Effectiveness of Hydrocolloid Dressings for Treating Pressure Ulcers in Adult Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
1.1. Description of the Condition
1.2. Description of Interventions
- basic dressings (e.g., low-adherence dressings; absorbent dressings);
- antibacterial dressings: (e.g., honey, iodine, or silver impregnated dressings; other dressings with anti-bacterial properties);
- advanced dressings (e.g., foam dressings consisting of hydrophilic polyurethane foam; alginate dressings made of calcium and sodium alginate; hydrogel dressings consisting of cross-linked insoluble starch or carboxymethocellulose polymers and water (up to 96%); occlusive hydrocolloid dressings (HDs) consisting of a hydrocolloid matrix attached to a vapor-permeable foil or foam base; foil/membrane dressings permeable to water vapor and oxygen but impermeable to water and microorganisms; capillary action dressings consisting of an absorbent core of hydrophilic fibers contained between two weakly adhering contact layers; odor absorbing dressings, usually containing charcoal);
- specialist dressings (e.g., protease-modulating matrix dressings designed to change the activity of proteolytic enzymes in chronic wounds, which promotes natural wound cleansing) [9].
1.3. Objectives
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
- clinical trials comparing the efficacy of hydrocolloid and alternative dressings (simple dressings, dressings according to the British National Formulary classification);
- studies in adults (>18 years old);
