Abstract
Healthcare workers (HCWs) have increased risk for latent tuberculosis infection (LTBI) and tuberculosis (TB) disease due to their occupational exposure. For some years now, interferon-γ release assays (IGRAs) have replaced the tuberculin skin test for the diagnosis of LTBI in many countries. This review examined the occupational risk of LTBI in HCWs with IGRA testing in low incidence countries. A systematic review and meta-analysis of studies from 2005 onwards provide data regarding the prevalence of LTBI in HCWs. In addition, the pooled effect estimates were calculated for individual regions and occupational groups. 57 studies with 31,431 HCWs from four regions and a total of 25 countries were analysed. The prevalence of LTBI varied from 0.9 to 85.5%. The pooled estimation found the lowest prevalence of LTBI for North American and West Pacific countries (<5%), and the highest prevalence for Eastern Mediterranean countries (19.4%). An increased risk for LTBI was found only for administrative employees. Studies on the occupational risk of LTBI continue to show increased prevalence of HCWs, even in low-incidence countries. Good quality studies will continue to be needed to describe occupational exposure.
1. Introduction
Healthcare workers (HCWs) have an elevated risk of latent tuberculosis infection (LTBI) and TB disease (tuberculosis (TB)) due to the nature of their jobs [1,2,3,4]. The reduction in TB incidence in high-income countries should correlate with a decrease in the risk of TB infection for HCWs. However, it appears that working in the healthcare sector even poses a risk in high-income countries with high hygiene standards [5,6]. In Germany, where TB incidence is low, TB in healthcare workers remains one of the most common infections reported to the compensation board [7].
Most of the reviews of the TB infection risk among HCWs were conducted in low and middle-income countries [3,8,9,10]. Furthermore, the occupational infection risk and the probability of causation have primarily been analysed while using the tuberculin skin test (TST) after Mendel and Mantoux. For several years, interferon-γ release assays (IGRAs) have also been used to diagnose latent tuberculosis infection. IGRAs have higher specificity and a good negative predictive value and are, therefore, a valid alternative to TST. In the review and meta-analysis by Diel et al. [11], the specificity of the IGRAs was found to be 98–100%. The negative predictive value was 97.8% for T-Spot TB and 99.8% for QFT-GIT. Thus, the IGRAs have strong advantage in the diagnosis of LTBI and they can more accurately exclude LTBI. Therefore, they replace the TST in most high income, low incidence countries.
This systematic review and meta-analysis examines the prevalence and occupational risk of LTBI assessed by IGRA in healthcare workers in low-incidence countries.
2. Methods
This literature review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [12]. The Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting checklist was also taken into account [13].
The question and the corresponding inclusion and exclusion criteria were formulated while using the PEO criteria:
Population: Healthcare workers from countries with low tuberculosis (TB) incidence
Exposure: Occupational exposure to TB pathogens, infected material, or an infected environment
Outcome: LTBI found in the occupational setting using IGRAs
- (1)
- How high is the prevalence of LTBI among healthcare workers in low-incidence countries, measured using IGRAs?
- (2)
- In which occupational groups or areas of work within the healthcare sectors is there an elevated risk of occupational LTBI?
2.1. Selection Criteria
The target population was defined as healthcare workers whose occupations meant that they had either direct contact with patients (doctors, nursing staff and assistants, students, various therapists) or indirect or no contact with patients, but were exposed to infected material or an infected environment (e.g., laboratory workers, cleaning staff, administrative employees). The review only included studies that were conducted in countries with a low incidence of TB, as defined by the WHO (estimated incidence rate <40 per 100,000 inhabitants [14]). Studies from low-incidence countries with a study population exclusively from high-incidence countries were excluded due to the selective group. Furthermore, the occupational exposure to LTBI had to have been investigated while using immuno-diagnostic tests as part of routine examinations or screenings. Studies that used both IGRAs and TSTs as diagnostic procedures were only included if the results of these two methods were recorded separately. Cases where IGRAs were used to confirm a positive TST were excluded from further analysis on the grounds of selection. Studies with contact investigations following the disclosure of active TB cases were likewise excluded.
Observational studies comprised cohort studies, case control studies, and cross-sectional studies. Reviews, editorials, comments, conference reports, case reports, and statements were excluded. Abstracts were only taken into account if they included all of the relevant information and the full text was not available. The initial study had no language restrictions. However, at the full text screening stage, only peer-reviewed publications in a language spoken by the study group were included (e.g., English, German, Dutch, French, Italian, Portuguese, and Spanish). A detailed overview of the inclusion and exclusion criteria can be found in Supplementary Table S1.
2.2. Sources of Information and Search Strategy
A systematic electronic search was conducted in the MEDLINE, PubMed, CINAHL, Web of Science and LIVIVO databases. The search string was initially devised for PubMed and then adapted to other databases. The keywords were developed in accordance with Medical Subject Headings (MeSH) and then combined with additional terms for the target population, exposure/diagnostic procedure, and the outcome. The detailed search strategy and database search in PubMed can be found in Supplementary Tables S2 and S3. The search spanned the publication period from 1 January 2005 to 31 January 2019 (last update: 15 August 2019).
2.3. Data Management, Study Selection and Data Extraction
The search results were sorted and managed while using the referencing software EndNote. First, the citations were automatically checked for duplicates; then, the search results were manually cleaned up. The titles and abstracts were selected by a reviewer (AK) using the predefined inclusion and exclusion criteria. Two reviewers checked the full text of the shortlisted articles for relevance (AK and CP). In the case of disagreements, an additional reviewer was consulted (AS) to reach a consensus through discussion. A manual search was conducted for other relevant sources in the references of the identified publications. Systematic reviews and meta-analyses were also included. One reviewer (CP) extracted the data, then compared and verified by a second reviewer (AK). The following information was extracted from all of the studies using a prescribed form: lead author, publication date, country, study design, study period, research setting, occupational group, age, diagnostic method, IGRA cut-off values, other risk factors for LTBI, and proportion of positive IGRA results. Finally, the studies were listed by WHO region.
