Association of Insufficient or Excess Sleep with Type 2 Diabetes Mellitus in the Presence of Periodontitis
Abstract
1. Introduction
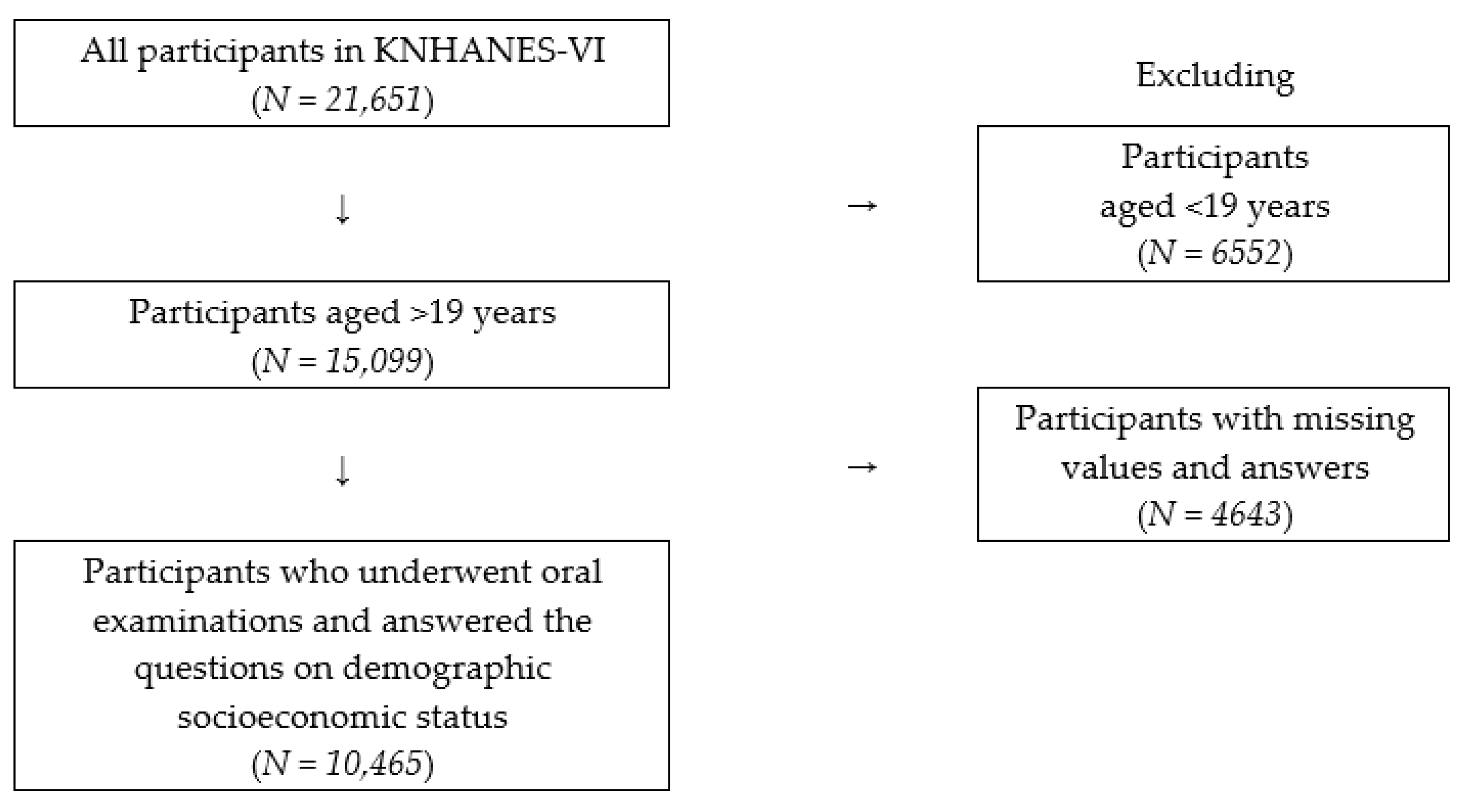
2. Materials and Methods
2.1. Data Sources
2.2. Variables
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Akerstedt, T.; Knutsson, A.; Westerholm, P.; Theorell, T.; Alfredsson, L.; Kecklund, G. Sleep Disturbances, Work Stress and Work Hours: A Cross-Sectional Study. J. Psychosom. Res. 2002, 53, 741–748. [Google Scholar] [CrossRef]
- Carskadon, M.A.; Dement, W.C. Normal Human Sleep: An Overview Principles and Practice of Sleep Medicine, 5th ed.; Elsevier Saunders: St. Louis, MO, USA, 2005; pp. 13–23. [Google Scholar]
- Liu, X.; Uchiyama, M.; Shibui, K.; Kim, K.; Kudo, Y.; Tagaya, H.; Suzuki, H.; Okawa, M. Diurnal preference, sleep habits, circadian sleep propensity and melatonin rhythm in healthy human subjects. Neurosci. Lett. 2000, 280, 199–202. [Google Scholar] [CrossRef]
- Besedovsky, L.; Lange, T.; Born, J. Sleep and immune function. Pflugers Arch. 2012, 463, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Cauter, E.V.; Spiegel, K.; Tasali, E.; Leproult, R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008, 9, S23–S28. [Google Scholar] [CrossRef]
- Gangwisch, J.E.; Heymsfield, S.B.; Boden-Albala, B.; Buijs, R.M.; Kreier, F.; Pickering, T.G.; Rundle, A.G.; Zammit, G.K.; Malaspina, D. Sleep duration as a risk factor for diabetes incidence in a large US sample. Sleep 2007, 30, 1667–1673. [Google Scholar] [CrossRef]
- Knutson, K.L.; Ryden, A.M.; Mander, B.A.; Cauter, E.V. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch. Intern. Med. 2006, 166, 1768–1774. [Google Scholar] [CrossRef]
- Knutson, K.L. Sleep Duration and Cardiometabolic Risk: A Review of the Epidemiologic Evidence. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 731–743. [Google Scholar] [CrossRef]
- Lee, H.J.; Jeong, Y.W.; Kim, H.J.; Jeong, R.H. Association between Sleep Duration and Prevalence of Dyslipidemia in Korean Adults: The Sixth Korean National Health and Nutrition Examination Survey. Korean J. Family Pract. 2019, 9, 485–491. [Google Scholar] [CrossRef]
- Knutsson, A.; Kempe, A. Shift work and diabetes—A systematic review. Chronobiol. Int. 2014, 31, 1146–1151. [Google Scholar] [CrossRef]