- the presence of at least one pressure ulcer (stage I, II, III, or IV), with no restrictions on the type of pressure ulcer classification scale [10];
- various healthcare settings (outpatient clinics, home care agencies; acute-care facilities, family homes, long-term care nursing homes, geriatric hospital wards, rehabilitation centers, palliative care centers).
2.3. Study Selection
2.4. Data Extraction and Analysis
2.5. Assessment of the Risk of Bias in the Included Studies
2.6. Statistical Analysis
3. Results
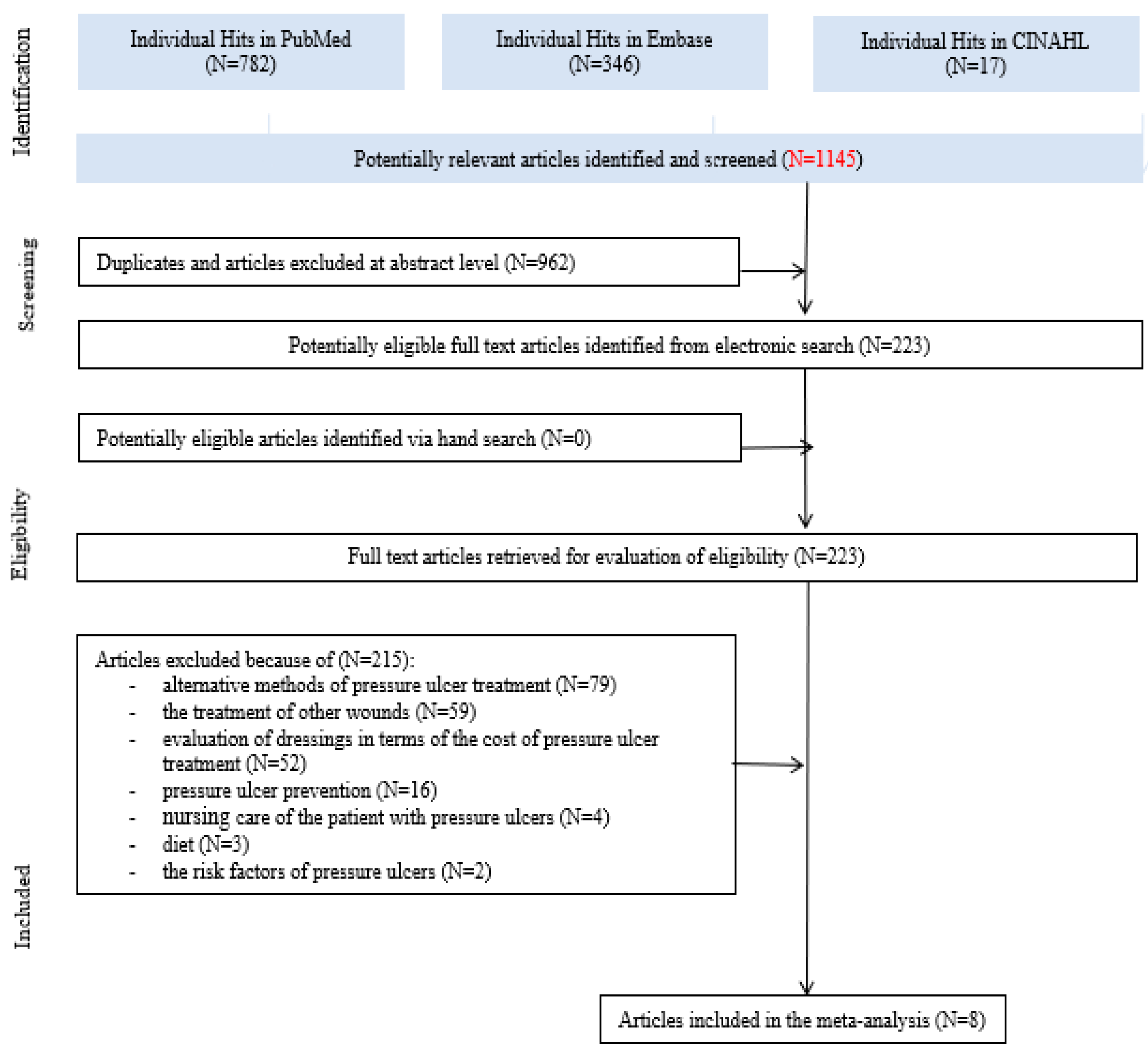
3.1. Search Results
3.2. Characteristics of the Included Studies
3.2.1. Characteristics of Pressure Ulcers
3.2.2. Characteristics of Interventions
3.2.3. Results of Interventions
3.3. The Risk of Bias Assessment
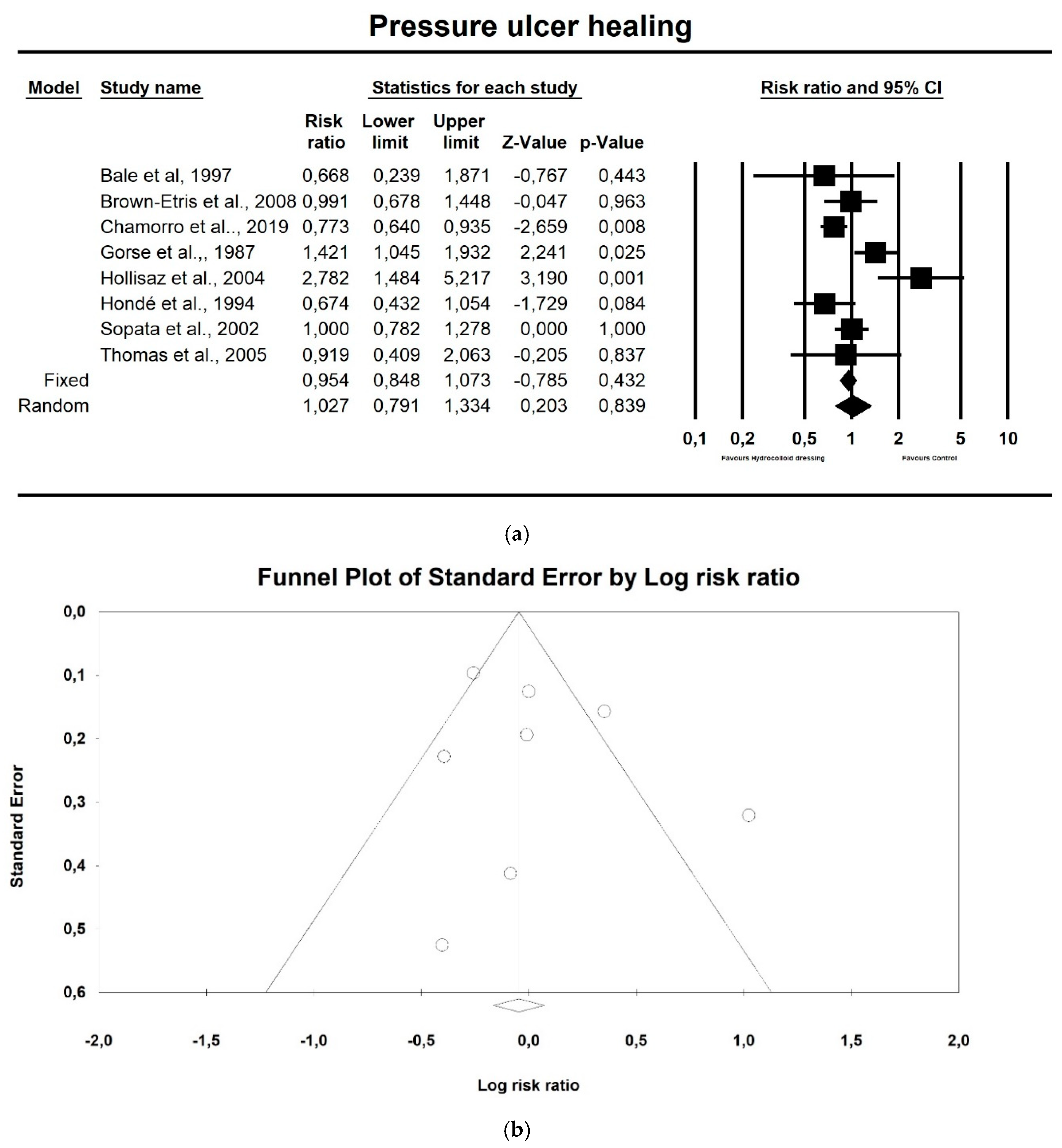
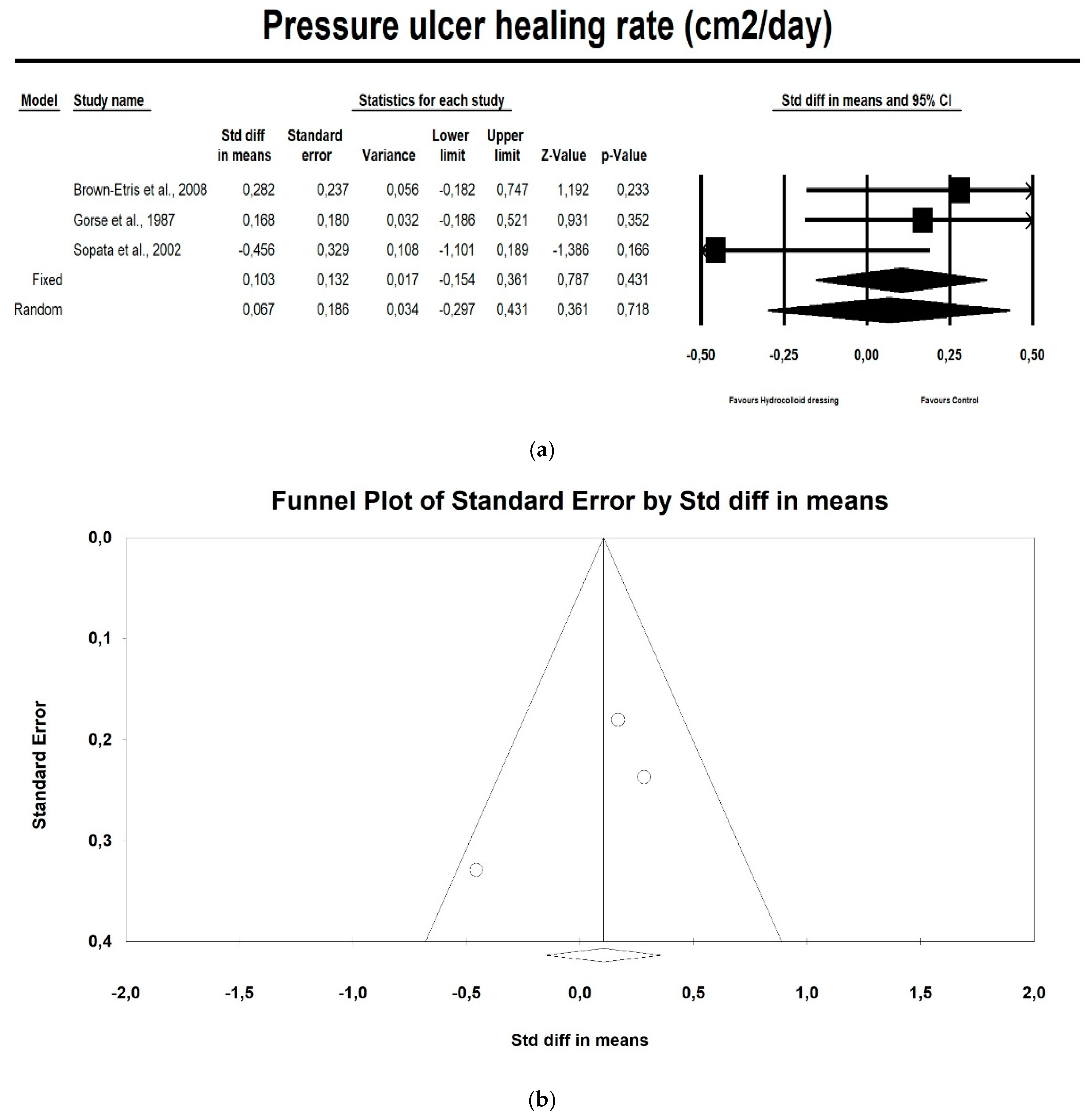
3.4. Meta-Analysis
4. Discussion
4.1. Main Findings
4.2. Differences between Ours and Other Published Studies
4.3. Strenghts and Limitations
4.4. Implications for Current Practice and Future Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- National Pressure Ulcer Advisory Panel. European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance (NPUAP/EPUAP/PPPIA). In Prevention and Treatment of Pressure Ulcers: Quick Reference Guide; Emily, H., Ed.; Cambridge Media: Osborne Park, Australia, 2014. [Google Scholar]
- Mervis, J.S.; Philips, T.J. Pressure ulcers: Pathophysiology, epidemiology, risk factors, and presentation. J. Am. Acad. Dermatol. 2019, 81, 881–890. [Google Scholar] [CrossRef]
