Are AAMA and GAMA Levels in Urine after Childbirth a Suitable Marker to Assess Exposure to Acrylamide from Passive Smoking during Pregnancy?—A Pilot Study
Abstract
1. Introduction
2. Material
2.1. The Study Group
2.2. Urine Sampling
2.3. Dietary Intake Assessment
3. Methods
3.1. Determination of AAMA and GAMA Levels in Urine
3.2. Determination of Urinary Creatinine Levels
3.3. Estimation of Exposure to Acrylamide from Food Based on Food Consumption Data
3.4. Estimation of Exposure to Acrylamide from Tobacco Smoke in a Group of Passive Smokers During Pregnancy
3.5. Estimation of Exposure to Acrylamide from Food and Tobacco Smoke Based on AAMA and GAMA Levels in Urine of Women Post-Partum
- Sum of AAMA + GAMA [µg/g creatinine];
- KW—excretion of creatinine [mg/kg b.w./day];
- MAA—molar mass of acrylamide (MAA = 71);
- 0.5—the conversion factor [28];
- MAAMA + GAMA—sum of molar masses AAMA and GAMA (MAAMA + GAMA = 476).
3.6. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization (WHO). WHO Report on the Global Tobacco Epidemic. 2019. Country Profile Poland. Available online: https://www.who.int/tobacco/surveillance/policy/country_profile/pol.pdf?ua=1 (accessed on 13 March 2020).
- EURO-PERISTAT. European Perinatal Health Report by the EURO-PERISTAT Project in Collaboration with SCPE, EUROCAT & EURONEOSTAT. 2015. Available online: https://www.europeristat.com/images/doc/EPHR/european-perinatal-health-report.pdf (accessed on 12 March 2020).
- Banderali, G.; Martelli, A.; Landi, M.; Moretti, F.; Betti, F.; Radaelli, G.; Lassandro, C.; Verduci, E. Short and long term health effects of parental tobacco smoking during pregnancy and lactation: A descriptive review. J. Transl. Med. 2015, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, C.; Gan, Y.; Jiang, H.; Fu, W.; Cao, S.; Lu, Z. Maternal smoking during pregnancy and the risk of strabismus in offspring: A meta-analysis. Acta Ophthalmol. 