2.4. Study Quality
The studies’ methodological quality was assessed using the JBI Critical Appraisal Checklist for Analytical Cross Sectional Studies [15]. This checklist consists of eight items which address biases in the design, execution, and analysis of the studies. One item (“Were objective, standardised criteria used to measure the findings?”) was replaced with another item from the JBI Checklist for Studies Reporting Prevalence Data (“Was the sample size adequate?”). While using the sample calculation formula by Naing et al. [16], which is cited in the description, a sample size of N = 139 was calculated for an anticipated LTBI prevalence of 10%. Consequently, in the quality appraisal, a point was awarded if at least this number of study participants was included in individual studies. The maximum score was eight points, one point for each question. High, medium, and low-quality studies were defined as scoring 7–8, 5–6, and less than 5 points, respectively. Two reviewers independently evaluated the study quality (CP, AS).
- 1st item: Criteria for inclusion in the sample clearly defined
- 2nd item: Study subjects and setting described in detail
- 3rd item: Exposure measured in a valid and reliable way
- 4th item: Sample size adequate (min. N = 139)
- 5th item: Confounding factors identified/considered
- 6th item: Strategies for dealing with confounding factors indicated
- 7th item: Outcomes measured in a valid and reliable way
- 8th item: Appropriate statistical analysis applied
2.5. Statistical Analysis
For the LTBI prevalence, the number of positive LTBI cases and total number of participating healthcare workers was extracted from the original studies into an Excel table that was developed by Neyeloff et al. [17]. On this basis, pooled prevalence estimates were calculated with a 95% confidence interval (CI). Random effects models were chosen to determine the pooled prevalence estimates for the individual WHO regions, as we assumed that the effects between studies were heterogeneous (European, Western Pacific, Eastern Mediterranean, and Americas).
The corresponding number of positive LTBI cases in the respective occupational group/field of work (e.g., nursing staff, doctors, administrative employees or laboratory workers) was compared with the case count in the whole study population. Only studies with good or very good methodological scores (≥5 points) were included in the meta-analysis. The Mantel-Haenszel method was used to calculate odds ratios (OR) for dichotomous outcomes. The model calculations are based on random effects. Forest plots were generated for each group comparison. Heterogeneity was quantified with the aid of the chi-square (χ2) and I2 statistic. The latter expresses the total variability between the studies as a percentage. The higher the percentage, the greater the degree of heterogeneity: I2 values between 0–40%, 30–60%, 50–90%, and 75–100% correspond to a low, medium, substantial, or high level of heterogeneity A p-value (χ2) of <0.10 was deemed to be statistically significant. Review Manager (version 5.3), which was the statistical software provided by Cochrane, was used for data analysis [18].
3. Results
3.1. Study Selection
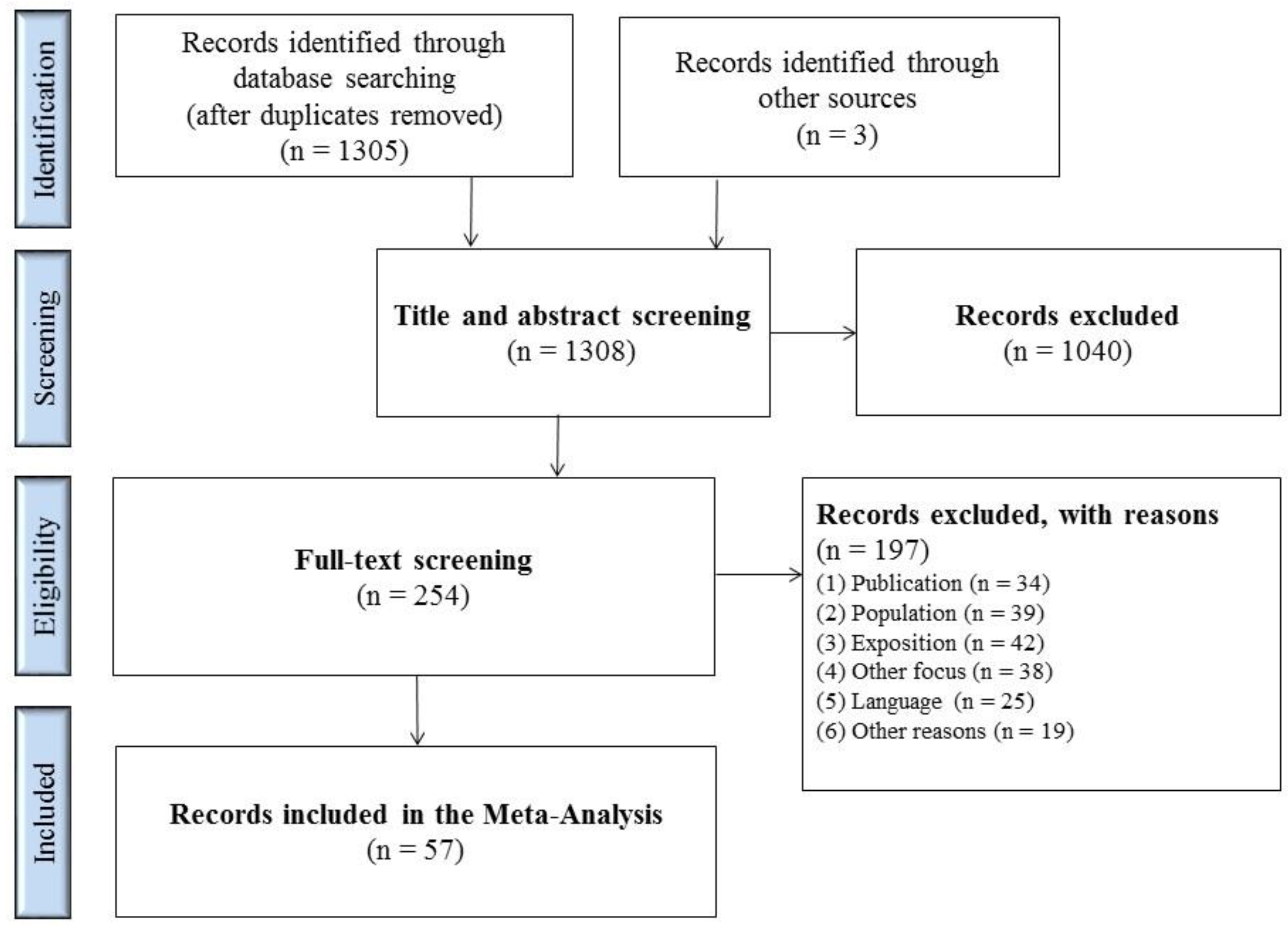
1308 matches were identified in the databases and from other sources (Figure 1). 254 full texts were screened after removing the duplicates and screening titles and abstracts. The most common reasons for exclusion were: publications covered contact investigations following TB exposure; there was no medical study population or the study took place in high-incidence countries; there was no use of IGRA tests or its use was only to confirm a positive TST; or, the exclusion was due to the type of publication. Table S1 lists the exact selection criteria.