- Pieper, B. Pressure Ulcers: Prevalence, Incidence, and Implications for the Future; National Pressure Ulcer Advisory Panel: Washington, DC, USA, 2013. [Google Scholar]
- Blackburn, J.; Ousey, K.; Taylor, L.; Moore, T.; Patton, D.; Moore, Z.; Avsar, P. The relationship between common risk factors and the pathology of pressure ulcer development: A systematic review. J. Wound Care 2020, 29, S4–S12. [Google Scholar] [CrossRef]
- Pelham, F.; Keith, M.; Smith, A.; Williams, D.V.; Powell, G. Pressure ulcer prevalence and cost in the U.S. population. J. Am. Med. Direct. Assoc. 2007, 8, B20. [Google Scholar] [CrossRef]
- Hall, J.; Buckley, H.L.; Lamb, K.A.; Stubbs, N.; Saramago, P.; Dumville, J.C.; Cullum, N.A. Point prevalence of complex wounds in a defined United Kingdom population. Wound Repair Regen. 2014, 22, 694–700. [Google Scholar] [CrossRef]
- Lahmann, N.A.; Halfens, R.J.; Dassen, T. Pressure ulcers in German nursing homes and acute care hospitals: Prevalence, frequency and ulcer characteristics. Ostomy Wound Manag. 2006, 52, 20–33. [Google Scholar]
- VanGilder, C.; Amlung, S.; Harrison, P.; Meyer, S. Results of the 2008–2009 International Pressure Ulcer Prevalence™ Survey and a 3-year, acute care, unit-specific analysis. Ostomy Wound Manag. 2009, 55, 39–45. [Google Scholar]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- British Medical Association. British Royal Pharmaceutical Society of Great Britain. British National Formulary (BNF). Available online: https://about.medicinescomplete.com/publication/british-national-formulary/ (accessed on 30 March 2020).