2018, 97, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Ruisch, I.H.; Dietrich, A.; Glennon, J.C.; Buitelaar, J.; Hoekstra, P.J. Maternal substance use during pregnancy and offspring conduct problems: A meta-analysis. Neurosci. Biobehav. Rev. 2018, 84, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wei, Y.; Tang, X.; Liu, B.; Shen, L.; Long, C.; Lin, T.; He, D.; Wu, S.; Wei, G. Correction to: Maternal smoking during pregnancy and risk of cryptorchidism: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2019, 178, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Leonardi-Bee, J.; Smyth, A.; Britton, J.; Coleman, T. Environmental tobacco smoke and fetal health: Systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F351–F361. [Google Scholar] [CrossRef] [PubMed]
- Kazdepka-Ziemińska, A.; Jagielski, I.; Stankiewicz, M.; Głogiewicz, M.; Tyloch, M.; Grabiec, M. Nicotinism among pregnant women with selected complications of pregnancy. Mod. Pharm. 2013, 6, 168–172. (In Polish) [Google Scholar]
- Talhout, R.; Schulz, T.; Florek, E.; Van Benthem, J.; Wester, P.; Opperhuizen, A. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public Health 2011, 8, 613–628. [Google Scholar] [CrossRef]
- Diekmann, J.; Wittig, A.; Stabbert, R. Gas chromatographic--mass spectrometric analysis of acrylamide and acetamide in cigarette mainstream smoke after on-column injection. J. Chromatogr. Sci. 2008, 46, 659–663. [Google Scholar] [CrossRef]
- Moldoveanu, S.C.; Gerardi, A.R. Acrylamide analysis in tobacco, alternative tobacco products, and cigarette smoke. J. Chromatogr. Sci. 2011, 49, 234–242. [Google Scholar] [CrossRef]
- Hagmar, L.; Wirfält, E.; Paulsson, B.; Törnqvist, M. Differences in hemoglobin adduct levels of acrylamide in the general population with respect to dietary intake, smoking habits and gender. Mutat. Res. Toxicol. Environ. Mutagen. 2005, 580, 157–165. [Google Scholar] [CrossRef]
- Schettgen, T.; Weiss, T.; Drexler, H.; Angerer, J. A first approach to estimate the internal exposure to acrylamide in smoking and non-smoking adults from Germany. Int. J. Hyg. Environ. Health 2003, 206, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.; Kavvadias, D.; Riedel, K.; Scherer, G.; Tricker, A.R. Urinary mercapturic acids and a hemoglobin adduct fort the dosimetry of acrylamide exposure in smokers and nonsmokers. Inhal. Toxicol. 