Associations of Dietary Bioactive Compounds with Maternal Adiposity and Inflammation in Gestational Diabetes: An Update on Observational and Clinical Studies
Abstract
1. Introduction
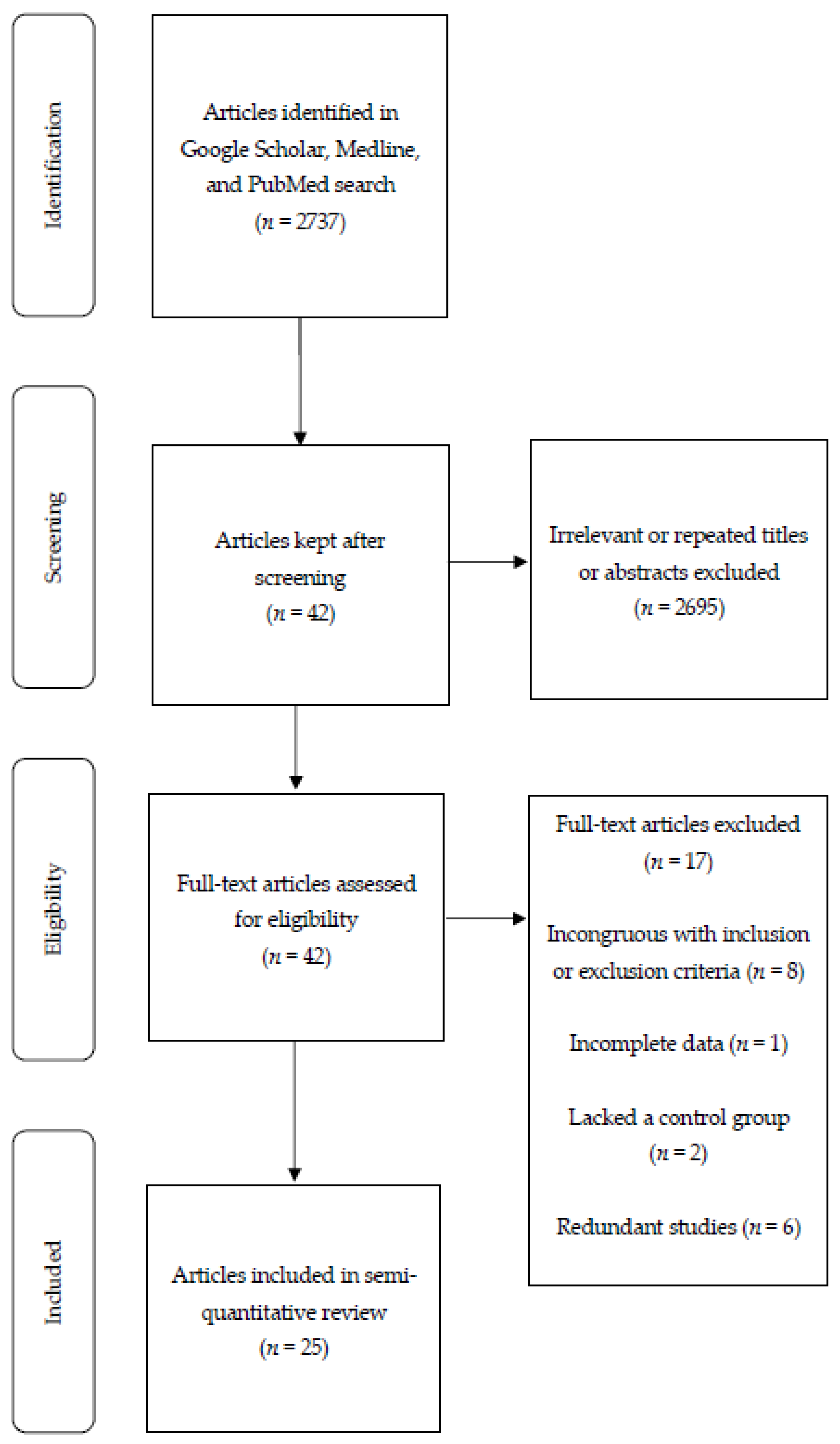
2. Materials and Methods
3. Results
3.1. Observational Studies
3.1.1. Nutrient Intake and Risk of GDM
3.1.2. Biomarkers of Adiposity, Antioxidant Vitamin and Mineral Status, Oxidative Stress, and Inflammation
3.2. Randomized Controlled Trials
3.2.1. Diet Therapy
3.2.2. Omega-3 (n-3) Fatty Acids
3.2.3. Probiotics and Synbiotics
3.2.4. Trace Elements
3.2.5. Vitamin D
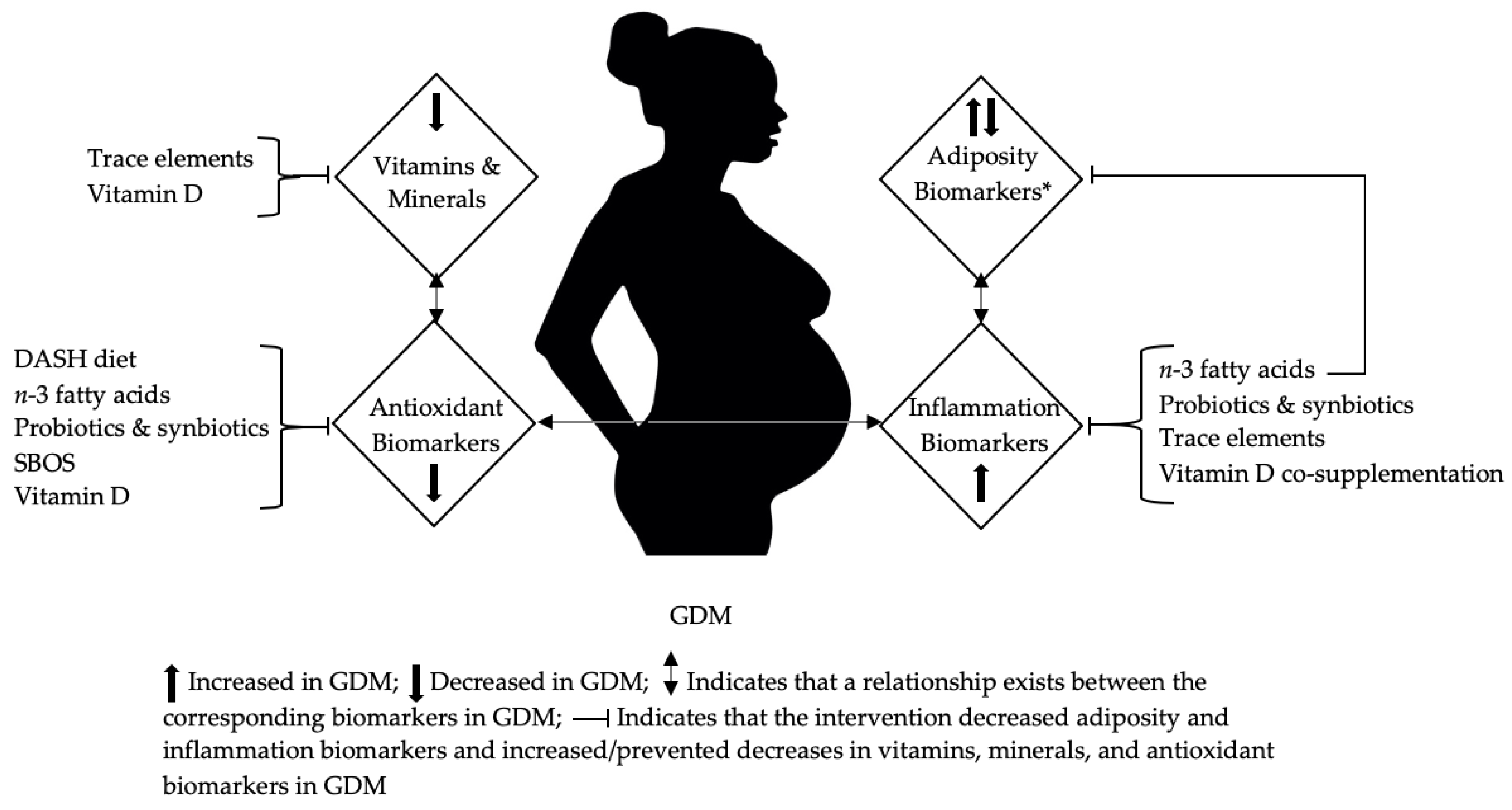
4. Discussion
4.1. Observational Studies
4.2. RCTs—Diet Therapy
4.3. RCTs—n-3 Fatty Acids
4.4. RCTs—Probiotic and Synbiotics
4.5. RCTs—Trace Elements
4.6. RCTs—Vitamin D
4.7. Strengths and Limitations
4.8. Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 2019, 5, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: A global perspective. Curr. Diab. Rep. 2016, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Moses, R.G.; Calvert, D. Pregnancy outcomes in women without gestational diabetes mellitus related to the maternal glucose level: Is there a continuum of risk? Dia Care 1995, 18, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Sermer, M.; Naylor, C.D.; Gare, D.J.; Kenshole, A.B.; Ritchie, J.W.K.; Farine, D.; Cohen, H.R.; McArthur, K.; Holzapfel, S.; Biringer, A.; et al. Impact of increasing carbohydrate intolerance on maternal-fetal outcomes in 3637 women without gestational diabetes: The Toronto tTri-Hospital Gestational Diabetes Project. Am. J. Obstet. Gynecol. 1995, 173, 146–156. [Google Scholar] [CrossRef]
- HAPO Study Cooperative Research Group; Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; et al. Hyperglycemia and Adverse Pregnancy Outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Cheung, N.W.; Byth, K. Population health significance of gestational diabetes. Dia Care 2003, 26, 2005–2009. [Google Scholar] [CrossRef] [PubMed]
- Aerts, L.; Van Assche, F.A. Animal evidence for the transgenerational development of diabetes mellitus. Int. J. Biochem. Cell Biol. 2006, 38, 894–903. [Google Scholar] [CrossRef]
- Clausen, T.D.; Mathiesen, E.R.; Hansen, T.; Pedersen, O.; Jensen, D.M.; Lauenborg, J.; Damm, P. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: The role of intrauterine hyperglycemia. Dia Care 2008, 31, 340–346. [Google Scholar] [CrossRef]
- Clausen, T.D.; Mathiesen, E.R.; Hansen, T.; Pedersen, O.; Jensen, D.M.; Lauenborg, J.; Schmidt, L.; Damm, P. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J. Clin. Endocrinol. Metab. 2009, 94, 2464–2470. [Google Scholar] [CrossRef]
- Lowe, W.L.; Scholtens, D.M.; Lowe, L.P.; Kuang, A.; Nodzenski, M.; Talbot, O.; Catalano, P.M.; Linder, B.; Brickman, W.J.; Clayton, P.; et al. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA 2018, 320, 1005–1016. [Google Scholar] [CrossRef]