- Westby, M.J.; Dumville, J.C.; Soares, M.O.; Stubbs, N.; Norman, G. Dressings and topical agents for treating pressure ulcers. Cochrane Database Syst Rev. 2017, 6, CD011947. [Google Scholar] [CrossRef]
- Hondé, C.; Derks, C.; Tudor, D. Local Treatment of Pressure Sores in the Elderly: Amino Acid Copolymer Membrane Versus Hydrocolloid Dressing. J. Am. Geriatr. Soc. 1994, 42, 1180–1183. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration. 2011. Available online: https://www.cochrane-handbook.org/ (accessed on 30 March 2020).
- Comprehensive Meta-Analysis V3. Biostat, New Jersey, USA. Available online: https://www.meta-analysis.com. (accessed on 31 August 2020).
- Bale, S.; Squires, D.; Varnon, T.; Walker, A.; Benbow, M.; Harding, K.G. A comparison of two dressings in pressure sore management. J. Wound Care 1997, 6, 463–466. [Google Scholar] [CrossRef]
- Brown-Etris, M.; Milne, C.; Orsted, H.; Gates, J.L.; Netsch, D.; Punchello, M.; Couture, N.; Albert, M.; Attrell, E.; Freyberg, J. A prospective, randomized, multisite clinical evaluation of a transparent absorbent acrylic dressing and a hydrocolloid dressing in the management of Stage II and shallow Stage III pressure ulcers. Adv. Skin Wound Care 2008, 21, 169–174. [Google Scholar] [CrossRef]
- Chamorro, A.M.; Vidal, T.M.C.; Mieras, A.S.; Leiva, A.; Martínes, M.P.; Yeste, M.M.S.H.; Grupo, U.P.P. Multicenter randomized controlled trial comparing the effectiveness and safety of hydrocellular and hydrocolloid dressings for treatment of category II pressure ulcers in patients at primary and long-term care institutions. Int. J. Nurs. Stud. 2019, 94, 179–185. [Google Scholar] [CrossRef]
- Gorse, G.J.; Messner, R.L. Improved pressure sore healing with hydrocolloid dressings. Arch. Dermatol. 1987, 123, 766–771. [Google Scholar] [CrossRef]
- Hollisaz, M.T.; Khedmat, H.; Yari, F. A randomized clinical trial comparing hydrocolloid, phenytoin and simple dressings for the treatment of pressure ulcers [ISRCTN33429693]. BMC Dermatol. 2004, 4, 18. [Google Scholar] [CrossRef]
- Sopata. M.; Luczak, J.; Ciupinska, M. Effect of bacteriological status on pressure ulcer healing in patients with advanced cancer. J. Wound Care 2002, 11, 107–110. [Google Scholar] [CrossRef]
- Thomas, D.R.; Diebold, M.R.; Eggmeyer, L.M. A controlled, randomized, comparative study of a radiant heat bandage on the healing of stage 3–4 pressure ulcers: A pilot study. J. Am. Med. Direct. Assoc. 2005, 6, 46–49. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Heyneman, A.; Beele, H.; Venderwee, K.; Defloor, T. A systematic review of the use of hydrocolloids in the treatmnet of pressure ulcers. J. Clin. Nurs. 2008, 17, 1164–1173. [Google Scholar] [CrossRef]
- Bouza, C.; Saz, Z.; Muñoz, A.; Amate, J.M. Efficacy of advanced dressings in the treatment of pressure ulcers: A systematic review. J. Wound Care 2005, 14, 193–199. [Google Scholar] [CrossRef]
- Huang, L.; Woo, K.Y.; Liu, L.B.; Wen, R.J.; Hu, A.L.; Shi, C.G. Dressings for preventing pressure ulcers: A meta-analysis. Adv. Skin Wound Care 2015, 28, 267–273. [Google Scholar] [CrossRef]
- Pott, F.S.; Meier, M.J.; Dorociak-Stocco, J.G.; Crozeta, K.; Ribas, J.D. The effectiveness of hydrocolloid dressings versus other dressings in the healing of pressure ulcers in adults and older adults: A systematic review and meta-analysis. Rev. Lat. Am. Enfermagem. 2014, 22, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Yarkony, G.M. Pressure ulcers: A review. Arch. Phys. Med. Rehabil. 1994, 75, 908–917. [Google Scholar] [CrossRef]
- Hutchinson, J.J.; Mc Guckin, M. Occlusive dressings: A microbiologic and clinical review. Am. J. Infect. Control 1990, 18, 257–268. [Google Scholar] [CrossRef]
- Eisenbud, D.; Hunter, H.; Kessler, L.; Zulkowski, K. Hydrogel wound dressings: Where do we stand in 2003? Ostomy Wound Manag. 2003, 49, 52–57. [Google Scholar]