2006, 18, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Mojska, H.; Gielecińska, I.; Cendrowski, A. Acrylamide content in cigarette mainstream smoke and estimation of exposure to acrylamide from tobacco smoke in Poland. Ann. Agric. Environ. Med. 2016, 23, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Stadler, R.H.; Scholz, G. Acrylamide: An update on current knowledge in analysis, levels in food, mechanisms of formation, and potential strategies of control. Nutr. Rev. 2004, 62, 449–467. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Scientific opinion on acrylamide in food. EFSA J. 2015, 13, 4104. [Google Scholar] [CrossRef]
- He, F.S.; Zhang, S.L.; Wang, H.L.; Li, G.; Zhang, Z.M.; Li, F.L.; Dong, X.M.; Hu, F.R. Neurological and electroneuromyographic assessment of the adverse effects of acrylamide on occupationally exposed workers. Scand. J. Work. Environ. Health 1989, 15, 125–129. [Google Scholar] [CrossRef]
- Friedman, M.A.; Dulak, L.H.; Stedham, M.A. A lifetime oncogenicity study in rats with acrylamide. Fundam. Appl. Toxicol. 1995, 27, 95–105. [Google Scholar] [CrossRef]
- Johnson, K.; Gorzinski, S.; Bodnar, K.; Campbell, R.; Wolf, C.; Friedman, M.; Mast, R. Chronic toxicity and oncogenicity study on acrylamide incorporated in the drinking water of Fisher 344 rats. Toxicol. Appl. Pharmacol. 1986, 85, 154–168. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Acrylamide, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Some Industrials Chemicals, vol. 60; International Agency for Research on Cancer: Lyon, France, 1994; pp. 389–433. Available online: http://www.iarc.fr/ENG/Databases/index.php (accessed on 12 March 2020).
- Annola, K.; Karttunen, V.; Keskirahkonen, P.; Myllynen, P.; Segerback, D.; Heinonen, S.; Vähäkangas, K. Transplacental transfer of acrylamide and glycidamide are comparable to that of antipyrine in perfused human placenta. Toxicol. Lett. 2008, 182, 50–56. [Google Scholar] [CrossRef]
