Association between Urinary Triclosan and Serum Testosterone Levels in U.S. Adult Males from NHANES, 2011–2012
Abstract
1. Introduction
2. Materials and Methods
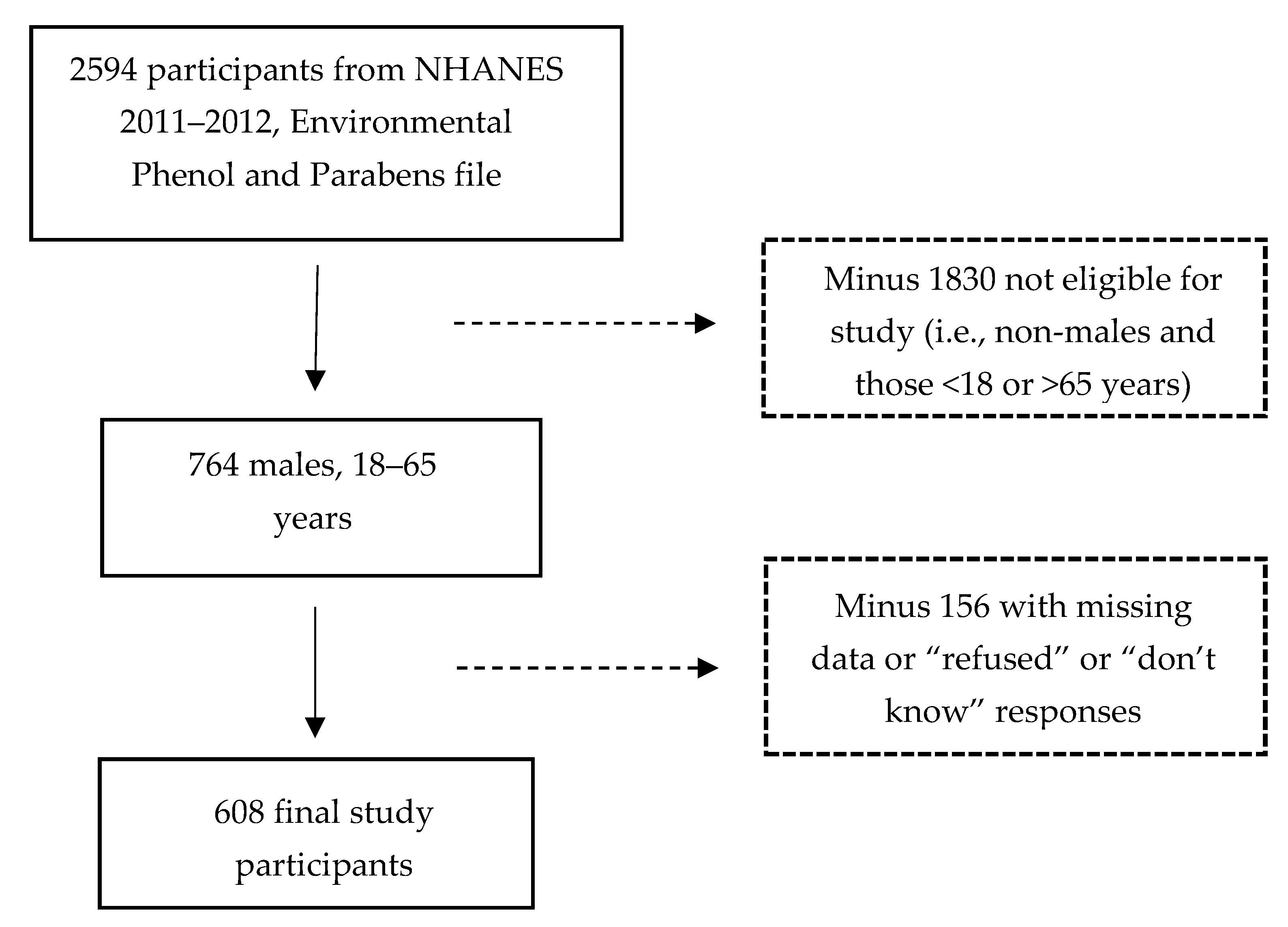
2.1. Sample Selection
2.2. Confounding Measures
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cooney, C. Personal care products: Triclosan comes under scrutiny. Environ. Health Perspect. 2010, 118, A242. [Google Scholar] [CrossRef] [PubMed]
- Dann, A.B.; Hontela, A. Triclosan: Environmental exposure, toxicity and mechanisms of action. J. Appl. Toxicol. 2011, 31, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, W.; Xia, Y.; Wang, X. Associations between urinary polycyclic aromatic hydrocarbon metabolites and serum testosterone in US adult males: National Health and nutrition examination survey 2011–2012. Environ. Sci. Pollut. Res. 2017, 24, 7707–7716. [Google Scholar] [CrossRef]
- Jurewicz, J.; Radwan, M.; Sobala, W.; Brzeznicki, S.; Ligocka, D.; Radwan, P.; Bochenek, M.; Hanke, W. Association between a biomarker of exposure to polycyclic aromatic hydrocarbons and semen quality. Int. J. Occup. Med. Environ. Health 2013, 26, 790–801. [Google Scholar] [CrossRef]
- Han, X.; Zhou, N.; Cui, Z.; Ma, M.; Li, L.; Cai, M.; Li, Y.; Lin, H.; Li, Y.; Ao, L.; et al. Association between urinary polycyclic aromatic hydrocarbon metabolites and sperm DNA damage: A population study in Chongqing, China. Environ. Health Perspect. 2011, 119, 652–657. [Google Scholar] [CrossRef]
- Fisher, J.S. Environmental anti-androgens and male reproductive health: Focus on phthalates and testicular dysgenesis syndrome. Reproduction 2004, 127, 305–315. [Google Scholar] [CrossRef]
- Jurewicz, J.; Radwan, M.; Wielgomas, B.; Kałużny, P.; Klimowska, A.; Radwan, P.; Hanke, W. Environmental levels of triclosan and male fertility. Environ. Sci. Pollut. Res. Int. 2018, 25, 5484–5490. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Usman, Z.; Rehman, S.; Aldraihem, M.; Rehman, N.; Rehman, I.; Ahmad, G. Endocrine disrupting chemicals and impact on male reproductive health. Transl. Androl. Urol. 2018, 7, 490–503. [Google Scholar] [CrossRef]
- Kumar, V.; Balomajumder, C.; Roy, P. Disruption of LH-induced testosterone biosynthesis in testicular leydig cells by triclosan: Probable mechanism of action. Toxicology 2008, 250, 124–131. [Google Scholar] [CrossRef]
- Kumar, V.; Chakraborty, A.; Kural, M.R.; Roy, P. Alteration of testicular steroidogenesis and histopathology of reproductive system in male rats treated with triclosan. Reprod. Toxicol. 2009, 27, 177–185. [Google Scholar] [CrossRef]