- Mulder, G.D.; Altman, M.; Seeley, J.E.; Tintle, T. Prospective randomized study of the efficacy of hydrogel, hydrocolloid, and saline solution-moistened dressings on the management of pressure ulcers. Wound Repair Regen. 1993, 1, 213–218. [Google Scholar] [CrossRef]
- Banks, V.; Hagelstein, S.; Thomas, N.; Bale, S.; Harding, K.G. Comparing hydrocolloid dressings in management of exuding wounds. Br. J. Nurs. 1999, 8, 640–646. [Google Scholar] [CrossRef]
- Baxter, H. A comparison of two hydrocolloid sheet dressings. Br. J. Community Nurs. 2000, 5, 572–577. [Google Scholar] [CrossRef]
- Walker, R.M.; Gillespie, B.M.; Thalib, L.; Higgins, N.S.; Whitty, J.A. Foam dressings for treating pressure ulcers. Cochrane Database Syst. Rev. 2017, 2017, CD011332. [Google Scholar] [CrossRef]

| Database | Search Strings with Medical Subject Headings |
|---|---|
| PubMed | (‘pressure ulcer’ [MeSH Terms] OR (‘pressure’ [All Fields] AND ‘ulcer’ [All Fields]) OR ‘pressure ulcer’ [All Fields] OR ‘decubitus’ [All Fields]) AND (‘adult’ [MeSH Terms] OR ‘adult’ [All Fields]) AND (‘bandages’ [MeSH Terms] OR ‘bandages’ [All Fields] OR ‘dressing’ [All Fields]) AND (‘therapy’ [Subheading] OR ‘therapy’ [All Fields] OR ‘treatment’ [All Fields] OR ‘therapeutics’ [MeSH Terms] OR ‘therapeutics’ [All Fields]). The following search terms with medical subject headings (MeSH–bold font) |
| Embase | (‘decubitus’/exp OR ‘bed sore’ OR ‘bedsore’ OR ‘decubital ulcer’ OR ‘decubital ulcus’ OR ‘decubitus’ OR ‘decubitus ulcer’ OR ‘decubitus ulceration’ OR ‘decubitus ulcers’ OR ‘decubitus ulcus’ OR ‘decubus ulcer’ OR ‘pressure sore’ OR ‘pressure ulcer’ OR ‘sore, pressure’ OR ‘ulcer, pressure’ OR ‘ulcus decubitus’) AND (‘human’/exp OR ‘homo sapiens’ OR ‘human’ OR ‘human being’ OR ‘human body’ OR ‘human race’ OR ‘human subject’ OR ‘humans’ OR ‘man (homo sapiens)’) AND (‘adult’/exp OR ‘adult’ OR ‘adults’ OR ‘grown-ups’ OR ‘grownup’ OR ‘grownups’) AND (‘wound dressing’/exp OR ‘amd telfa’ OR ‘adaptic (device)’ OR ‘adaptic touch’ OR ‘algisite’ OR ‘aquacel’ OR ‘aquacel-ag’ OR ‘askina calgitrol’ OR ‘atrauman’ OR ‘autologel’ OR ‘biopatch’ OR ‘biostep’ OR ‘curasorb’ OR ‘cutilin’ OR ‘cutimed sorbact’ OR ‘drymax’ OR ‘eclypse (device)’ OR ‘excellagen’ OR ‘flivasorb’ OR ‘graftskin’ OR ‘hemcon’ OR ‘hemcon bandage pro’ OR ‘hemcon dental dressing pro’ OR ‘hemcon nasal plug’ OR ‘hemcon strip pro’ OR ‘jaloskin’ OR ‘kerramax’ OR ‘leukomed’ OR ‘leukomed sorbact’ OR ‘medihoney’ OR ‘mepitel’ OR ‘mepore’ OR ‘mesorb’ OR ‘opsite (device)’ OR ‘primatrix ag’ OR ‘primapore’ OR ‘promogran’ OR ‘quickclot acs’ OR ‘seasorb’ OR ‘silvercel’ OR ‘sorbion’ OR ‘steri-strips’ OR ‘suprathel’ OR ‘surfasoft’ OR ‘telfa’ OR ‘veloderm’ OR ‘zetuvit’ OR ‘askina sorb’ OR ‘biobrane’ OR ‘dressing, wound’ OR ‘oasis (device)’ OR ‘wound dressing’ OR ‘wound dressing agent’) AND (‘therapy’/exp OR ‘combination therapy’ OR ‘disease therapy’ OR ‘disease treatment’ OR ‘diseases treatment’ OR ‘disorder treatment’ OR ‘disorders treatment’ OR ‘efficacy, therapeutic’ OR ‘illness treatment’ OR ‘medical therapy’ OR ‘medical treatment’ OR ‘multiple therapy’ OR ‘polytherapy’ OR ‘somatotherapy’ OR ‘therapeutic action’ OR ‘therapeutic efficacy’ OR ‘therapeutic trial’ OR ‘therapeutic trials’ OR ‘therapeutics’ OR ‘therapy’ OR ‘therapy, medical’ OR ‘treatment effectiveness’ OR ‘treatment efficacy’ OR ‘treatment, medical’). The following search terms with medical subject headings (MeSH–bold font) |
| CINAHL | (‘pressure ulcer’ OR ‘bedsore’ OR ‘decubitus ulcer’ OR ‘pressure sore’) AND (‘dressings’ OR ‘bandages’) AND (‘treatment’ OR ‘intervention’ OR ‘therapy’) AND (‘adults’ OR ‘adult’ OR ‘aged’ OR ‘elderly’) |