- Duarte-Salles, T.; Von Stedingk, H.; Granum, B.; Gützkow, K.B.; Rydberg, P.; Tornqvist, M.; Méndez, M.A.; Brunborg, G.; Brantsæter, A.L.; Meltzer, H.M.; et al. Dietary acrylamide intake during pregnancy and fetal growth—Results from the Norwegian mother and child cohort study (MoBa). Environ. Health Perspect. 2012, 121, 374–379. [Google Scholar] [CrossRef]
- Pedersen, M.; Von Stedingk, H.; Botsivali, M.; Agramunt, S.; Alexander, J.; Brunborg, G.; Chatzi, L.; Fleming, S.; Fthenou, E.; Granum, B.; et al. Birth weight, head circumference, and prenatal exposure to acrylamide from maternal diet: The European prospective mother–child study (NewGeneris). Environ. Health Perspect. 2012, 120, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Kadawathagedara, M.; Tong, A.C.H.; Heude, B.; Forhan, A.; Charles, M.-A.; Sirot, V.; Botton, J.; The EDEN Mother-Child Cohort Study Group. Dietary acrylamide intake during pregnancy and anthropometry at birth in the French EDEN mother-child cohort study. Environ. Res. 2016, 149, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Fennell, T.R.; Sumner, S.C.; Snyder, R.W.; Burgess, J.; Spicer, R.; Bridson, W.E.; Friedman, M.A. Metabolism and hemoglobin adduct formation of acrylamide in humans. Toxicol. Sci. 2004, 85, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Bergmark, E. Hemoglobin adducts of acrylamide and acrylonitrile in laboratory workers, smokers and nonsmokers. Chem. Res. Toxicol. 1997, 10, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, M.I.; Bolt, H.M.; Drexler, H.; Angerer, J. Excretion of mercapturic acids of acrylamide and glycidamide in human urine after single oral administration of deuterium-labelled acrylamide. Arch. Toxicol. 2005, 80, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Hays, S.; Aylward, L.L. Biomonitoring Equivalents (BE) dossier for acrylamide (AA) (CAS No. 79-06-1). Regul. Toxicol. Pharmacol. 2008, 51 (Suppl. 3), S57–S67. [Google Scholar] [CrossRef]
- Boettcher, M.I.; Schettgen, T.; Kütting, B.; Pischetsrieder, M.; Angerer, J. Mercapturic acids of acrylamide and glycidamide as biomarkers of the internal exposure to acrylamide in the general population. Mutat. Res. Toxicol. Environ. Mutagen. 2005, 580, 167–176. [Google Scholar] [CrossRef]