- Lowe, W.L.; Scholtens, D.M.; Kuang, A.; Linder, B.; Lawrence, J.M.; Lebenthal, Y.; McCance, D.; Hamilton, J.; Nodzenski, M.; Talbot, O.; et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-Up Study (HAPO FUS): Maternal gestational diabetes mellitus and childhood glucose metabolism. Dia Care 2019, 42, 372–380. [Google Scholar] [CrossRef]
- Grunnet, L.G.; Hansen, S.; Hjort, L.; Madsen, C.M.; Kampmann, F.B.; Thuesen, A.C.B.; Granstrømi, C.; Strøm, M.; Maslova, E.; Frikke-Schmidt, R.; et al. Adiposity, dysmetabolic traits, and earlier onset of female puberty in adolescent offspring of women with gestational diabetes mellitus: A clinical study within the Danish National Birth Cohort. Dia Care 2017, 40, 1746–1755. [Google Scholar] [CrossRef]
- Kelstrup, L.; Damm, P.; Mathiesen, E.R.; Hansen, T.; Vaag, A.A.; Pedersen, O.; Clausen, T.D. Insulin resistance and impaired pancreatic β-cell function in adult offspring of women with diabetes in pregnancy. J. Clin. Endocrinol. Metab. 2013, 98, 3793–3801. [Google Scholar] [CrossRef]
- Zhang, C.; Solomon, C.G.; Manson, J.E.; Hu, F.B. A prospective study of pregravid physical activity and sedentary behaviors in relation to the risk for gestational diabetes mellitus. Arch. Intern. Med. 2006, 166, 543–548. [Google Scholar] [CrossRef]
- Zhang, C.; Ning, Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: Review of epidemiologic evidence. Am. J. Clin. Nutr. 2011, 94, 1975S–1979S. [Google Scholar] [CrossRef]
- Gaston, A.; Cramp, A. Exercise during pregnancy: A review of patterns and determinants. J. Sci. Med. Sport 2011, 14, 299–305. [Google Scholar] [CrossRef]
- Fell, D.B.; Joseph, K.S.; Armson, B.A.; Dodds, L. The impact of pregnancy on physical activity level. Matern. Child Health J. 2008, 13, 597. [Google Scholar] [CrossRef]
- Bailey, R.L.; Pac, S.G.; Fulgoni, V.L.; Reidy, K.C.; Catalano, P.M. Estimation of total usual dietary intakes of pregnant women in the United States. JAMA Netw. Open 2019, 2, e195967. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Kunath, J.; Günther, J.; Rauh, K.; Hoffmann, J.; Stecher, L.; Rosenfeld, E.; Kick, L.; Ulm, K.; Hauner, H. Effects of a lifestyle intervention during pregnancy to prevent excessive gestational weight gain in routine care—The cluster-randomised GeliS trial. BMC Med. 2019, 17, 5. [Google Scholar] [CrossRef]
- Song, C.; Li, J.; Leng, J.; Ma, R.C.; Yang, X. Lifestyle intervention can reduce the risk of gestational diabetes: A meta-analysis of randomized controlled trials. Obes. Rev. 2016, 17, 960–969. [Google Scholar] [CrossRef]
- Guo, X.-Y.; Shu, J.; Fu, X.-H.; Chen, X.-P.; Zhang, L.; Ji, M.-X.; Liu, X.-M.; Yu, T.-T.; Sheng, J.-Z.; Huang, H.-F. Improving the effectiveness of lifestyle interventions for gestational diabetes prevention: A meta-analysis and meta-regression. BJOG 2019, 126, 311–320. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.; Solomon, C.G.; Hu, F.B. Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Dia Care 2006, 29, 2223–2230. [Google Scholar] [CrossRef]