| Characteristics of Included Studies | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Reference (Localization) | Age (years) (Mean ± SD) | Subjects/Males (n) | Healthcare Setting | Pressure Ulcers at the Beginning of the Study SG/CG (n) | Healed Pressure Ulcers SG/CG (n) | Healed Pressure Ulcers (n)/A Total Number of Pressure Ulcers (n) | Length of Follow up | Source of Funding | The Types of Dressings | |
| SG | CG | ||||||||||
| 1. | Bale et al., 1997 (the UK) | nd (median: SG: 74; CG: 73) | 60/27 | 5 centers in the UK | 31/29 | 5/7 | 12/60 | Up to 4 weeks (30 days). | Financial support from Smith and Nephew—industry funded. | A hydrocolloid dressing | A polyurethane foam dressing |
| 2. | Brown-Etris et al., 2008 (the USA, Canada) | SG: 72.7 (18.6); CG: 78.3 (14.7) | 72/32 | A variety of healthcare settings, including extended-care facilities, outpatient wound care clinics, and home care agencies | 37/35 | 22/21 | 43/72 | Up to 8 weeks (56 days). | Grant from 3M Company (manufacturers of Tegaderm)—industry funded. | A hydrocolloid dressing (DuoDERM CGF, ConvaTec, ER Squibb & Sons, Princeton, NJ) | A transparent absorbent acrylic dressing (3M Tegaderm Absorbent Clear Acrylic Dressing) |
| 3. | Chamorro et al., 2019 (Spain) | SG: 83.3 (8.7); CG: 79.2 (13.3) | 169/71 | Primary care centers and ling-term care institutions | 85/84 | 54/69 | 123/169 | Up to 8 weeks | Grant from the Ministry of Economy and Competitiveness, Carlos III Institute (ISCIII), as well as with financial support from the Health Promotion and Preventive Activities- Primary Health Care Network, the Ministry of Health ISCIII-RETIC), and the European Union Regional Development Funds – non-industry funded. | A hydrocolloid dressing (VARIHESIVE® GEL CONTROL (Convatec) | A hydrocellular dressing (ALLEVYN Adhesive® (Smith & Nephew) |
| 4. | Gorse et al., 1987 (the USA) | SG: 72.0 +/− 12.8; CG: 68.4–13.5 | 52/52 | The Huntington Veterans Administration Medical Center—acute-care facility | 76/52 | 54/26 | 80/128 | Approx. 11 weeks. | nd | A hydrocolloid dressing (Duoderm, Convatec, E.R. Squib and Sons Inc.) | A mesh-gauze wet-to-dry dressing (WDDs) |
| 5. | Hollisaz et al., 2004 (Iran) | 36.64 ± 6.04 | 83/83 | Family homes or nursing homes | 31/30 | 23/8 | 31/61 | Approx. 8 weeks (2 months). | Grant from the Jaonbazan Medical and Engineering Research Center, the medical and research section of the official governmental body responsible for spinal cord injury (SCI) war victims—non-industry funded. | A hydrocolloid dressing | A simple dressing |
| 6. | Hondé et al., 1994 (France) | general—82 yearsSG: 83.5 +/− 7.8 (years 64–101); CG: 80.4 +/− 8.2 (years 63-98); | 168/47 | Geriatric hospital wards | 88/80 | 23/31 | 54/168 | Up to 8 weeks. | Financial support from Synthelabo Recherche (manufacturers of Inerpan)—industry funded. | A hydrocolloid dressing, Comfeel™ (Coloplast) | A copolymer membrane, Inerpan™ (Synthélabo) |
| 7. | Sopata et al., 2002 (Poland) | general: 58.6 +/− 15.51; SG: 58.7 +/− 14.11; CG: 58.5 +/− 16.92 | 34/16 | Palliative Care Department at the University of Medical Sciences, Poznan, Poland | 17/17 | 15/15 | 30/38 | Up to 8 weeks. | This study was non-industry funded—declaration of interest: none. | A hydrogel dressing (Aquagel, Wytwórnia Opatrunków, Poland) | A polyurethane foam dressing Lyofoam (Seton, UK) |
| 8. | Thomas et al., 2005 (the USA) | General—75.5 +/− 12.6; SG: 77.0 +/− 11.5; CG:74.1 +/− 13.8 | 41/21 | Outpatient clinics, long-term care nursing homes, and a rehabilitation center | 20/21 | 7/8 | 15/41 | Up to 12 weeks | nd | A sterile control hydrocolloid dressing (Duoderm™ Convatec, Inc., Princeton, NJ) with or without a calcium alginate filler (Sorbasan,™ Smith-Nephew, Inc., Largo, FL) | A radiant-heat dressingdevice (Warm-Up™, Augustine Medical, Inc., Princeton, NJ) |
| No. | Reference (Localization) | Characteristics of the Pressure Ulcers | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pressure Ulcers (n) | Stage (n) | Localization (n) | Baseline Size cm2 (Mean ± SD) (cm2 +/− SD) | ||||||||||