Figure 1.
Flowchart of study selection process.
A total of 57 studies fulfilled the inclusion criteria and could be included in the analysis. Of these, 32 studies were conducted in Europe, five in America, nine in the Western Pacific region, and 11 in countries in the Mediterranean region (Table 1). The countries were allocated according to WHO regions.

Table 1.
Studies of occupational latent tuberculosis infection (LTBI) with interferon-γ release assays (IGRAs) by WHO regions
3.2. Study Characteristics
Most of the studies were conducted as cross-sectional studies and cohort studies in hospital settings between 2005 and 2015. Only staff from special infection or TB units were included in some of the investigations. Less commonly, the studies looked at university settings, laboratories, radiology departments, or geriatric care. The number of participants varied between 21 and 3823 employees. For the most part, the IGRA testing for LTBI used the QFT (various generations) with a cut-off value for INF-γ of 0.35 IU/mL. The T-SPOT assay was used six times and a non-commercial ELISpot was utilised, alongside the other two tests in the study by Girardi et al. [23]. An appraisal of the studies’ methodological quality resulted in a high score for 31 studies, while 15 studies were awarded a medium score. 10 studies were deemed to be of low methodological quality. For one study [26], only an abstract was available, so it could not be included in the evaluation. The most common shortcomings were insufficient study size, the approach for dealing with confounding factors and a lack of suitable statistical methods for the identification of risk factors (e.g., regression analysis).
3.3. Meta-Analysis—Prevalence of LTBI
The prevalence of LTBI in the individual studies was between 0.9% and 85.5%. The findings from Europe varied particularly widely, from 1.1 to 85.5%.
The pooled effect estimates for Europe showed an LTBI prevalence of 16.2% (95% CI 13.0–19.3) (Table 2). When all of the studies with high and medium methodological quality were considered, the estimate was 16.3%. The figure was 13.9% among those with at least 139 employee participants. For America, the joint effect estimate indicated a prevalence of 16.5%, while excluding low quality studies gave a prevalence of 19.3%. Excluding one study with a small number of participants reduced the estimate to 14.9% [54]. However, the prevalence was 4.5% if only North America is considered. The pooled prevalence for Western Pacific countries was 4.8%, while 19.4% was recorded for the Eastern Mediterranean region. Selection on the basis of high and medium study quality reduced the joint prevalence in the Mediterranean region to 16.1%; looking only at those with a sufficient cohort size resulted in 15.2%. Seven of the nine studies in the Western Pacific region were conducted in Japan. No studies were excluded here, so there was no variation in the findings.

Table 2.
Pooled prevalence estimations for LTBI by WHO regions.
3.4. Meta-Analysis—Occupational Risk for LTBI
23 studies were available for the meta-analysis to establish occupational exposure. Studies whose methodological quality was considered low were excluded from the analysis [29,47], as was another study, which focused solely on laboratory staff [73]. All in all, 20 studies with data from 2612 cases of LTBI among a total population of 15,262 workers were included in the meta-analysis (Table 3). The majority of the publications came from the Eastern Mediterranean and Western Pacific regions. It was not possible to extract any data to analyse the occupational exposure from the studies that were conducted in America.

Table 3.
Meta-analysis for job categories by WHO regions.