- Chen, L.; Hu, F.B.; Yeung, E.; Willett, W.; Zhang, C. Prospective study of pre-gravid sugar-sweetened beverage consumption and the risk of gestational diabetes mellitus. Dia Care 2009, 32, 2236–2241. [Google Scholar] [CrossRef]
- Bao, W.; Tobias, D.K.; Hu, F.B.; Chavarro, J.E.; Zhang, C. Pre-pregnancy potato consumption and risk of gestational diabetes mellitus: Prospective cohort study. BMJ 2016, 352. [Google Scholar] [CrossRef]
- Bao, W.; Tobias, D.K.; Olsen, S.F.; Zhang, C. Pre-pregnancy fried food consumption and the risk of gestational diabetes mellitus: A prospective cohort study. Diabetologia 2014, 57, 2485–2491. [Google Scholar] [CrossRef]
- Zhang, C.; Schulze, M.B.; Solomon, C.G.; Hu, F.B. A prospective study of dietary patterns, meat intake and the risk of gestational diabetes mellitus. Diabetologia 2006, 49, 2604–2613. [Google Scholar] [CrossRef]
- Bowers, K.; Tobias, D.K.; Yeung, E.; Hu, F.B.; Zhang, C. A prospective study of prepregnancy dietary fat intake and risk of gestational diabetes. Am. J. Clin. Nutr. 2012, 95, 446–453. [Google Scholar] [CrossRef]
- Bao, W.; Bowers, K.; Tobias, D.K.; Hu, F.B.; Zhang, C. Prepregnancy dietary protein intake, major dietary protein sources, and the risk of gestational diabetes mellitus. Dia Care 2013, 36, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Bowers, K.; Yeung, E.; Williams, M.A.; Qi, L.; Tobias, D.K.; Hu, F.B.; Zhang, C. A prospective study of prepregnancy dietary iron intake and risk for gestational diabetes mellitus. Dia Care 2011, 34, 1557–1563. [Google Scholar] [CrossRef]
- Looman, M.; Schoenaker, D.A.J.M.; Soedamah-Muthu, S.S.; Geelen, A.; Feskens, E.J.M.; Mishra, G.D. Pre-pregnancy dietary carbohydrate quantity and quality, and risk of developing gestational diabetes: The Australian Longitudinal Study on Women’s Health. Br. J. Nutr. 2018, 120, 435–444. [Google Scholar] [CrossRef]
- Santangelo, C.; Zicari, A.; Mandosi, E.; Scazzocchio, B.; Mari, E.; Morano, S.; Masella, R. Could gestational diabetes mellitus be managed through dietary bioactive compounds? Current knowledge and future perspectives. Br. J. Nutr. 2016, 115, 1129–1144. [Google Scholar] [CrossRef]
- Sun, Y.-L.; Zhou, F.-M.; Wang, H.-R. Mechanism of pomegranate ellagic polyphenols reducing insulin resistance on gestational diabetes mellitus rats. Am. J. Transl. Res. 2019, 11, 5487–5500. [Google Scholar]
- Pham, N.M.; Do, V.V.; Lee, A.H. Polyphenol-rich foods and risk of gestational diabetes: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2019, 73, 647–656. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Hales, C.M.; Caroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018; NCHS Data Brief; National Center for Health Statistics: Hyattsville, MD, USA, 2020; p. 8.