- Bjellaas, T.; Stølen, L.H.; Haugen, M.; Paulsen, J.E.; Alexander, J.; Lundanes, E.; Becher, G. Urinary acrylamide metabolites as biomarkers for short-term dietary exposure to acrylamide. Food Chem. Toxicol. 2007, 45, 1020–1026. [Google Scholar] [CrossRef]
- Choi, S.Y.; Ko, A.; Kang, H.-S.; Hwang, M.-S.; Lee, H.-S. Association of urinary acrylamide concentration with lifestyle and demographic factors in a population of South Korean children and adolescents. Environ. Sci. Pollut. Res. 2019, 26, 18247–18255. [Google Scholar] [CrossRef]
- Heudorf, U.; Hartmann, E.; Angerer, J. Acrylamide in children—Exposure assessment via urinary acrylamide metabolites as biomarkers. Int. J. Hyg. Environ. Health 2009, 212, 135–141. [Google Scholar] [CrossRef]
- Brisson, B.; Ayotte, P.; Normandin, L.; Gaudreau, E.; Bienvenu, J.-F.; Fennell, T.R.; Blanchet, C.; Phaneuf, D.; Lapointe, C.; Bonvalot, Y.; et al. Relation between dietary acrylamide exposure and biomarkers of internal dose in Canadian teenagers. J. Expo. Sci. Environ. Epidemiol. 2013, 24, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Kang, S.; Lee, G.; Lee, S.; Jo, A.; Kwak, K.; Kim, D.; Kho, D.; Lee, S.; Kim, S.; et al. Urinary levels of N-acetyl-S-(2-carbamoylethyl)-cysteine (AAMA), an acrylamide metabolite, in Korean children and their association with food consumption. Sci. Total Environ. 2013, 456, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.J.; Li, C.-M.; Wu, C.-F.; Jao, S.-P.; Wu, K.-Y. Analysis of urinary N-acetyl-S-(propionamide)-cysteine as a biomarker for the assessment of acrylamide exposure in smokers. Environ. Res. 2007, 104, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Mojska, H.; Gielecińska, I.; Zielińska, A.; Winiarek, J.; Sawicki, W. Estimation of exposure to dietary acrylamide based on mercapturic acids level in urine of Polish women post partum and an assessment of health risk. J. Expo. Sci. Environ. Epidemiol. 2015, 26, 288–295. [Google Scholar] [CrossRef]
- Szponar, L.; Wolnicka, K.; Rychlik, E. Album of Photographs of Food Products and Dishes; Food and Nutrition Institute: Warsaw, Poland, 2000. [Google Scholar]