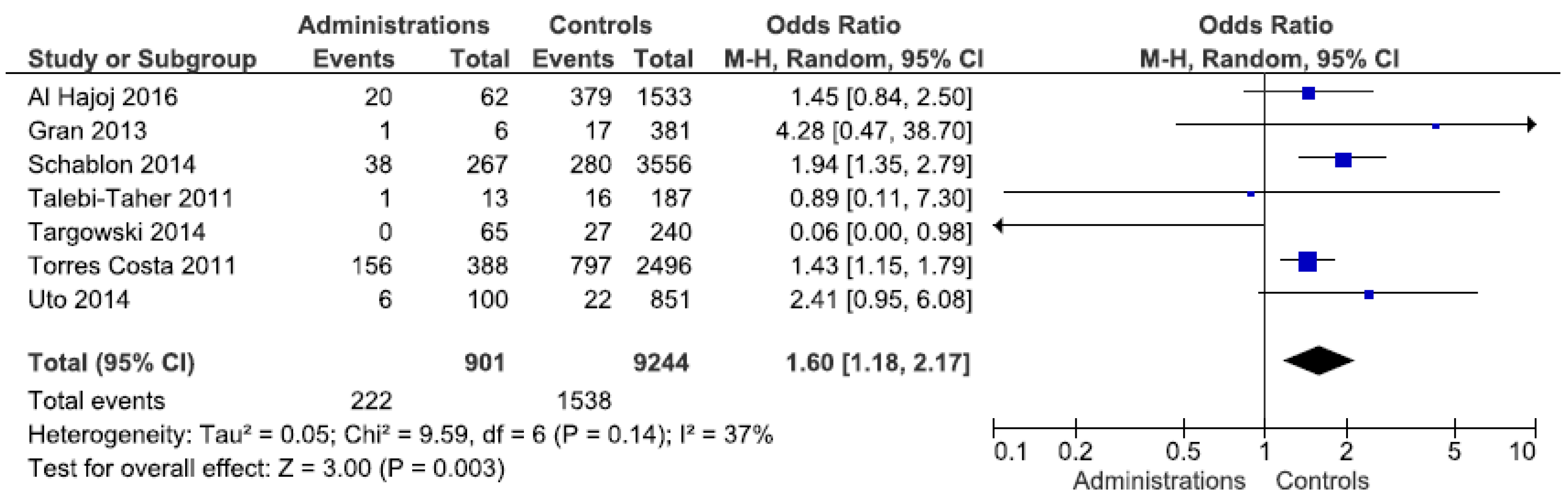
Stratification by occupation revealed a statistically significant exposure risk for administrative employees, with an OR of 1.6 (95% CI 1.18–2.17) (Figure 2). Forest plots for other occupations can be found in Supplementary Figures S1–S3. The studies from Europe confirm this finding (OR 1.7). In the other regions, only a few studies showed an elevated, but not statistically significant OR. An elevated risk (OR 2.6) was identified for laboratory staff in the Western Pacific region, which was not evident in the other regions. Doctors and nurses were not found to be at any greater risk of exposure than other workers. By contrast, a statistically insignificant protective effect was identified among the nursing staff. Even after regional differentiation, this was still observed for Europe and the Western Pacific region. The heterogeneity of the meta-analyses can be largely seen as moderate.
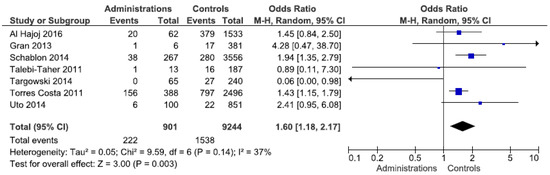
Figure 2.
Forest plot of the LTBI prevalence in administrative employees by IGRA in low incidence countries.
4. Discussion
57 studies investigated the occupational LTBI risk of medical personnel in low-incidence countries using the IGRA tests. The prevalence varied between 0.9% and 85.5%. Regional stratification revealed the lowest pooled effect estimates for North American and Western Pacific countries (<5%) and the highest for the Eastern Mediterranean (19.4%). The job-related analysis showed an increased infection risk for administrative staff, whereas there was no indication of an elevated risk for doctors and nurses in these studies.
In the review by Apriani et al. [9], the highest prevalence of 60% for LTBI that was measured with IGRA tests was identified among general service staff, followed by doctors (35%) and nurses (34%). General service staff included cleaners, drivers, and housekeepers. However, this review targeted low and middle-income countries; high-incidence countries were not an exclusion criterion. In a meta-analysis, Uden et al. [8] investigated the LTBI prevalence among medical staff with a comparison group consisting of employees with no direct patient contact or the general population. This found an elevated risk of LTBI and a higher incidence of active TB among healthcare workers. However, there was no stratification for the individual occupational groups. The pooled prevalence estimate was 37% and the risk estimate put the OR at 2.3 (95% CI 1.6–3.21) for LTBI. However, the TST and IGRA results were analysed together and no constraints were applied to the TB incidence. Baussano et al. [76] also identified an elevated risk for healthcare workers as compared with the general population in low-incidence countries. In a further review, the LTBI risk in high-burden countries was investigated using TSTs. This found a pooled LTBI prevalence of 57% for medical personnel [10].
Many of the studies analysed individual risk factors alongside LTBI prevalence. This resulted in 16 studies identifying higher age as a risk factor, while eight studies found staff from high-incidence countries to be at greater risk. In an occupational context, length of service, workplaces in units with high exposure, and specific job categories were repeatedly listed. The various studies’ findings differ with regard to job category. For instance, Torres Costa [49] and Bukhary et al. [68] found that doctors had an elevated risk vis-à-vis administrative staff. Two further studies identified an elevated risk for doctors and nurses [64,77]. However, another study showed elevated risk for administrative staff [36]. Our meta-analysis found no risks for medical professionals such as doctors, nurses and laboratory staff, but it did show an occupational risk of LTBI for administrative employees. However, most of the literature does not exactly define who was included in this group. It could encompass receptionists, administrative staff, or service and managerial employees, for example. This makes it difficult to venture an explanation for this finding.
In this review, studies of LTBI prevalence in low-incidence countries using the IGRA diagnostic procedure were collectively viewed. Regions and occupational groups were also analysed. Seidler et al. [5] previously formulated the same study objective in relation to TSTs. The authors found an elevated risk for various medical professions and/or units, although they describe the epidemiological evidence for the occupational groups as limited, except in the case of nurses. One reason was the lack of methodologically suitable studies with sufficient power.
Numerous studies were available for this review based on the use of IGRA testing among healthcare workers. Combining the studies offered greater statistical power and more meaningful findings than was possible with individual pieces of research. However, the result of the joint effect estimate can only be interpreted in relation to the underlying data of the individual publications. Conducting a quality appraisal of the studies, completing sub-group analyses and using the random effects model addressed the problem of statistical heterogeneity. Other criteria—such as the known risk factors of age or origins in a high-incidence country—could not be taken into account when calculating the pooled estimates due to the heterogeneity of the data. Most of the studies used convenience samples, which were tested with IGRA, due to some kind of risk assessment. Therefore, it is difficult to define an unexposed group, rending risk analysis unreliable.