- Hajifaraji, M.; Jahanjou, F.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Abbasi, M.M.; Dolatkhah, N. Effect of probiotic supplements in women with gestational diabetes mellitus on inflammation and oxidative stress biomarkers: A randomized clinical trial. Asia Pac. J. Clin. Nutr. 2018, 27. [Google Scholar] [CrossRef]
- Dolatkhah, N.; Hajifaraji, M.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Mehrabi, Y.; Mesgari Abbasi, M. Is there a value for probiotic supplements in gestational diabetes mellitus? A randomized clinical trial. J. Health Popul. Nutr. 2015, 33, 25. [Google Scholar] [CrossRef]
- Shivappa, N.; Hébert, J.R.; Akhoundan, M.; Mirmiran, P.; Rashidkhani, B. Association between inflammatory potential of diet and odds of gestational diabetes mellitus among Iranian women. J. Matern. Fetal Neonatal Med. 2019, 32, 3552–3558. [Google Scholar] [CrossRef]
- Parast, V.M.; Paknahad, Z. Antioxidant status and risk of gestational diabetes mellitus: A case-control study. Clin. Nutr. Res. 2017, 6, 81. [Google Scholar] [CrossRef]
- Haidari, F.; Jalali, M.-T.; Shahbazian, N.; Haghighizadeh, M.-H.; Azadegan, E. Comparison of serum levels of vitamin d and inflammatory markers between women with gestational diabetes mellitus and healthy pregnant control. J. Fam. Reprod. Health 2016, 10, 1–8. [Google Scholar]
- McManus, R.; Summers, K.; de Vrijer, B.; Cohen, N.; Thompson, A.; Giroux, I. Maternal, umbilical arterial and umbilical venous 25-hydroxyvitamin D and adipocytokine concentrations in pregnancies with and without gestational diabetes. Clin. Endocrinol. 2014, 80, 635–641. [Google Scholar] [CrossRef]
- Javadian, P.; Alimohamadi, S.; Gharedaghi, M.H.; Hantoushzadeh, S. Gestational diabetes mellitus and iron supplement; effects on pregnancy outcome. Acta Med. Iran. 2014, 52, 385–389. [Google Scholar]
- Park, S.; Kim, M.-Y.; Baik, S.H.; Woo, J.-T.; Kwon, Y.J.; Daily, J.W.; Park, Y.-M.; Yang, J.-H.; Kim, S.-H. Gestational diabetes is associated with high energy and saturated fat intakes and with low plasma visfatin and adiponectin levels independent of prepregnancy BMI. Eur. J. Clin. Nutr. 2013, 67, 196–201. [Google Scholar] [CrossRef]
- Suhail, M.; Patil, S.; Khan, S.; Siddiqui, S. Antioxidant vitamins and lipoperoxidation in non-pregnant, pregnant, and gestational diabetic women: Erythrocytes osmotic fragility profiles. J. Clin. Med. Res. 2010, 2, 266–273. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Y.; He, M.; Li, T.; Ma, Z.; Cheng, H. Effect of various doses of vitamin D supplementation on pregnant women with gestational diabetes mellitus: A randomized controlled trial. Exp. Ther. Med. 2016, 12, 1889–1895. [Google Scholar] [CrossRef]
- Fei, B.; Ling, L.; Hua, C.; Ren, S. Effects of soybean oligosaccharides on antioxidant enzyme activities and insulin resistance in pregnant women with gestational diabetes mellitus. Food Chem. 2014, 158, 429–432. [Google Scholar] [CrossRef]
- Jamilian, M.; Tabassi, Z.; Reiner, Ž.; Panahandeh, I.; Naderi, F.; Aghadavod, E.; Amirani, E.; Taghizadeh, M.; Shafabakhsh, R.; Satari, M.; et al. The effects of n-3 fatty acids from flaxseed oil on genetic and metabolic profiles in patients with gestational diabetes mellitus: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2020, 123, 792–799. [Google Scholar] [CrossRef]
- Jamilian, M.; Mirhosseini, N.; Eslahi, M.; Bahmani, F.; Shokrpour, M.; Chamani, M.; Asemi, Z. The effects of magnesium-zinc-calcium-vitamin D co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. BMC Pregnancy Childbirth 2019, 19, 107. [Google Scholar] [CrossRef]
- Jamilian, M.; Amirani, E.; Asemi, Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 2098–2105. [Google Scholar] [CrossRef]
- Jamilian, M.; Samimi, M.; Mirhosseini, N.; Afshar Ebrahimi, F.; Aghadavod, E.; Taghizadeh, M.; Asemi, Z. A randomized double-blinded, placebo-controlled trial investigating the effect of fish oil supplementation on gene expression related to insulin action, blood lipids, and inflammation in gestational diabetes mellitus-fish oil supplementation and gestational diabetes. Nutrients 2018, 10, 163. [Google Scholar] [CrossRef]
- Karamali, M.; Nasiri, N.; Taghavi Shavazi, N.; Jamilian, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Asemi, Z. The effects of synbiotic supplementation on pregnancy outcomes in gestational diabetes. Probiotics Antimicrob. Proteins 2018, 10, 496–503. [Google Scholar] [CrossRef]