- OECD iLibrary. OECD Social Indicators. Available online: https://doi.org/10.1787/19991290 (accessed on 1 June 2020).
- Löe, H. Periodontal disease: The sixth complication of diabetes mellitus. Diabetes Care 1993, 16, 329–334. [Google Scholar] [CrossRef]
- Saini, R.; Saini, S.; Sugandha, R. Periodontal disease: The sixth complication of diabetes. J. Family Community Med. 2011, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Casanova, L.; Hughes, F.J.; Preshaw, P.M. Diabetes and periodontal disease: A two-way relationship. Br. Dent. J. 2014, 217, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.; Hayes, C.; Taylor, G.W. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent. Oral Epidemiol. 2002, 30, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Mattout, C.; Bourgeois, D.; Bouchard, P. Type 2 diabetes and periodontal indicators: Epidemiology in France 2002–2003. J. Periodontal Res. 2006, 41, 253–258. [Google Scholar] [CrossRef]
- Gurav, A.; Jadhav, V. Periodontitis and risk of diabetes mellitus. J. Diabetes 2011, 3, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ship, J.A. Diabetes and oral health: An overview. J. Am. Dent. Assoc. 2003, 134, 4S–10S. [Google Scholar] [CrossRef] [PubMed]
- Ainamo, J.; Barmes, D.; Beagrie, G.; Cutress, T.; Martin, J.; Sardo-Infirri, J. Development of the World Health Organization (WHO) community periodontal index of treatment needs (CPITN). Int. Dent. J. 1982, 32, 281–291. [Google Scholar]
- Brown, R.E.; Basheer, R.; McKenna, J.T.; Strecker, R.E.; McCarley, R.W. Control of sleep and wakefulness. Physiol. Rev. 2012, 92, 1087–1187. [Google Scholar] [CrossRef]
- Kim, Y.; Wilkens, L.R.; Schembre, S.M.; Henderson, B.E.; Kolonel, L.N.; Goodman, M.T. Insufficient and excessive amounts of sleep increase the risk of premature death from cardiovascular and other diseases: The Multiethnic Cohort Study. Prev. Med. 2013, 57, 377–385. [Google Scholar] [CrossRef]
- Irwin, M.R. Why sleep is important for health: A psychoneuroimmunology perspective. Annu. Rev. Psychol. 2015, 66, 143–172. [Google Scholar] [CrossRef]
- Grover, V.; Malhotra, R.; Kaur, H. Exploring association between sleep deprivation and chronic periodontitis: A pilot study. J. Indian Soc. Periodontol. 2015, 19, 304–307. [Google Scholar] [CrossRef]