Our strict inclusion criteria regarding the consideration of age or occupation in the individual studies must be viewed as a limitation. Nevertheless, although many of the included studies investigated these factors, they did not publish data on them. For this reason, the large studies from North America [51,52,53], for example, could be included in the overview, but not in the meta-analysis. A further limitation is the non-consideration of the results of the repeated test with the IGRA. For serial testing studies, we have only included the baseline test results for analysis in this review to ensure comparability with studies that only involved a single IGRA test. Repeated testing can lead to reversions and thus, if not taken into account, can lead to a possible overestimation of the risk of LTBI in HCWs.
5. Conclusions
This systematic review presents studies on the occupational LTBI risk of healthcare workers in low-incidence countries, as measured using the IGRA diagnostic procedure. It became apparent that numerous studies had been conducted on this topic and that prevalence is widespread. Regional differentiation showed the lowest LTBI prevalence for the North American and Western Pacific region and the highest for Eastern Mediterranean countries. A breakdown by occupational group identified an elevated infection risk for administrative employees, which was unexpected. Well-designed cohort studies are warranted for describing the occupational risk for LTBI in healthcare workers in low incidence countries.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/17/2/581/s1, Table S1: Eligibility criteria for the inclusion of studies. Table S2. Keywords included in the search strategy for all databases. Table S3. PubMed specific search strategy. Figure S1. Forest plot of the LTBI prevalence in nurses by IGRA in low incidence countries. Figure S2. Forest plot of the LTBI prevalence in physicians by IGRA in low incidence countries. Figure S3. Forest plot of the LTBI prevalence in laboratory workers by IGRA in low incidence countries.
Author Contributions
C.P. participated in the conception of the study, the literature search, data extraction, quality assessment, analysis and interpretation, and was in charge of writing the manuscript. A.K. participated in the literature search, data extraction and interpretation of the data and contributed to writing the manuscript. A.N. participated in the conception of the study, revised the manuscript critically for important intellectual content and gave final approval for the version to be published. A.S. participated in the conception of the study, quality assessment, and interpretation of the data and contributed to writing the manuscript. All authors read and approved the final manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Menzies, D.; Joshi, R.; Pai, M. Risk of tuberculosis infection and disease associated with work in health care settings. Int. J. Tuberc. Lung Dis. 2007, 11, 593–605. [Google Scholar] [PubMed]
- Pai, M.; Zwerling, A.; Menzies, D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: An update. Ann. Intern. Med. 2008, 149, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Zwerling, A.; van den Hof, S.; Scholten, J.; Cobelens, F.; Menzies, D.; Pai, M. Interferon-gamma release assays for tuberculosis screening of healthcare workers: A systematic review. Thorax 2012, 67, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.W.; Hur, M. Interferon-gamma Release Assays for the Diagnosis of Latent Tuberculosis Infection: An Updated Review. Ann. Clin. Lab. Sci. 2013, 43, 221–229. [Google Scholar] [PubMed]
- Seidler, A.; Nienhaus, A.; Diel, R. Review of epidemiological studies on the occupational risk of tuberculosis in low-incidence areas. Respiration 2005, 72, 431–446. [Google Scholar] [CrossRef]
- Nienhaus, A.; Ringshausen, F.C.; Costa, J.T.; Schablon, A.; Tripodi, D. IFN-gamma release assay versus tuberculin skin test for monitoring TB infection in healthcare workers. Expert Rev. Anti Infect. Ther. 2013, 11, 37–48. [Google Scholar] [CrossRef]
- Dulon, M.; Wendeler, D.; Nienhaus, A. Occupational infectious diseases in employees in the healthcare service 2017, Claims data from the Occupational Accident Insurance Association for healthcare and welfare services. Zbl. Arbeitsmed. 2019, 69, 16–22. [Google Scholar] [CrossRef]
- Uden, L.; Barber, E.; Ford, N.; Cooke, G.S. Risk of Tuberculosis Infection and Disease for Health Care Workers: An Updated Meta-Analysis. Open Forum Infect. Dis. 2017, 4, ofx137. [Google Scholar] [CrossRef]
- Apriani, L.; McAllister, S.; Sharples, K.; Alisjahbana, B.; Ruslami, R.; Hill, P.C.; Menzies, D. Latent tuberculosis infection in health care workers in low and middle-income countries: An updated systematic review. Eur. Respir. J. 2019, 53, 1801789. [Google Scholar] [CrossRef]
- Nasreen, S.; Shokoohi, M.; Malvankar-Mehta, M.S. Prevalence of Latent Tuberculosis among Health Care Workers in High Burden Countries: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0164034. [Google Scholar] [CrossRef]
- Diel, R.; Goletti, D.; Ferrara, G.; Bothamley, G.; Cirillo, D.; Kampmann, B.; Lange, C.; Losi, M.; Markova, R.; Migliori, G.B.; et al. Interferon-gamma release assays for the diagnosis of latent Mycobacterium tuberculosis infection: A systematic review and meta-analysis. Eur. Respir. J. 2011, 37, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. World Health Organization (WHO) Estimates of Tuberculosis Incidence by Country. 2017. Available online: https://webarchive.nationalarchives.gov.uk/20191003045845/https://www.gov.uk/government/publications/tuberculosis-tb-by-country-rates-per-100000-people (accessed on 12 December 2019).
- The Joanna Briggs Institute. Critical Appraisal Tools for Use in JBI Systematic Reviews. Checklist for Prevalence Studies. Available online: http://joannabriggs.org/research/critical-appraisal-tools.html (accessed on 12 December 2019).