- Nabhani, Z.; Hezaveh, S.J.G.; Razmpoosh, E.; Asghari-Jafarabadi, M.; Gargari, B.P. The effects of synbiotic supplementation on insulin resistance/sensitivity, lipid profile and total antioxidant capacity in women with gestational diabetes mellitus: A randomized double blind placebo controlled clinical trial. Diabetes Res. Clin. Pract. 2018, 138, 149–157. [Google Scholar] [CrossRef]
- Razavi, M.; Jamilian, M.; Samimi, M.; Afshar Ebrahimi, F.; Taghizadeh, M.; Bekhradi, R.; Seyed Hosseini, E.; Haddad Kashani, H.; Karamali, M.; Asemi, Z. The effects of vitamin D and omega-3 fatty acids co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in patients with gestational diabetes. Nutr. Metab. (Lond.) 2017, 14, 80. [Google Scholar] [CrossRef]
- Jafarnejad, S.; Saremi, S.; Jafarnejad, F.; Arab, A. Effects of a multispecies probiotic mixture on glycemic control and inflammatory status in women with gestational diabetes: A randomized controlled clinical trial. J. Nutr. Metab. 2016, 2016. [Google Scholar] [CrossRef]
- Karamali, M.; Heidarzadeh, Z.; Seifati, S.-M.; Samimi, M.; Tabassi, Z.; Talaee, N.; Bahardoost, H.; Asemi, Z. Zinc supplementation and the effects on pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Exp. Clin. Endocrinol. Diabetes 2016, 124, 28–33. [Google Scholar] [CrossRef]
- Yazdchi, R.; Gargari, B.P.; Asghari-Jafarabadi, M.; Sahhaf, F. Effects of vitamin D supplementation on metabolic indices and hs-CRP levels in gestational diabetes mellitus patients: A randomized, double-blinded, placebo-controlled clinical trial. Nutr. Res. Pract. 2016, 10, 328. [Google Scholar] [CrossRef][Green Version]
- Asemi, Z.; Jamilian, M.; Mesdaghinia, E.; Esmaillzadeh, A. Effects of selenium supplementation on glucose homeostasis, inflammation, and oxidative stress in gestational diabetes: Randomized, double-blind, placebo-controlled trial. Nutrition 2015, 31, 1235–1242. [Google Scholar] [CrossRef]
- Jamilian, M.; Asemi, Z. The effect of soy intake on metabolic profiles of women with gestational diabetes mellitus. J. Clin. Endocrinol. Metab. 2015, 100, 4654–4661. [Google Scholar] [CrossRef]
- Asemi, Z.; Samimi, M.; Tabassi, Z.; Sabihi, S.; Esmaillzadeh, A. A randomized controlled clinical trial investigating the effect of DASH diet on insulin resistance, inflammation, and oxidative stress in gestational diabetes. Nutrition 2013, 29, 619–624. [Google Scholar] [CrossRef]
- Hernandez, T.L.; Van Pelt, R.E.; Anderson, M.A.; Reece, M.S.; Reynolds, R.M.; de la Houssaye, B.A.; Heerwagen, M.; Donahoo, W.T.; Daniels, L.J.; Chartier-Logan, C.; et al. Women with gestational diabetes mellitus randomized to a higher-complex carbohydrate/low-fat diet manifest lower adipose tissue insulin resistance, inflammation, glucose, and free fatty acids: A pilot study. Diabetes Care 2016, 39, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Panse, S.; Falkner, B. The role of adiponectin in metabolic and vascular disease: A review. Clin. Nephrol. 2011, 75, 26–33. [Google Scholar]
- Vrachnis, N.; Belitsos, P.; Sifakis, S.; Dafopoulos, K.; Siristatidis, C.; Pappa, K.I.; Iliodromiti, Z. Role of adipokines and other inflammatory mediators in gestational diabetes mellitus and previous gestational diabetes mellitus. Int. J. Endocrinol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Vahid, F.; Shivappa, N.; Karamati, M.; Naeini, A.J.; Hebert, J.R.; Davoodi, S.H. Association between Dietary Inflammatory Index (DII) and risk of prediabetes: A case-control study. Appl. Physiol. Nutr. Metab. 2017, 42, 399–404. [Google Scholar] [CrossRef]
- Cruz, N.G.; Sousa, L.P.; Sousa, M.O.; Pietrani, N.T.; Fernandes, A.P.; Gomes, K.B. The linkage between inflammation and type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2013, 99, 85–92. [Google Scholar] [CrossRef]
- King, G.L. The role of inflammatory cytokines in diabetes and its complications. J. Periodontol. 2008, 79, 1527–1534. [Google Scholar] [CrossRef]
- Ngo, D.T.; Sverdlov, A.L.; McNeil, J.J.; Horowitz, J.D. Does vitamin D modulate asymmetric dimethylarginine and C-reactive protein concentrations? Am. J. Med. 2010, 123, 335–341. [Google Scholar] [CrossRef]
- Chen, N.; Wan, Z.; Han, S.-F.; Li, B.-Y.; Zhang, Z.-L.; Qin, L.-Q. Effect of vitamin D supplementation on the level of circulating high-sensitivity c-reactive protein: A meta-analysis of randomized controlled trials. Nutrients 2014, 6, 2206–2216. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Raghavamenon, A.; Garelnabi, M.O.; Santanam, N. Oxidized low-density lipoprotein. Methods Mol. Biol. 2010, 610, 403–417. [Google Scholar] [CrossRef]
- NHLBI; NIH. National Heart, Lung, and Blood Institute DASH Eating Plan. Available online: https://www.nhlbi.nih.gov/health-topics/dash-eating-plan (accessed on 21 July 2020).