- Ciba-Geigy, A.G. Teiband körperflüssigkeiten. In Wissenschaftliche Tabellen Geigy, 8th ed.; Leitner, C., Ed.; Ciba-Geigy AG: Basel, Switzerland, 1977; pp. 51–97. [Google Scholar]
- Kantar Public. Report from a Nationwide Survey on Attitudes towards Smoking; Kantar Public for the Chief Sanitary Inspectorate: Warsaw, Poland, 2019. [Google Scholar]
- Cunningham, F.; Fiebelkorn, S.; Johnson, M.; Meredith, C. A novel application of the Margin of Exposure approach: Segregation of tobacco smoke toxicants. Food Chem. Toxicol. 2011, 49, 2921–2933. [Google Scholar] [CrossRef]
- Mojska, H.; Gielecińska, I.; Szponar, L.; Ołtarzewski, M. Estimation of the dietary acrylamide exposure of the Polish population. Food Chem. Toxicol. 2010, 48, 2090–2096. [Google Scholar] [CrossRef]
- Tyl, R. Effects of acrylamide on rodent reproductive performance. Reprod. Toxicol. 2003, 17, 1–13. [Google Scholar] [CrossRef]
- Da Costa, G.G.; Churchwell, M.I.; Hamilton, L.P.; Von Tungeln, L.S.; Beland, F.A.; Marques, M.M.; Doerge, D.R. DNA Adduct formation from acrylamide via conversion to Glycidamide in adult and neonatal mice. Chem. Res. Toxicol. 2003, 16, 1328–1337. [Google Scholar] [CrossRef]
- Von Tungeln, L.S.; Doerge, D.R.; Da Costa, G.G.; Marques, M.M.; Witt, W.M.; Koturbash, I.; Pogribny, I.P.; Beland, F.A. Tumorigenicity of acrylamide and its metabolite glycidamide in the neonatal mouse bioassay. Int. J. Cancer 2012, 131, 2008–2015. [Google Scholar] [CrossRef]
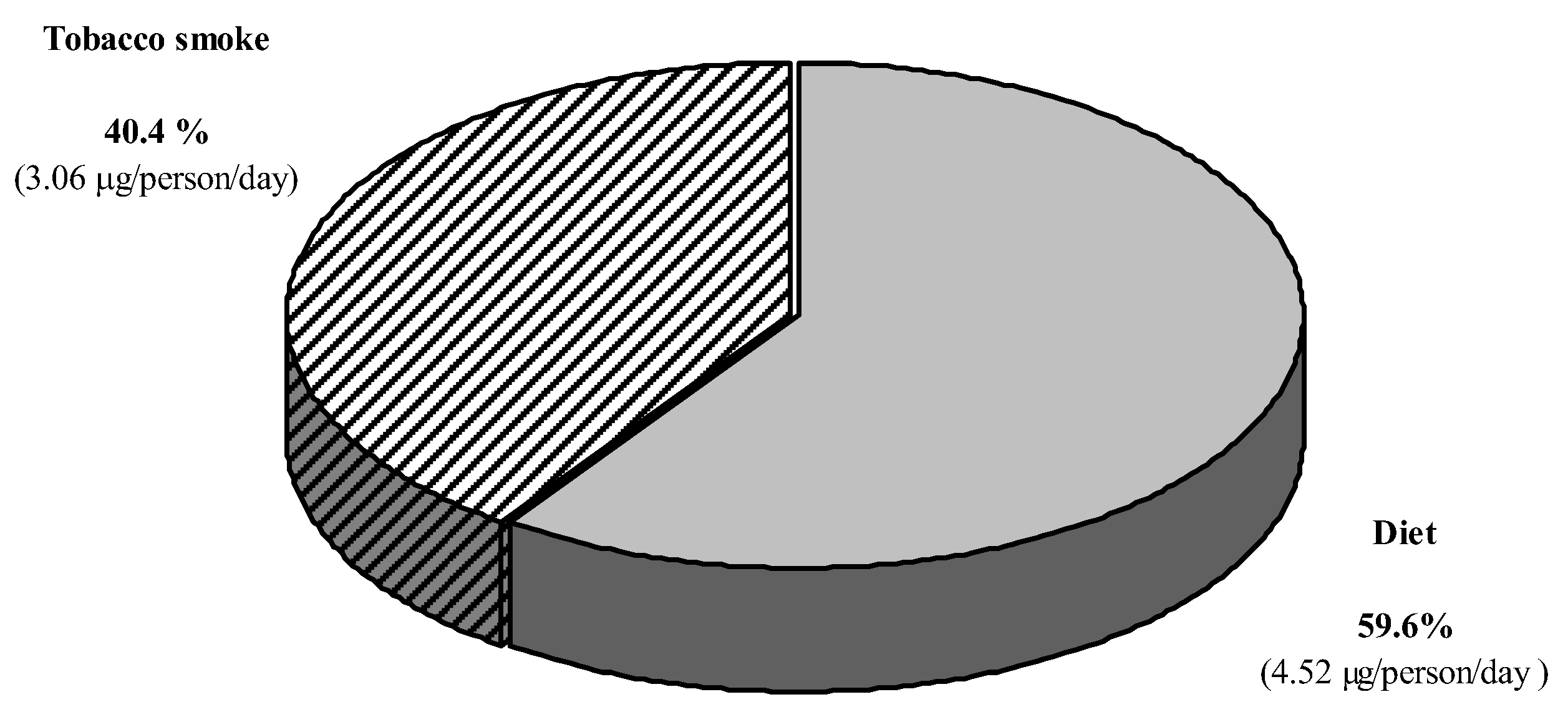
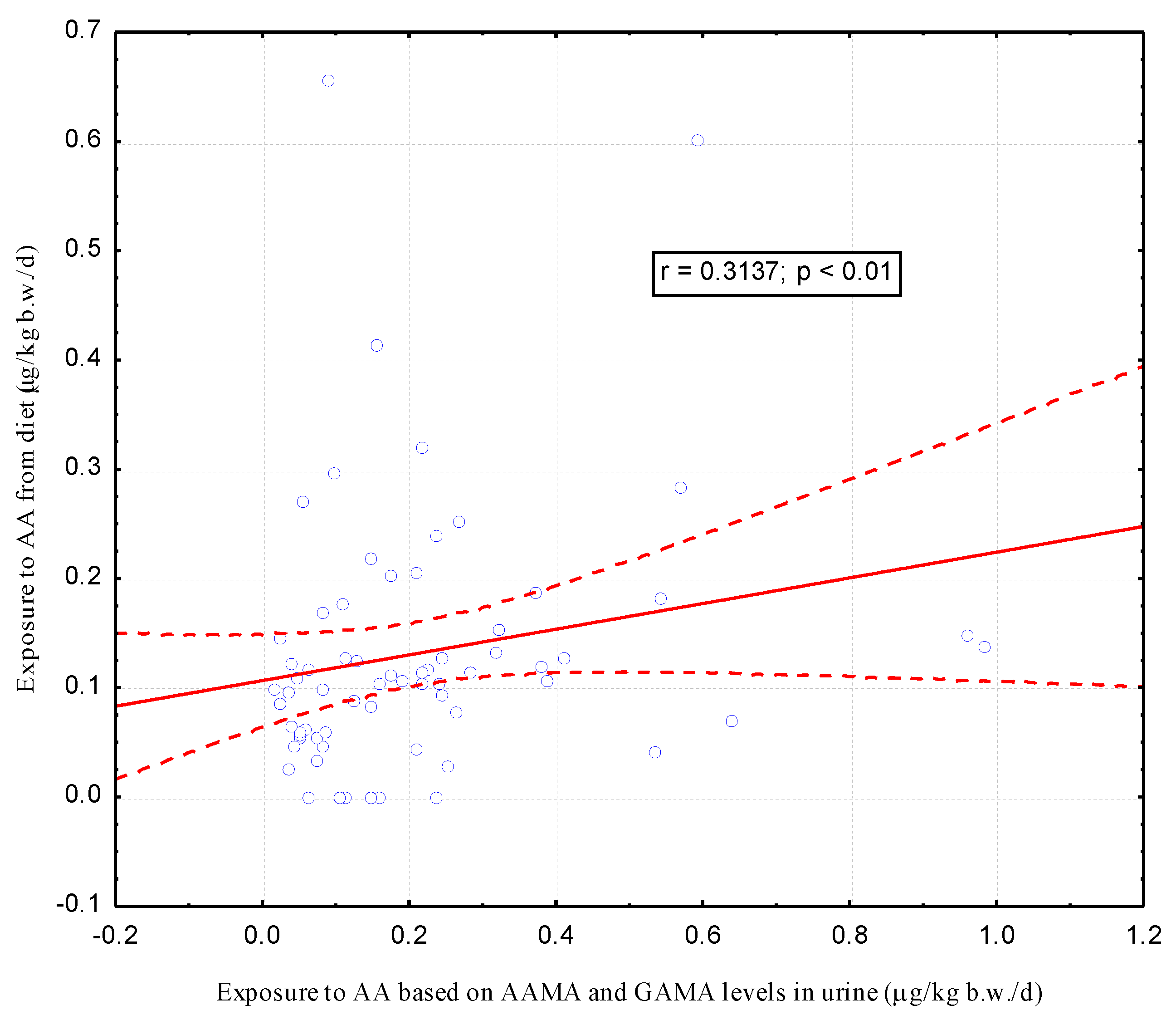
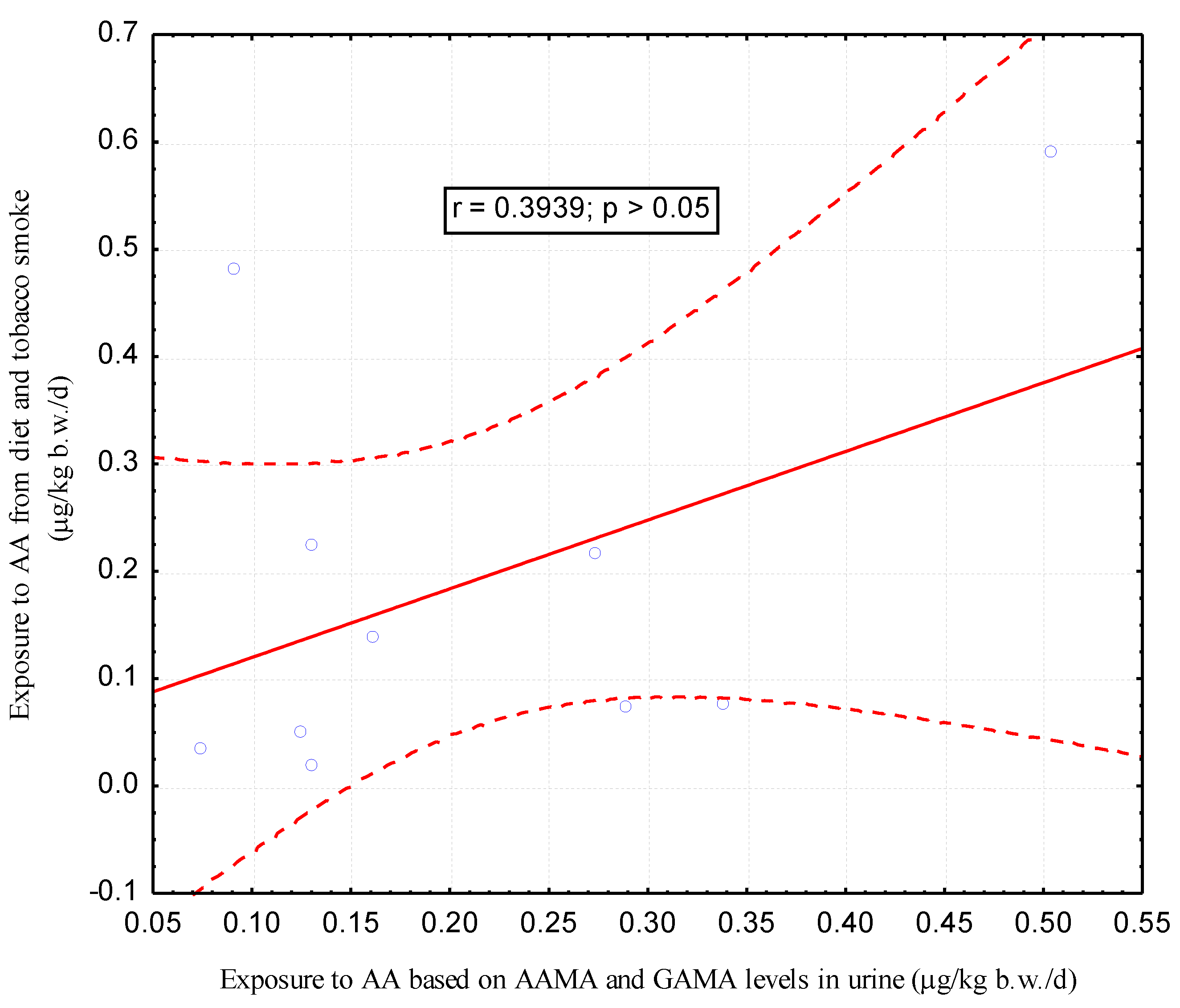
| Parameters | Passive Smokers (n = 10) | Non-Smokers (n= 67) | p |
|---|---|---|---|
| Average (range) | |||
| Age (years) | 28 (24–35) | 31 (21–40) | <0.05 |
| Body weight (kg) | 78 (62–95) | 72 (51–111) | n.s. |
| Creatinine content in urine (mg/L) | 947 (198–1785) | 773 (77–2893) | n.s. |
| Pregnancy length (weeks) | 40 (38–41) | 39 (33–41) | n.s. |
| Number of persons (%) | |||
| Type of labour: | |||
| Natural | 5 (50) | 44 (65.7) | n.s. |