| I | II | III | IV | Sacrum/ Coccyx | Foot/ Heel | Buttock | Ischium | Trochanter | Other | ||||
| Bale et al., 1997 (the UK) | 60 | 0 | 45 | 15 | 0 | 31 | 16 | nd | nd | 2 | 11 | (sore area (cm2) >5: n = 24 (sore area (cm2) 5–<10: n = 12 (sore area (cm2) 10–<20: n = 13 (sore area (cm2) ≥ 20: n = 11 | |
| Brown-Etris et al., 2008 (the USA, Canada) | 72 | 0 | 45 | 27 | 0 | 22 | 8 | 14 | 12 | nd | 16 | SG = 2.5 +/− 4.86 (cm2 +/− SD) CG = 1.5 +/− 1.69 (cm2 +/− SD) | |
| Chamorro et al., 2019 (Spain) | 169 | 0 | 169 | 0 | 0 | 75 | 19 | 40 | 0 | 15 | 20 | nd | |
| Gorse et al., 1987 (the USA) | 128 | 0 | 107 | 21 | 56 | nd | nd | 22 | 29 | 21 | nd | ||
| Hollisaz et al., 2004 (Iran) | 61 | 24 | 37 | 0 | 0 | 15 | 0 | 14 | 32 | 0 | 0 | SG = 7.26 +/− 15.4 (cm2 +/− SD) CG = 10.27 +/− 15.32 (cm2 +/− SD) | |
| Hondé et al., 1994 (France) | 168 | 0 | nd | nd | nd | 61 | 92* | 0 | 0 | 5 | 10 | SG = 6.85 cm2 CG = 8.99 cm2 | |
| Sopata et al., 2002 (Poland) | 38 | 0 | 12 | 26 | 0 | 17 | nd | 12 | nd | nd | nd | SG = 8.28 +/− 13.90 (cm2 +/− SD) CG = 11.04 +/− 11.65 (cm2 +/− SD) | |
| Thomas et al., 2005 (the USA) | 41 | 0 | 0 | 22 | 19 | 23 ** | nd | nd | 9 ** | nd | 9 ** | SG = 12.1 +/− 18.2 (cm2 +/− SD) CG = 11.0 +/− 9.5 (cm2 +/− SD) | |
| No. | Reference (Localization) | Wound Healing (Rate) | Frequency of Dressing Change Dressing Wear Time | Adverse Events (n) | Conclusion | |||
|---|---|---|---|---|---|---|---|---|
| SG | CG | SG | CG | SG | CG | |||
| Bale et al., 1997 (the UK) | nd | nd | Mean wear times: SG: 3.2 days; The maximum wear time for an individual dressing was 11 days. | Mean wear times: CG: 3.8 days The maximum wear time for an individual dressing was 13 days. | 0 | 1 (skin rash) | SG and CG are easy and convenient to apply; absorbency and ease of removal were significantly better with CG than SG; wear times were similar. | |
| Brown-Etris et al., 2008 (the USA, Canada) | Linear healing rate, cm/wk Mean (SD): 0.12 (0.136). | Linear healing rate, cm/wk Mean (SD): 0.10 (0.205). | Mean (SD) wear time was: 4.7 (2.29) days. | Mean (SD) wear time was: 5.7 (2.55) days. | 8 None of the adverse events were related to the study dressings under evaluation. | 10 None of the adverse events were related to the study dressings under evaluation. | Performance results favored the CG over the SG as standard treatment for stage II and shallow stage III pressure ulcers. | |
| Chamorro et al., 2019 (Spain) | nd | nd | The dressing was changed every 7 days. | The dressing was changed every 7 days. | 0 | 6 (infection, erythema, dressing hypersensitivity) | CG were superior to SG in terms of healing at 8 weeks and time required for healing. These two dressings had similar safety profiles. | |
| Gorse et al., 1987 (the USA) | Completely healed Rate of decrease, cm2/d: 0.72 ± 1.22 Days to resolution: 10.0 ± 10.5 | Completely healed Rate of decrease, cm2/d: 0.55 ± 0.59 Days to resolution: 8.7 ± 6.2 | The dressing was changed routinely every four days or more frequently if the membrane became contaminated with stool, became nonocclusive, or if signs and symptoms of systemic infection developed. | The dressings were changed every eight hours. | 1 (infection) | 0 | SG regimen was more efficacious even in a subgroup of patients who did not receive adequate nutritional support during treatment. Adequate nutritional support during the study was associated with better healing in both SG and CG. | |
| Hollisaz et al., 2004 (Iran) | n (%) The completion of healing, regardless of location and stage: 23/31 (74.19%). Completion of healing of stage I ulcers: 11/13 (85%). Completion of healing of stage II ulcers: 12/18 (67%). | n (%) The completion of healing, regardless of location and stage: 8/30 (26.66%). Completion of healing of stage I ulcers: 5/11 (45%). Completion of healing of stage II ulcers: 3/19 (16%). | Twice a day. | Twice a day. | 0 | 0 | SG is the most effective method investigated for treating stage I and II pressure ulcers in young paraplegic men. | |