- Kim, E.-K.; Lee, S.G.; Choi, Y.H.; Won, K.C.; Moon, J.S.; Merchant, A.T.; Lee, H.-K. Association between diabetes-related factors and clinical periodontal parameters in type-2 diabetes mellitus. BMC Oral Health 2013, 13, 64. [Google Scholar] [CrossRef]
- Wiener, R.C. Relationship of routine inadequate sleep duration and periodontitis in a nationally representative sample. Sleep Disord. 2016, 2016, 9158195. [Google Scholar] [CrossRef] [PubMed]
- Hackett, R.A.; Dal, Z.; Steptoe, A. The relationship between sleep problems and cortisol in people with type 2 diabetes. Psychoneuroendocrinology 2020, 117, 104688. [Google Scholar] [CrossRef] [PubMed]
- Do, K.-Y.; Lee, E.-S. Relationship between Sleep Duration and Periodontitis in Korean Adult Women: Data from Korea National Health and Nutrition Examination Survey 2014. J. Dent. Hyg. Sci. 2017, 17, 298–305. [Google Scholar] [CrossRef]
- Nakada, T.; Kato, T.; Numabe, Y. Effects of fatigue from sleep deprivation on experimental periodontitis in rats. Comp. Study 2015, 50, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, A.L.; Toma, C.; Lascu, V. Associations among sleep disturbance, vitality, fatigue and oral health. Oral Health Prev. Dent. 2010, 8, 323–330. [Google Scholar]
| Total (%) | No Periodontitis (%) | Periodontitis (%) | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | DM | IFG | NDM | DM | IFG | NDM | DM | IFG | NDM | |
| Sex | <0.001 | |||||||||
| Male | 8.4 | 24.4 | 67.2 | 5.3 | 21.5 | 73.3 | 15.6 | 31.2 | 53.3 | |
| Female | 5.7 | 16.2 | 78.2 | 3.9 | 13.9 | 82.2 | 12.3 | 24.9 | 62.9 | |
| Age group (years) | <0.001 | |||||||||
| 19–34 | 1.1 | 9.1 | 89.8 | 0.9 | 9.0 | 90.1 | 3.5 | 10.2 | 86.3 | |
| 35–44 | 4.5 | 20.7 | 74.8 | 3.6 | 18.6 | 77.7 | 7.3 | 28.1 | 64.5 | |
| 45–54 | 8.3 | 26.8 | 64.8 | 5.8 | 24.4 | 69.8 | 12.6 | 31.0 | 56.4 | |
| 55–64 | 16.0 | 31.8 | 52.2 | 12.0 | 30.8 | 57.2 | 20.9 | 33.0 | 46.2 | |
| >65 | 21.2 | 28.3 | 50.5 | 19.6 | 28.6 | 51.8 | 22.8 | 28.1 | 49.2 | |
| Education level | <0.001 | |||||||||
| ≤Junior high school | 16.2 | 28.9 | 55.0 | 13.4 | 26.0 | 60.5 | 19.4 | 32.2 | 48.5 | |
| High school | 5.5 | 20.0 | 74.5 | 3.2 | 17.3 | 79.5 | 12.9 | 28.6 | 58.5 | |
| ≥College | 4.6 | 16.8 | 78.6 | 3.2 | 15.0 | 81.8 | 10.4 | 24.7 | 64.9 | |