| Caesarean section | 5 (50) | 23 (34.3) | |
| Labour order: | |||
| First | 6 (60) | 34 (50.7) | n.s. |
| Subsequent | 4 (40) | 33 (49.3) | |
| Median (min–max) | |||
| Acrylamide intake from the postpartum diet: | |||
| μg/person/day | 4.52 (0.00–51.30) | 7.33 (0.00–49.63) | n.s |
| μg/kg b.w./day | 0.06 (0.00–0.59) | 0.11 (0.00–0.66) | n.s. |
| <10 Cigarettes (n = 7) | ≥10 Cigarettes (n = 3) | p | ||||
|---|---|---|---|---|---|---|
| Exposure to AA | Number of Cigarettes (pcs) | Exposure to AA | Number of Cigarettes (pcs) | |||
| μg/Person/Day | μg/kg b.w./Day | μg/Person/Day | μg/kg b.w./Day | |||
| 0.12 | 0.001 | 0.2 | 6.8 | 0.10 | 10 | |
| 1.36 | 0.02 | 2 | 13.6 | 0.14 | 20 | |
| 1.70 | 0.02 | 2.5 | 13.6 | 0.16 | 20 | |
| 1.70 | 0.03 | 2.5 | - | - | - | |
| 2.72 | 0.03 | 4 | - | - | - | |
| 3.40 | 0.05 | 5 | - | - | - | |
| 4.08 | 0.05 | 6 | - | - | - | |
| Me = 1.70 | Me = 0.03 | x = 3.2 | Me = 13.6 | Me = 0.14 | x = 16.7 | <0.05 |
| Non-Smokers (n = 67) | Passive Smokers | p *1 | p *2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| In Total (n = 10) | <10 Cigarettes (n = 7) | ≥10 Cigarettes (n = 3) | ||||||||
| Median | Min–Max | Median | Min–Max | Median | Min–Max | Median | Min–Max | |||
| On volume basis [μg/L] | ||||||||||
| AAMA | 19.5 | 2.3–114 | 21.6 | 9.0–73.9 | 22.3 | 9.0–73.9 | 20.9 | 10.9–42.1 | n.s. | n.s. |
| GAMA | 7.2 | 1.3–50.7 | 9.4 | 3.5–30.9 | 10.1 | 5.0–30.9 | 8.8 | 3.5–14.1 | n.s. | n.s. |
| AAMA + GAMA | 26.9 | 3.7–164.7 | 31.0 | 14.0–104.8 | 32.4 | 14.0–104.8 | 29.7 | 14.4–56.2 | n.s. | n.s. |
| GAMA − : AAMA | 0.38 | 0.13 – 1.01 | 0.41 | 0.26–0.74 | 0.42 | 0.26–0.74 | 0.33 | 0.32–0.42 | n.s. | n.s. |
| On creatinine basis [μg/g] | ||||||||||
| AAMA | 30.7 | 7.8–138.2 | 25.2 | 8.6–122.8 | 25.0 | 8.6–122.8 | 25.4 | 17.5–55.1 | n.s. | n.s. |
| GAMA | 11.4 | 4.9–26.7 | 10.3 | 6.4–51.3 | 10.6 | 6.4–51.3 | 8.54 | 7.3–17.6 | n.s. | n.s. |
| AAMA + GAMA | 43.5 | 15.7–162.0 | 35.4 | 15.1–174.1 | 37.0 | 15.1–174.1 | 33.8 | 24.8–72.7 | n.s. | n.s. |
| Exposure to Acrylamide Estimated on the Basis of: | [μg/kg b.w./Day] | p | |
|---|---|---|---|
| Median (Min-Max) | |||
