ragab WM, Benjadid MS, Al Awaji NN, Elserougy HR. A Comparative Study on the Effect of Task Specific Training on Right Versus Left Chronic Stroke Patients. International Journal of Environmental Research and Public Health. 2020; 17(21):7950. https://doi.org/10.3390/ijerph17217950
Chicago/Turabian StyleAlwhaibi, Reem M., Noha F. Mahmoud, Hoda M. Zakaria, Wanees M. Badawy, Mahmoud Y. Elzanaty, Walaa M. Ragab, Maher S. Benjadid, Nisreen N. Al Awaji, and Hager R. Elserougy. 2020. "A Comparative Study on the Effect of Task Specific Training on Right Versus Left Chronic Stroke Patients" International Journal of Environmental Research and Public Health 17, no. 21: 7950. https://doi.org/10.3390/ijerph17217950
APA StyleAlwhaibi, R. M., Mahmoud, N. F., Zakaria, H. M., Badawy, W. M., Elzanaty, M. Y., Ragab, W. M., Benjadid, M. S., Al Awaji, N. N., & Elserougy, H. R. (2020). A Comparative Study on the Effect of Task Specific Training on Right Versus Left Chronic Stroke Patients. International Journal of Environmental Research and Public Health, 17(21), 7950. https://doi.org/10.3390/ijerph17217950