- Naing, L.; Winn, T.; Nordin, R. Pratical Issues in Calculating the Sample Size for Prevalence Studies. Arch. Orofac. Sci. 2006, 1, 9–14. [Google Scholar]
- Neyeloff, J.L.; Fuchs, S.C.; Moreira, L.B. Meta-analyses and Forest plots using a microsoft excel spreadsheet: Step-by-step guide focusing on descriptive data analysis. BMC Res. Notes 2012, 5, 52. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]; The Cochrane Collaboration: Melbourne, Australia, 2011; Available online: www.handbook.cochrane.org (accessed on 12 December 2019).
- Fox, B.D.; Kramer, M.R.; Mor, Z.; Preiss, R.; Rusanov, V.; Fuks, L.; Peled, N.; Haim, I.; Raz, M.; Shitrit, D. The QuantiFERON-TB-GOLD assay for tuberculosis screening in healthcare workers: A cost-comparison analysis. Lung 2009, 187, 413–419. [Google Scholar] [CrossRef]
- Soborg, B.; Andersen, A.B.; Larsen, H.K.; Weldingh, K.; Andersen, P.; Kofoed, K.; Ravn, P. Detecting a low prevalence of latent tuberculosis among health care workers in Denmark detected by M. tuberculosis specific IFN-gamma whole-blood test. Scand. J. Infect. Dis. 2007, 39, 554–559. [Google Scholar] [CrossRef]
- Gran, G.; Assmus, J.; Dyrhol-Riise, A.M. Screening for latent tuberculosis in Norwegian health care workers: High frequency of discordant tuberculin skin test positive and interferon-gamma release assay negative results. BMC Public Health 2013, 13, 353. [Google Scholar] [CrossRef]
- Ciaschetti, A.; Franchi, A.; Richeldi, L.; Rumpianesi, F.; Meacci, M.; Valente, A.; Franco, G. Screening of latent tuberculosis infection in health care workers by QuantiFERON-TB and tuberculin skin test. G. Ital. Med. Lav. Ergon. 2007, 29, 406–407. [Google Scholar]
- Girardi, E.; Angeletti, C.; Puro, V.; Sorrentino, R.; Magnavita, N.; Vincenti, D.; Carrara, S.; Butera, O.; Ciufoli, A.M.; Squarcione, S.; et al. Estimating diagnostic accuracy of tests for latent tuberculosis infection without a gold standard among healthcare workers. Eurosurveillance 2009, 14, 19373. [Google Scholar]
- Larcher, C.; Frizzera, E.; Pretto, P.; Lang, M.; Sonnleitner, N.; Huemer, H.P. Immunosurveillance for Mycobacterium tuberculosis of health care personnel in a third level care hospital. Med. Lav. 2012, 103, 26–36. [Google Scholar] [PubMed]
- Sauzullo, I.; Mastroianni, C.M.; Mengoni, F.; Ermocida, A.; Mascia, C.; Salotti, A.; Falciano, M.; Vullo, V. Long-term IFN-gamma and IL-2 response for detection of latent tuberculosis infection in healthcare workers with discordant immunologic results. J. Immunol. Methods 2014, 414, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Magrini, A.; Coppeta, L.; Somma, G.; Neri, A.; Gentili, S.; Fiocco, G.; Pietroiusti, A. Risk of tuberculosis in healthcare workers: Risk assessment and medical surveillance. Ig. Sanita Pubblica 2016, 72, 137–143. [Google Scholar]
- Stebler, A.; Iseli, P.; Muhlemann, K.; Bodmer, T. Whole-blood interferon-gamma release assay for baseline tuberculosis screening of healthcare workers at a Swiss university hospital. Infect. Control Hosp. Epidemiol. 2008, 29, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, D.; Brunet-Courtois, B.; Nael, V.; Audrain, M.; Chailleux, E.; Germaud, P.; Naudin, F.; Muller, J.Y.; Bourrut-Lacouture, M.; Durand-Perdriel, M.H.; et al. Evaluation of the tuberculin skin test and the interferon-gamma release assay for TB screening in French healthcare workers. J. Occup. Med. Toxicol. 2009, 4, 30. [Google Scholar] [CrossRef]
- Faibis, F.; Castelain, D.; Moreau, M.C.; Tellier, J.; Dekimeche, A.; Ittah-Desmeulles, H.; Fiacre, A.; Demachy, M.C. Prevalence of latent tuberculosis infection among health care workers from the emergency department of Meaux hospital using an interferon gamma release assay. Presse Med. 2011, 40, e516–e520. [Google Scholar] [CrossRef]
- Moucaut, A.; Nienhaus, A.; Courtois, B.; Nael, V.; Longuenesse, C.; Ripault, B.; Rucay, P.; Moisan, S.; Roquelaure, Y.; Tripodi, D. The effect of introducing IGRA to screen French healthcare workers for tuberculosis and potential conclusions for the work organisation. J. Occup. Med. Toxicol. 2013, 8, 12. [Google Scholar] [CrossRef]
- Nienhaus, A.; Gariepy, P.K.; Trouve, C.; Lhaumet, C.; Toureau, J.; Peters, C. Tuberculosis screening at the Sainte-Anne Hospital in Paris—Results of first and second IGRA. J. Occup. Med. Toxicol. 2014, 9, 24. [Google Scholar] [CrossRef]
- Lucet, J.C.; Abiteboul, D.; Estellat, C.; Roy, C.; Chollet-Martin, S.; Tubach, F.; Carcelain, G. Interferon-gamma release assay vs. tuberculin skin test for tuberculosis screening in exposed healthcare workers: A longitudinal multicenter comparative study. Infect. Control Hosp. Epidemiol. 2015, 36, 569–574. [Google Scholar] [CrossRef]