| Household income level | <0.001 | |||||||||
| Low | 13.3 | 22.2 | 64.5 | 7.7 | 19.2 | 73.1 | 22.8 | 27.2 | 50.1 | |
| Low-Middle | 8.4 | 19.6 | 72.0 | 5.6 | 17.3 | 77.1 | 15.9 | 25.6 | 58.4 | |
| Middle-High | 5.4 | 20.2 | 74.5 | 3.8 | 16.7 | 79.5 | 10.5 | 31.1 | 58.4 | |
| High | 5.6 | 20.3 | 74.1 | 3.8 | 17.7 | 78.6 | 12.1 | 29.3 | 58.6 | |
| Smoking status | <0.001 | |||||||||
| Current smoker | 7.7 | 24.1 | 68.2 | 4.2 | 21.0 | 74.8 | 14.3 | 29.8 | 55.9 | |
| Past smoker | 9.7 | 25.4 | 64.9 | 6.1 | 23.0 | 70.9 | 17.5 | 30.7 | 51.7 | |
| Non-smoker | 5.9 | 17.1 | 77.0 | 4.2 | 14.7 | 81.1 | 12.5 | 26.6 | 60.9 | |
| Estimate sleeping duration | <0.001 | |||||||||
| ≤5 h | 9.3 | 23.2 | 67.5 | 6.3 | 20.3 | 73.4 | 16.2 | 30.0 | 53.8 | |
| 6–7 h | 6.2 | 20.3 | 73.5 | 4.4 | 17.3 | 78.3 | 11.9 | 29.4 | 58.7 | |
| ≥8 h | 7.5 | 18.8 | 73.6 | 4.1 | 16.4 | 79.5 | 17.8 | 26.0 | 56.1 | |
| Variables | IFG | DM |
|---|---|---|
| Crude OR (95% CI) | Crude OR (95% CI) | |
| Sex | ||
| Male | 1.74 (1.53–1.99) | 1.51 (1.21–1.90) |
| Female | 1.00 | 1.00 |
| Age | 0.61 (0.58–0.65) | 0.41 (0.38–0.45) |
| Education level | ||
| ≤Junior high school | 2.35 (1.97–2.81) | 5.66 (4.27–7.51) |
| High school | 1.19 (1.03–1.39) | 1.03 (0.77–1.37) |
| ≥College | 1.00 | 1.00 |
| Household income level | ||
| Low | 1.17 (0.92–1.47) | 2.20 (1.55–3.11) |
| Low-Middle | 1.00 (0.83–1.19) | 1.51 (1.12–2.03) |
| Middle-High | 0.93 (0.79–1.10) | 0.99 (0.73–1.36) |
| High | 1.00 | 1.00 |
| Smoking status | ||
| Current smoker | 1.55 (1.30–1.84) | 1.07 (0.78–1.47) |
| Past smoker | 1.79 (1.50–2.13) | 1.66 (1.24–2.23) |
| Non-smoker | 1.00 | 1.00 |
| Estimate sleeping duration | ||
| ≤5 h | 1.25 (1.03–1.52) | 1.53 (1.14–2.07) |
| ≥8 h | 0.94 (0.80–1.10) | 0.91 (0.70–1.20) |
| 6–7 h | 1.00 | 1.00 |
| Variables | IFG | DM |
|---|---|---|
| Crude OR (95% CI) | Crude OR (95% CI) | |
| Sex | ||
| Male | 1.48 (1.22–1.79) | 1.50 (1.18–1.90) |
| Female | 1.00 | 1.00 |
| Age | 0.76 (0.70–0.82) | 0.56 (0.51–0.63) |
| Education level | ||
| ≤Junior high school | 1.74 (1.37–2.22) | 2.48 (1.82–3.38) |
| High school | 1.28 (1.01–1.64) | 1.37 (0.98–1.91) |
| ≥College | 1.00 | 1.00 |
| Household income level | ||
| Low | 1.08 (0.81–1.45) | 2.21 (1.56–3.12) |
| Low-Middle | 0.88 (0.68–1.14) | 1.32 (0.96–1.83) |