- Zorrilla, L.M.; Gibson, E.K.; Jeffay, S.C.; Crofton, K.M.; Setzer, W.R.; Cooper, R.L.; Stoker, T.E. The effects of triclosan on puberty and thyroid hormones in male wistar rats. Toxicol. Sci. 2009, 107, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Haring, R.; Volzke, H.; Steveling, A.; Krebs, A.; Felix, S.B.; Schoft, C.; Dorr, M.; Nauck, M.; Wallaschofski, H. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20–79. Eur. Heart J. 2010, 31, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.C.; Johns, L.E.; Meeker, J.D. Serum biomarkers of exposure to perfluoroalkyl substances in relation to serum testosterone and measures of thyroid function among adults and adolescents from NHANES 2011–2012. Int. J. Environ. Res. Public Health 2015, 12, 6098–6114. [Google Scholar] [CrossRef] [PubMed]
- Traish, A.M.; Kypreos, K.E. Testosterone and cardiovascular disease: An old idea with modern clinical implications. Atherosclerosis 2010, 201, 244–248. [Google Scholar] [CrossRef]
- Malkin, C.J.; Pugh, P.J.; Morris, P.D.; Asif, S.; Jones, T.H.; Channer, K.S. Low serum testosterone and increased mortality in men with coronary heart disease. Heart 2010, 96, 1821–1825. [Google Scholar] [CrossRef]
- Almeida, O.P.; Yeap, B.B.; Hankey, G.J.; Jamrozik, K.; Flicker, L. Low free testosterone concentration as a potentially treatable cause of depressive symptoms in older men. Arch. Gen. Psychiatry 2008, 65, 283–289. [Google Scholar] [CrossRef]
- Yan, J.; Joseph, M.A.; Reynolds, S.A.; Veenema, R.J.; Geer, L.A. Association between triclosan levels and white blood cell counts in US adults from NHANES, 2011–2012. Environ. Res. Commun. 2020, 2, 055001. [Google Scholar] [CrossRef]
- Scinicariello, F.; Buser, M.C. Serum testosterone concentrations and urinary bisphenol A, benzophenon-3, triclosan, and paraben levels in male and female children and adolescents: NHANES 2011–2012. Environ. Health Perspect. 2016, 124, 1898–1904. [Google Scholar] [CrossRef]
- Adgent, M.A.; Rogan, W.J. Triclosan and prescription antibiotic exposures and enterolactone production in adults. Environ. Res. 2015, 142, 66–71. [Google Scholar] [CrossRef]
- Fang, J.L.; Stingley, R.L.; Beland, F.A.; Harrouk, W.; Lumpkins, D.L.; Howard, P. Occurrence, efficacy, metabolism, and toxicity of triclosan. J. Environ. Sci. Health Part C 2010, 28, 147–171. [Google Scholar] [CrossRef]
- National Health and Nutrition Examination Survey (NHANES). Laboratory Procedure Manual. Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/2011-12_laboratory_procedures_manual.pdf (accessed on 31 July 2018).