- Soltani, S.; Chitsazi, M.J.; Salehi-Abargouei, A. The effect of Dietary Approaches To Stop Hypertension (DASH) on serum inflammatory markers: A systematic review and meta-analysis of randomized trials. Clin. Nutr. 2018, 37, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Khodarahmi, M.; Jafarabadi, M.A.; Moludi, J.; Abbasalizad Farhangi, M. A systematic review and meta-analysis of the effects of soy on serum hs-CRP. Clin. Nutr. 2019, 38, 996–1011. [Google Scholar] [CrossRef]
- Ghani, M.A.; Barril, C.; Bedgood, D.R.; Prenzler, P.D. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef]
- Achari, A.E.; Jain, S.K. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef]
- Backes, J.; Anzalone, D.; Hilleman, D.; Catini, J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016, 15. [Google Scholar] [CrossRef]
- O’Mahoney, L.L.; Matu, J.; Price, O.J.; Birch, K.M.; Ajjan, R.A.; Farrar, D.; Tapp, R.; West, D.J.; Deighton, K.; Campbell, M.D. Omega-3 polyunsaturated fatty acids favourably modulate cardiometabolic biomarkers in type 2 diabetes: A meta-analysis and meta-regression of randomized controlled trials. Cardiovasc. Diabetol. 2018, 17, 98. [Google Scholar] [CrossRef] [PubMed]
- Miraglia del Giudice, M.; Maiello, N.; Allegorico, A.; Iavarazzo, L.; Capasso, M.; Capristo, C.; Ciprandi, G. Lactobacillus reuteri DSM 17938 plus vitamin D3 as ancillary treatment in allergic children with asthma- ClinicalKey. Ann. Allergy Asthma Immunol. 2016, 117, 703–727. [Google Scholar] [CrossRef]
- Kekkonen, R.A.; Lummela, N.; Karjalainen, H.; Latvala, S.; Tynkkynen, S.; Järvenpää, S.; Kautiainen, H.; Julkunen, I.; Vapaatalo, H.; Korpela, R. Probiotic intervention has strain-specific anti-inflammatory effects in healthy adults. World J. Gastroenterol. 2008, 14, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yue, R.; Zhang, B.; Li, Z.; Shui, J.; Huang, X. Effects of probiotics on blood glucose, biomarkers of inflammation and oxidative stress in pregnant women with gestational diabetes mellitus: A meta-analysis of randomized controlled trials. Med. Clin. 2020, 154, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Farrokhian, A.; Raygan, F.; Bahmani, F.; Talari, H.R.; Esfandiari, R.; Esmaillzadeh, A.; Asemi, Z. Long-term vitamin D supplementation affects metabolic status in vitamin D-deficient type 2 diabetic patients with coronary artery disease. J. Nutr. 2017, 147, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef]
- Lin, M.-Y.; Yen, C.-L. Antioxidative ability of lactic acid bacteria. J. Agric. Food Chem. 1999, 47, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Puddu, A.; Sanguineti, R.; Montecucco, F.; Viviani, G.L. Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediat. Inflamm. 2014, 2014, 162021. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Fabersani, E.; Marquez, A.; Gauffin-Cano, P. Adipose tissue inflammation and metabolic syndrome. The proactive role of probiotics. Eur. J. Nutr. 2019, 58, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Hasani, M.; Djalalinia, S.; Khazdooz, M.; Asayesh, H.; Zarei, M.; Gorabi, A.M.; Ansari, H.; Qorbani, M.; Heshmat, R. Effect of selenium supplementation on antioxidant markers: A systematic review and meta-analysis of randomized controlled trials. Hormones (Athens) 2019, 18, 451–462. [Google Scholar] [CrossRef]
- Arribas, L.; Almansa, I.; Miranda, M.; Muriach, M.; Romero, F.J.; Villar, V.M. Serum malondialdehyde concentration and glutathione peroxidase activity in a longitudinal study of gestational diabetes. PLoS ONE 2016, 11, e0155353. [Google Scholar] [CrossRef][Green Version]
- Shang, M.; Dong, X.; Hou, L. Correlation of adipokines and markers of oxidative stress in women with gestational diabetes mellitus and their newborns: Adipokines, oxidative stress and GDM. J. Obstet. Gynaecol. Res. 2018, 44, 637–646. [Google Scholar] [CrossRef]
- Lekva, T.; Norwitz, E.R.; Aukrust, P.; Ueland, T. Impact of systemic inflammation on the progression of gestational diabetes mellitus. Curr. Diab. Rep. 2016, 16, 26. [Google Scholar] [CrossRef]
- Mokkala, K.; Vahlberg, T.; Houttu, N.; Koivuniemi, E.; Laitinen, K. Distinct metabolomic profile because of gestational diabetes and its treatment mode in women with overweight and obesity. Obesity 2020, 28, 1637–1644. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, L.; Wang, M.; Chen, D.; Chen, Z.; Jiang, S.-W. A critical review of proteomic studies in gestational diabetes mellitus. J. Diabetes Res. 2020, 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
| Authors, Year (Country) | Study Design | Participants | Assessment of Nutritional Status | Vitamins and Minerals | Anthropometrics and/or Body Composition | Adiposity | Oxidative Stress and Inflammation |