- Barsegian, V.; Mathias, K.D.; Wrighton-Smith, P.; Grosse-Wilde, H.; Lindemann, M. Prevalence of latent tuberculosis infection in German radiologists. J. Hosp. Infect. 2008, 69, 69–76. [Google Scholar] [CrossRef]
- Schablon, A.; Beckmann, G.; Harling, M.; Diel, R.; Nienhaus, A. Prevalence of latent tuberculosis infection among health care workers in a hospital for pulmonary diseases. J. Occup. Med. Toxicol. 2009, 4, 1. [Google Scholar] [CrossRef]
- Schablon, A.; Diel, R.; Diner, G.; Anske, U.; Pankow, W.; Ringshausen, F.C.; Nienhaus, A. Specificity of a whole blood IGRA in German nursing students. BMC Infect. Dis. 2011, 11, 245. [Google Scholar] [CrossRef]
- Schablon, A.; Nienhaus, A.; Ringshausen, F.C.; Preisser, A.M.; Peters, C. Occupational screening for tuberculosis and the use of a borderline zone for interpretation of the IGRA in German healthcare workers. PLoS ONE 2014, 9, e115322. [Google Scholar] [CrossRef] [PubMed]
- Herzmann, C.; Sotgiu, G.; Bellinger, O.; Diel, R.; Gerdes, S.; Goetsch, U.; Heykes-Uden, H.; Schaberg, T.; Lange, C. Risk for latent and active tuberculosis in Germany. Infection 2017, 45, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Nikolayevskyy, V.; Warburton, F.; Dobson, E.; Drobniewski, F. Rate of Latent Tuberculosis Infection Detected by Occupational Health Screening of Nurses New to a London Teaching Hospital. Infect. Control Hosp. Epidemiol. 2009, 30, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Leon, E.E.; Espinosa-Vega, E.; Santana-Rodriguez, E.; Molina-Cabrillana, J.M.; Perez-Arellano, J.L.; Caminero, J.A.; Serrano-Aguilar, P. Screening for tuberculosis infection in spanish healthcare workers: Comparison of the QuantiFERON-TB gold in-tube test with the tuberculin skin test. Infect. Control Hosp. Epidemiol. 2009, 30, 876–883. [Google Scholar] [CrossRef]
- Casas, I.; Latorre, I.; Esteve, M.; Ruiz-Manzano, J.; Rodriguez, D.; Prat, C.; Garcia-Olive, I.; Lacoma, A.; Ausina, V.; Dominguez, J. Evaluation of interferon-gamma release assays in the diagnosis of recent tuberculosis infection in health care workers. PLoS ONE 2009, 4, e6686. [Google Scholar] [CrossRef]
- Martinez-Lacasa, X.; Font, R.; Gonzalez, S.; Sallent, S.; Jaen, A.; Lite, J.; Cuchi, E. Usefulness of Quantiferon-TB Gold in Tube(R) in screening for latent tuberculosis infection in health workers. Enferm. Infecc. Microbiol. Clin. 2015, 33, 525–531. [Google Scholar] [CrossRef]
- Topic, R.Z.; Dodig, S.; Zoricic-Letoja, I. Interferon-gamma and immunoglobulins in latent tuberculosis infection. Arch. Med. Res. 2009, 40, 103–108. [Google Scholar] [CrossRef]
- Targowski, T.; Chelstowska, S.; Plusa, T. Tuberculin skin test and interferon-gamma release assay in the detection of latent tuberculosis infection among Polish health care workers. Pol. Arch. Med. Wewn. 2014, 124, 36–42. [Google Scholar]
- Ozdemir, D.; Annakkaya, A.N.; Tarhan, G.; Sencan, I.; Cesur, S.; Balbay, O.; Guclu, E. Comparison of the tuberculin skin test and the quantiferon test for latent Mycobacterium tuberculosis infections in health care workers in Turkey. Jpn. J. Infect. Dis. 2007, 60, 102–105. [Google Scholar] [PubMed]
- Caglayan, V.; Ak, O.; Dabak, G.; Damadoglu, E.; Ketenci, B.; Ozdemir, M.; Ozer, S.; Saygi, A. Comparison of tuberculin skin testing and QuantiFERON-TB Gold-In Tube test in health care workers. Tuberk. Toraks 2011, 59, 43–47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Babayigit, C.; Ozer, B.; Ozer, C.; Inandi, T.; Duran, N.; Gocmen, O. Performance of QuantiFERON-TB Gold In-Tube test and Tuberculin Skin Test for diagnosis of latent tuberculosis infection in BCG vaccinated health care workers. Med. Sci. Monit. 2014, 20, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Bozkanat, E.; Kaya, H.; Sezer, O.; Caliskan, T.; Kilic, E.; Ciftci, F.; Gumus, S.; Kartaloglu, Z. Comparison of tuberculin skin test and quantiferon-TB gold in tube test for diagnosis of latent tuberculosis infection in health care workers: A cross sectional study. J. Pak. Med. Assoc. 2016, 66, 270–274. [Google Scholar]
- Kargi, A.; Ilgazli, A.H.; Yildiz, F.; Boyaci, H.; Basyigit, I.E. Latent tuberculosis infection in healthcare workers at a tertiary care center. Biomed. Res. India 2017, 28, 657–662. [Google Scholar]
- Torres Costa, J.; Silva, R.; Ringshausen, F.C.; Nienhaus, A. Screening for tuberculosis and prediction of disease in Portuguese healthcare workers. J. Occup. Med. Toxicol. 2011, 6, 19. [Google Scholar] [CrossRef]
- Nikolova, M.; Markova, R.; Drenska, R.; Muhtarova, M.; Todorova, Y.; Dimitrov, V.; Taskov, H.; Saltini, C.; Amicosante, M. Antigen-specific CD4- and CD8-positive signatures in different phases of Mycobacterium tuberculosis infection. Diagn. Microbiol. Infect. Dis. 2013, 75, 277–281. [Google Scholar] [CrossRef]