- Khairullah, A.; Klein, L.C.; Ingle, S.M.; May, M.T.; Whetzel, C.A.; Susman, E.J.; Paus, T. Testostrone trajectories and reference ranges in a large longitudinal sample of male adolescents. PLoS ONE 2014, 9, e108838. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, T.W.; Li, L.Q.; Mitchell, R.T.; Whelan, A.; Anderson, R.A.; Wallace, W.H. A validated age-related normative model for male total testosterone shows increasing variance but no decline after age 40 years. PLoS ONE 2015, 10, e0117674. [Google Scholar] [CrossRef] [PubMed]
- Yeap, B.B. Are declining testosterone levels a major risk factor for ill-health in aging men? Int. J. Impot. Res. 2009, 21, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Juric, A.; Singh, K.; Hu, X.F.; Chan, H.M. Exposure to triclosan among the Canadian population: Results of the Canadian health measures survey (2009–2013). Environ. Int. 2019, 123, 29–38. [Google Scholar] [CrossRef]
- Mortensen, M.D.; Calafat, A.M.; Ye, X.; Wong, L.Y.; Wright, D.J.; Pirkle, J.L.; Merrill, L.S.; Moye, J. Urinary concentrations of environmental phenols in pregnant women in a pilot study of the national children’s study. Environ. Res. 2014, 123, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Lankester, J.; Patel, C.; Cullen, M.R.; Ley, C.; Parsonnet, J. Urinary triclosan is associated with elevated body mass index in NHANES. PLoS ONE 2013, 8, e80057. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef] [PubMed]
- MacIsaac, J.K.; Gerona, R.R.; Blanc, P.D.; Apatira, L.; Friesen, M.W.; Coppolino, M.; Janssen, S. Healthcare workers exposure to triclosan. J. Occup. Environ. Med. 2014, 56, 834–839. [Google Scholar] [CrossRef]
- Jain, R.B. Impact of pregnancy and other factors including smoking on the urinary levels of triclosan. Toxicol. Environ. Chem. 2015, 97, 1276–1287. [Google Scholar] [CrossRef]
- Kim, K.; Park, H.; Yang, W.; Lee, J.H. Urinary concentrations of bisphenol A and triclosan and associations with demographic factors in the Korean population. Environ. Res. 2011, 111, 1280–1285. [Google Scholar] [CrossRef]
- Flegal, K.M.; Kit, B.K.; Graubard, B.I. Body mass index categories in observational studies of weight and risk of death. Am. J. Epidemiol. 2014, 180, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Hamilton, C.; Hamman, B.; Ng, H.K.T.; Grayburn, P. Categorizing BMI may lead to biased results in studies investigating in-hospital mortality after isolated CABG. J. Clin. Epidemiol. 2007, 60, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Preston, S.H.; Fishman, E.; Stokes, A. Effects of categorization and self-report bias on estimates of the association between obesity and mortality. Ann. Epidemiol. 2015, 25, 907.e2–911.e2. [Google Scholar] [CrossRef] [PubMed]
- CDC (Center for Disease Control and Prevention). Biomonitoring Summary: Cotinine. CAS No.486-56-6. 2013. Available online: https://www.cdc.gov/biomonitoring/Cotinine_BiomonitoringSummary.html (accessed on 7 August 2018).