|---|---|---|---|---|---|---|---|
| Shivappa et al., 2019 (Iran) [41] | Case Control | Women with GDM (n = 121) Age: 29.6 ± 4.5 years; BMI: 27.3 ± 3.8 kg/m2 Healthy pregnant CON (n = 266) Age: 29.8 ± 4.3 years; BMI: 24.6 ± 3.3 kg/m2 | Dietary inflammatory index (DII) FFQ (147 items) Blood samples | NR | NR | NR | ↑ odds of GDM with higher DII scores |
| Parast et al., 2017 (Iran) [42] | Case Control | Women with GDM (n = 40) Age: 29.4 ± 4.9 years; BMI: 25.2 ± 2.3 kg/m2 Healthy pregnant CON (n = 40) Age: 28.9 ± 5.2 years; BMI: 24.5 ± 2.8 kg/m2 | FFQ (168 items) Blood samples | GDM: intake of ↓ vit E, Se, Zn NS—β-carotene, vit C ↓ odds of GDM with ↑ intake of vit E and Zn | NR | NR | ↓ TAC in GDM vs. CON ↓ odds of GDM with ↑ TAC |
| Haidari et al., 2016 (Iran) [43] | Cross Sectional | Women with GDM (n = 45) Age: 29.3 ± 4.3 years; BMI: 25.7 ± 3.1 kg/m2 Healthy pregnant CON (n = 45) Age: 27.5 ± 4.9 years; BMI: 24.3 ± 3.0 kg/m2 | No dietary data Blood samples | GDM: ↓ 25(OH)D | GDM and CON: 25(OH)D negatively correlated with pre-pregnancy BMI | NR | GDM and CON: 25(OH)D negatively correlated with hs-CRP NS—hs-CRP, TNF-α |
| McManus et al., 2014 (Canada) [44] | Case Control | Women with GDM (n = 36) Age: 31.6 ± 5.0 years; BMI: 28.7 ± 5.5 kg/m2 Healthy pregnant CON (n = 37) Age: 30.2 ± 4.1 years; BMI: 27.2 ± 7.2 kg/m2 | No dietary data Blood samples | GDM: ↓ 25(OH)D | NR | GDM: ↓ adiponectin, resistin NS—leptin | NS—hs-CRP, IL-6, IL-8, MCP-1, PAI-1, TNF-α |
| Javadian et al., 2014 (Iran) [45] | Case Control | Women with GDM (n = 52) Age: 31.2 ± 6.7 years; BMI: 26.9 ± 3.8 kg/m2 Healthy pregnant CON (n = 50) Age: 28.9 ± 6.7 years; BMI: 25.7 ± 4.4 kg/m2 | No dietary data Blood samples | GDM: ↑ ferritin | NR | NR | GDM: ↑ MDA |
| Park et al., 2013 (South Korea) [46] | Case Control | Women with normal weight and GDM (n = 98) Women with overweight and GDM (n = 117) Healthy pregnant CON with normal weight (n = 395) Healthy pregnant CON with overweight (n = 136) Combined age NR; Combined BMI: 23.29 ± 3.59 kg/m2 | Interviewer-administered FFQ 24 h recall Blood samples | NR | NR | Groups with overweight vs. groups with normal weight: ↑ leptin, adipsin GDM + overweight vs. other groups: ↓ visfatin GDM vs. CON: ↓ adiponectin | GDM + overweight vs. other groups: ↑ CRP |
| Suhail et al., 2010 (India) [47] | Cross Sectional | Women with GDM (n = 23) Age: 28.0 ± 4.0 years; BMI at delivery: 21.0 ± 2.4 kg/m2 Healthy pregnant CON (n = 23) Age: 27.0 ± 4.0 years; BMI at delivery: 20.1 ± 1/4 kg/m2 Non-pregnant CON (n = 23) Age: 26.0 ± 5.0 years; BMI at delivery: 20.4 ± 2.6 kg/m2 | No dietary data Blood samples | GDM vs. healthy, pregnant CON: ↓ vit C, vit E GDM and healthy, pregnant CON vs. non-pregnant CON: ↓ vit A | NR | NR | GDM and healthy, pregnant CON vs. non-pregnant CON: ↑ MDA GDM vs. healthy, pregnant CON: ↑ MDA |
| Authors, Year (Country) | Trial Design | Participants | Dietary Intervention and Duration 1 | Antioxidant Vitamins and Minerals | Anthropometrics and/or Body Composition | Adiposity | Oxidative Stress and Inflammation |
|---|---|---|---|---|---|---|---|
| Jamilian et al., 2020 (Iran) [50] | Double-blind RCT | Women with GDM (n = 51) Age: 29.0 ± 4.6 years; BMI: 28.1 ± 4.5 kg/m2 | 2000 mg n-3 FA from flaxseed oil (800 mg ALA), daily Capsules, 6 weeks | NR | NS—height, mass, BMI | NR | ↓ hs-CRP, MDA ↑ GSH, total nitrite |
| Jamilian et al., 2019 (Iran) [51] | Double-blind RCT | Women with GDM (n = 60) Age: 28.4 ± 4.1 years; BMI: ± 25.6 3.1 kg/m2 | 200 mg Mg + 8 mg Zn + 800 mg Ca + 400 IU vit D, daily Tablets, 6 weeks | ↑ Mg, Zn, Ca, 25(OH)D | NS—height, mass, BMI | NR | ↓ hs-CRP, MDA ↑ TAC NS—total nitrite, GSH |
| Jamilian et al., 2019 (Iran) [52] | Double-blind RCT | Women with GDM (n = 87) Age: 30.0 ± 5.4 years; BMI: 27.2 ± 4.2 kg/m2 | G1: 50,000 IU vit D (biweekly) + probiotic (daily) G2: Probiotic (daily) + vit D PBO (biweekly) G3: Vit D PBO + probiotic PBO Capsules, 6 weeks | G1 vs. G2 and PBO; G2 vs. PBO: ↑ 25(OH)D | NS—height, mass, BMI | NR | G1 vs. PBO; G2 vs. PBO: ↓ hs-CRP, MDA G1 vs. G2 and PBO: ↑ TAC G1 vs. G2: ↑ GSH |
| Hajifaraji et al., 2018 (Iran) [39] and Dolatkhah et al., 2015 (Iran) [40] | Double-blind RCT | Women with GDM (n = 56) Age: 27.3 ± 5.8 years; BMI: 30.7 ± 3.7 kg/m2 | Probiotic, daily Capsules, 8 weeks | NR | ↓ body mass gained in the last two and four weeks of the study | NR | ↓ hs-CRP, lnTNF-α, MDA ↑ erythrocyte GPx, GSHR NS—erythrocyte SOD, IL-6, TAC, uric acid |
| Jamilian et al., 2018 (Iran) [53] | Double-blind RCT | Women with GDM (n = 40) Age: 30.7 ± 3.1 years; BMI: 27.5 ± 2.9 kg/m2 | 2000 mg fish oil (360 mg EPA and 240 mg DHA), daily Capsules, 6 weeks | NR | NS—height, mass, BMI | NR | ↓ hs-CRP |