| Middle-High | 1.06 (0.83–1.36) | 0.87 (0.61–1.23) |
| High | 1.00 | 1.00 |
| Smoking status | ||
| Current smoker | 1.22 (0.97–1.53) | 1.24 (0.93–1.67) |
| Past smoker | 1.36 (1.07–1.72) | 1.65 (1.25–2.19) |
| Non-smoker | 1.00 | 1.00 |
| Estimate sleeping duration | ||
| ≤5 h | 1.11 (0.85–1.46) | 1.49 (1.09–2.04) |
| ≥8 h | 0.93 (0.74–1.16) | 1.57 (1.19–2.07) |
| 6–7 h | 1.00 | 1.00 |
| Variables | IFG | DM | ||
|---|---|---|---|---|
| Adjusted OR (95% CI) | Adjusted OR (95% CI) | |||
| Total | Periodontitis | Yes | 1.28 (1.13–1.46) | 1.86 (1.55–2.23) |
| No | 1.00 | 1.00 | ||
| Sleep duration | ≤5 h | 1.09 (0.92–1.30) | 1.17 (0.93–1.48) | |
| ≥8 h | 1.03 (0.90–1.18) | 1.29 (1.05–1.57) | ||
| 6–7 h | 1.00 | 1.00 | ||
| Non-Periodontitis | Sleep duration | ≤5 h | 1.12 (0.91–1.38) | 1.49 (1.09–2.04) |
| ≥8 h | 1.13 (0.95–1.34) | 1.57 (1.19–2.07) | ||
| 6–7 h | 1.00 | 1.00 | ||
| Periodontitis | Sleep duration | ≤5 h | 1.06 (0.80–1.41) | 1.24 (0.89–1.73) |
| ≥8 h | 0.90 (0.72–1.14) | 1.49 (1.12–1.98) | ||
| 6–7 h | 1.00 | 1.00 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-Y.; Kim, J.-S.; Byon, M.-J.; Kang, H.K.; Kim, J.-B. Association of Insufficient or Excess Sleep with Type 2 Diabetes Mellitus in the Presence of Periodontitis. Int. J. Environ. Res. Public Health 2020, 17, 7670. https://doi.org/10.3390/ijerph17207670
Kim S-Y, Kim J-S, Byon M-J, Kang HK, Kim J-B. Association of Insufficient or Excess Sleep with Type 2 Diabetes Mellitus in the Presence of Periodontitis. International Journal of Environmental Research and Public Health. 2020; 17(20):7670. https://doi.org/10.3390/ijerph17207670
Chicago/Turabian StyleKim, Se-Yeon, Ji-Soo Kim, Min-Ji Byon, Hyun Kyung Kang, and Jin-Bom Kim. 2020. "Association of Insufficient or Excess Sleep with Type 2 Diabetes Mellitus in the Presence of Periodontitis" International Journal of Environmental Research and Public Health 17, no. 20: 7670. https://doi.org/10.3390/ijerph17207670
APA StyleKim, S.-Y., Kim, J.-S., Byon, M.-J., Kang, H. K., & Kim, J.-B. (2020). Association of Insufficient or Excess Sleep with Type 2 Diabetes Mellitus in the Presence of Periodontitis. International Journal of Environmental Research and Public Health, 17(20), 7670. https://doi.org/10.3390/ijerph17207670