- Duque, A.; Martinez, P.; Giraldo, A.; Gualtero, D.F.; Ardila, C.M.; Contreras, A.; Duarte, S.; Lafaurie, G. Accuracy of cotinine serum test to detect the smoking habit and its association with periodontal disease in a multicenter study. Med. Oral Patol. Oral Cir. Bucal 2017, 22, 3525–3531. [Google Scholar] [CrossRef] [PubMed]
- Hays, S.M.; Aylward, L.L.; Blount, B.D. Variations in urinary flow rates according to demographic characteristics and body mass index in NHANES: Potential confounding of associations between health outcomes and urinary biomarker concentrations. Environ. Health Perspect. 2015, 123, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, G.; Greenland, S. Simulation study of confounder-selection strategies. Am. J. Epidemiol. 1993, 138, 923–936. [Google Scholar] [CrossRef]
- Miles, J. Tolerance and variance inflation factor. In Encyclopedia of Statistics in Behavioral Science; Everitt, B.S., Howell, D., Eds.; John Wiley: Chichester, UK, 2005; pp. 2055–2056. [Google Scholar]
- Rao, J.N.K.; Scott, A.J. The analysis of categorical data from complex sample surveys: Chi-squared tests for goodness-of-fit and independence in two-way tables. J. Am. Stat. Assoc. 1981, 76, 221–230. [Google Scholar] [CrossRef]
- Rao, J.N.K.; Scott, A.J. On chi-squared tests for multi-way tables with cell proportions estimated from survey data. Ann. Stat. 1994, 12, 46–60. [Google Scholar] [CrossRef]
- National Center for Health Statistics. Module 3: Weighting. Available online: https://wwwn.cdc.gov/nchs/nhanes/tutorials/module3.aspx (accessed on 7 August 2018).
- Choi, J.C.; Chung, M.; Lee, Y. Modulation of pain sensation by stress-related testosterone and cortisol. Anaesthesia 2012, 67, 1146–1151. [Google Scholar] [CrossRef]
- Matsumoto, A.M.; Bremner, W.J. Serum testosterone assays—Accuracy matters. J. Clin. Endocrinol. Metab. 2004, 89, 520–524. [Google Scholar] [CrossRef]
- Sakaguchi, K.; Oki, M.; Honma, S.; Uehara, H.; Hasegawa, T. The lower salivary testosterone levels among unmarried and married sexually active men. J. Ethol. 2007, 25, 223–229. [Google Scholar] [CrossRef]
- FDA (U.S. Food and Drug Administration). 21 CFR Part 310: Safety and Effectiveness of Consumer Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use. Final Rule. Fed. Regist. 2016, 81, 61106–61130. [Google Scholar]
- Halden, R.U.; Lindeman, A.E.; Aiello, A.E.; Andrews, D.; Arnold, W.A.; Fair, P.; Fuoco, R.E.; Geer, L.A.; Johnson, P.I.; Lohmann, R.; et al. The Florence statement on triclosan and triclocarban. Environ. Health Perspect. 2017, 125, 064501. [Google Scholar] [CrossRef] [PubMed]
- EPA (Environmental Protection Agency). 21 July 2017. Available online: https://www.epa.gov/ingredients-used-pesticide-products/triclosan (accessed on 30 September 2018).
- U.S. Food & Drug Administration. Consumer Updates—5 Things to Know About Triclosan. 2017. Available online: https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm205999.htm (accessed on 31 July 2018).