| Passive Smokers (n = 10) | Non-Smokers (n = 67) | ||
| Dietary recall and content in tobacco smoke * | 0.11 (0.02–0.59) | 0.11 (0.00–0.66) | n.s. |
| AAMA and GAMA levels in urine | 0.15 (0.07–0.50) | 0.16 (0.02–0.98) | n.s. |
| Parameters | Average (Range) or Number of Persons (%) | ||
|---|---|---|---|
| Non-Smokers (n = 67) | Passive Smokers (n = 10) | p | |
| Birth weight (g) | 3433 (1860–4750) | 3552 (3000–4290) | n.s. |
| Birth body length (cm) | 55 (48–60) | 55 (48–58) | n.s. |
| Apgar score in the first minute of life *: | |||
| 10 | 55 (82.1) | 9 (90) | |
| 9 | 8 (11.9) | 1 (10) | |
| ≤8 | 4 (6.0) | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mojska, H.; Gielecińska, I.; Jasińska-Melon, E.; Winiarek, J.; Sawicki, W. Are AAMA and GAMA Levels in Urine after Childbirth a Suitable Marker to Assess Exposure to Acrylamide from Passive Smoking during Pregnancy?—A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 7391. https://doi.org/10.3390/ijerph17207391
Mojska H, Gielecińska I, Jasińska-Melon E, Winiarek J, Sawicki W. Are AAMA and GAMA Levels in Urine after Childbirth a Suitable Marker to Assess Exposure to Acrylamide from Passive Smoking during Pregnancy?—A Pilot Study. International Journal of Environmental Research and Public Health. 2020; 17(20):7391. https://doi.org/10.3390/ijerph17207391
Chicago/Turabian StyleMojska, Hanna, Iwona Gielecińska, Edyta Jasińska-Melon, Joanna Winiarek, and Włodzimierz Sawicki. 2020. "Are AAMA and GAMA Levels in Urine after Childbirth a Suitable Marker to Assess Exposure to Acrylamide from Passive Smoking during Pregnancy?—A Pilot Study" International Journal of Environmental Research and Public Health 17, no. 20: 7391. https://doi.org/10.3390/ijerph17207391
APA StyleMojska, H., Gielecińska, I., Jasińska-Melon, E., Winiarek, J., & Sawicki, W. (2020). Are AAMA and GAMA Levels in Urine after Childbirth a Suitable Marker to Assess Exposure to Acrylamide from Passive Smoking during Pregnancy?—A Pilot Study. International Journal of Environmental Research and Public Health, 17(20), 7391. https://doi.org/10.3390/ijerph17207391