| Karamali et al., 2018 (Iran) [54] | Double-blind RCT | Women with GDM (n = 60) Age: 26.7 ± 4.7 years; BMI: 28.5 ± 3.5 kg/m2 | Synbiotic + 800 mg inulin, daily Capsules, 6 weeks | NR | NS—height, mass, BMI | NR | ↓ hs-CRP, MDA ↑ GSH, TAC NS—NO |
| Nabhani et al., 2018 (Iran) [55] | Double-blind RCT | Women with GDM (n = 90) Age: 29.9 ± 5.7 years; BMI: 27.3 ± 4.5 kg/m2 | Synbiotic Capsules, 6 weeks | NR | NS—body mass gained, BMI, hip circumference | NR | ↑ TAC |
| Razavi et al., 2017 (Iran) [56] | Double-blind RCT | Women with GDM (n = 120) Age: 29.7 ± 4.0 years; BMI: 29.0 ± 3.7 kg/m2 | G1: 2000 mg n-3 FA (360 mg EPA, 240mg DHA, daily) + vit D PBO (biweekly) G2: n-3 FA PBO + 50,000 IU vit D (biweekly) G3: 2000 mg n-3 FA (daily) + 50,000 IU vit D (biweekly) G4: n-3 PBO (daily) + vit D PBO (biweekly) Capsules, 6 weeks | ↑ 25(OH)D (G3) | NS—height, mass, BMI | NR | G3: ↓ hs-CRP, MDA G3: ↑ GSH, NO, TAC |
| Hernandez et al., 2016 (USA) [63] | RCT | Women with GDM (n = 12) Age: 29.0 ± 1.8 years; BMI: 33.9 ± 1.5 kg/m2 | CHOICE diet vs. LC/CONV diet 6 weeks | NR | NS—mass, BMI | NR | NS—OxLDL |
| Jafarnejad et al., 2016 (Iran) [57] | Double-blind RCT | Women with GDM (n = 72) Age: 32.2 ± 3.6 years; BMI: 27.1 ± 2.9 kg/m2 | Probiotic mixture, daily Capsules, 8 weeks | NR | NS—height, mass, BMI | NR | ↓ hs-CRP, IL-6, TNF-α NS—IFN-γ, IL-10 |
| Karamali et al., 2016 (Iran) [58] | Double-blind RCT | Women with GDM (n = 50) Age: 29.6 ± 4.4 years; BMI: 28.2 ± 3.6 kg/m2 | 233 mg Zn gluconate (30 mg zinc), daily Tablets, 6 weeks | NS—Zn | NS—height, mass, BMI | NR | ↓ hs-CRP ↑ TAC NS—GSH, NO, MDA |
| Yazdchi et al., 2016 (Iran) [59] | Double-blind RCT | Women with GDM (n = 72) Age: 31.9 ± 4.0 years; BMI: 31.5 ± 3.7 kg/m2 | 50,000 IU vit D (biweekly) Capsules, 8 weeks | ↑ 25(OH)D | NS—height, mass, BMI | NR | NS—hs-CRP |
| Zhang et al., 2016 (China) [48] | Double-blind RCT | Women with GDM (n = 133) Age: 29.9 ± 4.8 years; BMI: 30.9 ± 4.0 kg/m2 | G1: 200 IU vit D (daily) G2: 2000 IU vit D (daily, 25 days, total of 50,000 IU) G3: 4000 IU vit D (daily, 12.5 days, total of 50,000 IU) Capsules, enrollment through delivery | NR | NR | NR | G1, G2, and G3: ↑ GSH, TAC NS—hs-CRP |
| Asemi et al., 2015 (Iran) [60] | Double-blind RCT | Women with GDM (n = 70) Age: 28.6 ± 4.6 years; BMI: 27.9 ± 4.0 kg/m2 | 200 μg Se (daily) Capsules, 6 weeks | NR | NS—height, mass, BMI | NR | ↓ hs-CRP ↑ GSH, TAC NS—MDA, NO |
| Jamilian et al., 2015 (Iran) [61] | RCT | Women with GDM (n = 68) Age: 28.8 ± 4.4 years; BMI: 28.7 ± 4.3 kg/m2 | Diet with 35% soy, 35% animal, and 30% other plant protein vs. CON diet with 70% animal and 30% other plant protein 0.8 g protein/kg body mass in each diet 6 weeks | NR | NS—height, mass, BMI | NR | CON vs. Soy (Between): ↑ MDA NS—GSH, hs-CRP, NO, TAC |
| Fei et al., 2014 (China) [49] | RCT | Women with GDM (n = 97) Age: > 20 years; BMI: NR | 10 g soybean oligosaccharides 200–300 mL water (daily) Orally, 8 weeks | NR | NR | ↑ adiponectin | ↑ SOD, CAT, GPx ↓ TBARS |
| Asemi et al., 2013 (Iran) [62] | Double-blind RCT | Women with GDM (n = 32) Age: 28.7 ± 5.5 years; BMI: 30.0 ± 3.9 kg/m2 | DASH diet 4 weeks | NR | NS—height, mass, BMI | NR | ↑ GSH, TAC NS—hs-CRP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, D.W.; Crew, J.; Planinic, P.; Alexander, J.M.; Basu, A. Associations of Dietary Bioactive Compounds with Maternal Adiposity and Inflammation in Gestational Diabetes: An Update on Observational and Clinical Studies. Int. J. Environ. Res. Public Health 2020, 17, 7528. https://doi.org/10.3390/ijerph17207528
Davis DW, Crew J, Planinic P, Alexander JM, Basu A. Associations of Dietary Bioactive Compounds with Maternal Adiposity and Inflammation in Gestational Diabetes: An Update on Observational and Clinical Studies. International Journal of Environmental Research and Public Health. 2020; 17(20):7528. https://doi.org/10.3390/ijerph17207528
Chicago/Turabian StyleDavis, Dustin W., Jeannette Crew, Petar Planinic, James M. Alexander, and Arpita Basu. 2020. "Associations of Dietary Bioactive Compounds with Maternal Adiposity and Inflammation in Gestational Diabetes: An Update on Observational and Clinical Studies" International Journal of Environmental Research and Public Health 17, no. 20: 7528. https://doi.org/10.3390/ijerph17207528
APA StyleDavis, D. W., Crew, J., Planinic, P., Alexander, J. M., & Basu, A. (2020). Associations of Dietary Bioactive Compounds with Maternal Adiposity and Inflammation in Gestational Diabetes: An Update on Observational and Clinical Studies. International Journal of Environmental Research and Public Health, 17(20), 7528. https://doi.org/10.3390/ijerph17207528