| Category of Variable | Variable Name | Type of Variable | Description |
|---|---|---|---|
| Response variable | Total serum testosterone (ng/dL) | Continuous | Lab values |
| Main predictor variable | Urinary triclosan (ng/mL) | Continuous | Lab values |
| Covariates | Race | Categorical | Black, White (reference), Asian, Mexican-American/Hispanic/Other |
| Body mass index (kg/m2) | Continuous | Lab values | |
| Annual income | Categorical | Low income (≤ 25th percentile), | |
| Middle income (25th–75th percentile) | |||
| High income (≥ 75th percentile) | |||
| Cotinine levels (ng/mL) (as a proxy for smoking status) | Binary | Non-smoker (lower than 10 ng/mL) | |
| Smoker (10 ng/mL or greater) | |||
| Creatinine levels (mg/dL) | Continuous | Lab values |
| Weighted Mean (SE) | |||||||||
| Independent Variables | Categories | Unweighted N (%) | Weighted N (%) | Mean | p | Urinary Triclosan, ng/mL | p | Total Serum Testosterone, ng/dL | p |
| Age, years | N/A | 608 | 40.7 (0.96) | <0.0001 | 112.0 (24.7) | 415.1 (12.5) | |||
| BMI, kg/m2 | 80,795,632 | 28.7 (0.36) | <0.0001 | 0.0003 | <0.0001 | ||||
| Creatinine, mg/dL | 137.6 (5.55) | <0.0001 | |||||||
| Race | |||||||||
| Mexican-American/Hispanic/Other | 149 (24.5%) | 15,215,210 (18.8%) | 162.0 (36.8) | 0.0004 | 413.6 (11.2) | <0.0001 | |||
| Black | 154 (25.3%) | 7,781,869 (9.6%) | 49.4 (11.3) | 0.0004 | 417.4 (17.8) | <0.0001 | |||
| Asian | 93 (15.3%) | 3,659,140 (4.5%) | 79.5 (35.8) | 0.0404 | 406.1 (16.4) | <0.0001 | |||
| White | 212 (34.9%) | 54,139,413 (67.0%) | 109.1 (35.9) | 0.0074 | 415.7 (17.2) | <0.0001 | |||
| Income | |||||||||
| Low | 176 (29.0%) | 15,354,875 (19.0%) | 53.8 (18.2) | 0.0089 | 421.0 (12.7) | <0.0001 | |||
| Middle | 232 (38.2%) | 30,292,907 (37.5%) | 113.7 (29.2) | 0.0012 | 433.6 (23.2) | <0.0001 | |||
| High | 200 (32.9%) | 35,147,851 (43.5%) | 135.8 (47.1) | 0.0104 | 396.5 (18.0) | <0.0001 | |||
| Smoking status | |||||||||
| Smoker | 170 (25.0%) | 21,638,149 (26.8%) | 43.5 (10.5) | 0.0007 | 432.9 (11.6) | <0.0001 | |||
| Non-smoker | 438 (72.0%) | 59,157,484 (73.2%) | 137.0 (36.1) | 0.0015 | 408.5 (16.2) | <0.0001 | |||
| Independent Variables | Total Serum Testosterone Level | ||
|---|---|---|---|
| β-coefficient (SE) | 95% CI | p | |
| Log (urinary triclosan) | < 0.001 (0.012) | −0.024, 0.025 | 0.982 |
| Log (BMI) | −0.868 (0.145) | −1.174, −0.563 | <0.0001 |
| Log (creatinine) | −0.025 (0.044) | −0.117, −0.067 | 0.579 |
| Smoking | |||
| Smoker | Reference | Reference | Reference |
| Non-smoker | −0.032 (0.044) | −0.124, 0.061 | 0.483 |
| Income categories | |||
| Low | −0.026 (0.049) | −0.129, 0.077 | 0.602 |
| Middle | 0.062 (0.053) | −0.051, 0.174 | 0.263 |
| High | Reference | Reference | Reference |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Joseph, M.A.; Reynolds, S.A.; Geer, L.A. Association between Urinary Triclosan and Serum Testosterone Levels in U.S. Adult Males from NHANES, 2011–2012. Int. J. Environ. Res. Public Health 2020, 17, 7412. https://doi.org/10.3390/ijerph17207412
Yan J, Joseph MA, Reynolds SA, Geer LA. Association between Urinary Triclosan and Serum Testosterone Levels in U.S. Adult Males from NHANES, 2011–2012. International Journal of Environmental Research and Public Health. 2020; 17(20):7412. https://doi.org/10.3390/ijerph17207412
Chicago/Turabian StyleYan, Judy, Michael A. Joseph, Simone A. Reynolds, and Laura A. Geer. 2020. "Association between Urinary Triclosan and Serum Testosterone Levels in U.S. Adult Males from NHANES, 2011–2012" International Journal of Environmental Research and Public Health 17, no. 20: 7412. https://doi.org/10.3390/ijerph17207412
APA StyleYan, J., Joseph, M. A., Reynolds, S. A., & Geer, L. A. (2020). Association between Urinary Triclosan and Serum Testosterone Levels in U.S. Adult Males from NHANES, 2011–2012. International Journal of Environmental Research and Public Health, 17(20), 7412. https://doi.org/10.3390/ijerph17207412