| Hondé et al., 1994 (France) | The median healing time was 38 (range 11–63) days. | The median healing time was 32 (range 13–59) days. | nd | nd | 6 (infection) | 6 (infection) | GK is easy to use, safeguards the healing process, and is of particular value in the management of pressure sores. | |
| Sopata et al., 2002 (Poland) | Rate of healing (cm2/day): 0.67 ± 0.37 cm2/day (grade II) and 0.31 ± 0.21 cm2/day (grade III). “Improved” ulcers (grade III only) healed at 0.27 ± 0.11 cm2/day. Treatment times (days):Medium time: 20.10 ± 14.70 (n = 20) | Rate of healing (cm2/day): 1.23 ± 1.33 cm2/day (grade II) and 0.44 ± 0.27 cm2/day (grade III). “Improved” ulcers (grade III only) healed at 0.70 ± 0.63 cm2/day. Treatment times (days): Medium time: 25.77 ± 14.15 (n = 18) | Dressings were changed according to clinical need. | Dressings were changed according to clinical need. | 0 | 0 | There was no statistical difference between SG and CG in efficacy, healing rates, and treatment times. | |
| Thomas et al., 2005 (the USA) | n (%): 7 (44%) with complete healing of their pressure ulcer. | n (%): 8 (57%) with complete healing of their pressure ulcer. | The dressing was changed every 7 days or when the occlusive seal was broken. | The dressing was changed every 7 days or when the occlusive seal was broken. | nd (adverse events and serious adverse events were assessed at each weekly visit). | nd (adverse events and serious adverse events were assessed at each weekly visit). | There was no statistical difference between SG and CG. However, at almost all points along the healing curve, the proportion not healed was higher in SG. | |
| Reference (Localization) | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data | Selective Reporting (Reporting Bias) | Other Sources of Bias | Number of Low Risk of Bias Assessments |
|---|---|---|---|---|---|---|---|---|
| Bale et al., 1997 (the UK) | ? | H | H | H | H | L | L | 2 |
| Brown-Etris et al., 2008 (the USA, Canada) | ? | ? | H | H | L | L | L | 3 |
| Chamorro et al., 2019 (Spain) | ? | H | H | L | H | H | H | 1 |
| Gorse et al., 1987 (the USA) | ? | H | ? | ? | L | L | H | 2 |
| Hollisaz et al., 2004 (Iran) | L | L | L | L | L | L | ? | 6 |
| Hondé et al., 1994 (France) | ? | ? | H | H | H | H | ? | 0 |
| Sopata et al., 2002 (Poland) | ? | ? | ? | ? | L | L | H | 2 |
| Thomas et al., 2005 (the USA) | ? | ? | H | H | H | L | L | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamińska, M.S.; Cybulska, A.M.; Skonieczna-Żydecka, K.; Augustyniuk, K.; Grochans, E.; Karakiewicz, B. Effectiveness of Hydrocolloid Dressings for Treating Pressure Ulcers in Adult Patients: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 7881. https://doi.org/10.3390/ijerph17217881
Kamińska MS, Cybulska AM, Skonieczna-Żydecka K, Augustyniuk K, Grochans E, Karakiewicz B. Effectiveness of Hydrocolloid Dressings for Treating Pressure Ulcers in Adult Patients: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2020; 17(21):7881. https://doi.org/10.3390/ijerph17217881
Chicago/Turabian StyleKamińska, Magdalena Sylwia, Anna Maria Cybulska, Karolina Skonieczna-Żydecka, Katarzyna Augustyniuk, Elżbieta Grochans, and Beata Karakiewicz. 2020. "Effectiveness of Hydrocolloid Dressings for Treating Pressure Ulcers in Adult Patients: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 17, no. 21: 7881. https://doi.org/10.3390/ijerph17217881
APA StyleKamińska, M. S., Cybulska, A. M., Skonieczna-Żydecka, K., Augustyniuk, K., Grochans, E., & Karakiewicz, B. (2020). Effectiveness of Hydrocolloid Dressings for Treating Pressure Ulcers in Adult Patients: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 17(21), 7881. https://doi.org/10.3390/ijerph17217881