- Joshi, M.; Monson, T.P.; Woods, G.L. Use of interferon-gamma release assays in a health care worker screening program: Experience from a tertiary care centre in the United States. Can. Respir. J. 2012, 19, 84–88. [Google Scholar] [CrossRef]
- Dorman, S.E.; Belknap, R.; Graviss, E.A.; Reves, R.; Schluger, N.; Weinfurter, P.; Wang, Y.; Cronin, W.; Hirsch-Moverman, Y.; Teeter, L.D.; et al. Interferon-gamma release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am. J. Respir. Crit. Care Med. 2014, 189, 77–87. [Google Scholar] [CrossRef]
- Zwerling, A.; Cojocariu, M.; McIntosh, F.; Pietrangelo, F.; Behr, M.A.; Schwartzman, K.; Benedetti, A.; Dendukuri, N.; Menzies, D.; Pai, M. TB screening in Canadian health care workers using interferon-gamma release assays. PLoS ONE 2012, 7, e43014. [Google Scholar] [CrossRef]
- Hernandez, M.; Casar, C.; Garcia, P.; Morales, V.; Mamani, N.; Gomez-Cofre, N.; Pizarro, P.; Balcells, M.E. Latent tuberculosis infection screening in healthcare workers in four large hospitals in Santiago, Chile. Rev. Chil. Infectol. 2014, 31, 254–260. [Google Scholar] [CrossRef][Green Version]
- Ochoa, J.; Leon, A.L.; Ramirez, I.C.; Lopera, C.M.; Bernal, E.; Arbelaez, M.P. Prevalence of tuberculosis infection in healthcare workers of the public hospital network in Medellin, Colombia: A Bayesian approach. Epidemiol. Infect. 2017, 145, 1095–1106. [Google Scholar] [CrossRef]
- Vinton, P.; Mihrshahi, S.; Johnson, P.; Jenkin, G.A.; Jolley, D.; Biggs, B.A. Comparison of QuantiFERON-TB Gold In-Tube Test and tuberculin skin test for identification of latent Mycobacterium tuberculosis infection in healthcare staff and association between positive test results and known risk factors for infection. Infect. Control Hosp. Epidemiol. 2009, 30, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.T.; Marshall, R.J.; Newton, S.; Austin, P.; Taylor, S.; Chew, T.C.; Gavaghan, S.; Roberts, S.A. Screening for Mycobacterium tuberculosis infection among healthcare workers in New Zealand: Prospective comparison between the tuberculin skin test and the QuantiFERON-TB Gold In-Tube assay. N. Z. Med. J. 2012, 125, 21–29. [Google Scholar] [PubMed]
- Harada, N.; Nakajima, Y.; Higuchi, K.; Sekiya, Y.; Rothel, J.; Mori, T. Screening for tuberculosis infection using whole-blood interferon-gamma and Mantoux testing among Japanese healthcare workers. Infect. Control Hosp. Epidemiol. 2006, 27, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Ogura, T.; Nishii, K.; Kodani, T.; Onishi, M.; Shimizu, Y.; Kanehiro, A.; Kiura, K.; Tanimoto, M.; Tobe, K. Whole blood interferon-gamma assay for baseline tuberculosis screening among Japanese healthcare students. PLoS ONE 2007, 2, e803. [Google Scholar] [CrossRef] [PubMed]
- Adachi, E.; Kogayu, M.; Fujii, T.; Mae, H.; Shimizu, S.; Iwai, Y.; Shibata, H.; Suzuki, M.; Imai, K.; Koibuchi, T. Tuberculosis examination using whole blood interferon-gamma release assay among health care workers in a Japanese hospital without tuberculosis-specific wards. Springerplus 2013, 2, 440. [Google Scholar] [CrossRef]
- Ogiwara, T.; Kimura, T.; Tokue, Y.; Watanabe, R.; Nara, M.; Obuchi, T.; Yaegashi, A.; Yomoda, S.; Ohshima, K.; Murakami, M. Tuberculosis screening using a T-cell interferon-gamma release assay in Japanese medical students and non-Japanese international students. Tohoku J. Exp. Med. 2013, 230, 87–91. [Google Scholar] [CrossRef]
- Uto, T.; Yasuda, K.; Sagisaka, S.; Sato, J.; Imokawa, S.; Uemura, N.; Suda, T.; Chida, K. Serial QuantiFERON TB-2G testing over a four-year period in healthcare workers at a city hospital. Intern. Med. 2014, 53, 1119–1124. [Google Scholar] [CrossRef]
- Mukai, S.; Shigemura, K.; Yamamichi, F.; Kitagawa, K.; Takami, N.; Nomi, M.; Arakawa, S.; Fujisawa, M. Comparison of cost-effectiveness between the quantiFERON-TB Gold-In-Tube and T-Spot tests for screening health-care workers for latent tuberculosis infection. Int. J. Mycobacteriol. 2017, 6, 83–86. [Google Scholar] [CrossRef]
- Tanabe, M.; Nakamura, A.; Arai, A.; Yamasaki, D.; Hirano, K.; Kobayashi, T.; Taguchi, O.; Kaneko, T.; Ito, M. The Direct Comparison of Two Interferon-gamma Release Assays in the Tuberculosis Screening of Japanese Healthcare Workers. Intern. Med. 2017, 56, 773–779. [Google Scholar] [CrossRef] [PubMed]
- El-Helaly, M.; Khan, W.; El-Saed, A.; Balkhy, H.H. Pre-employment screening of latent tuberculosis infection among healthcare workers using tuberculin skin test and QuantiFERON-TB Gold test at a tertiary care hospital in Saudi Arabia. J. Infect. Public Health 2014, 7, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.I.; Diab, A.E. Detection of latent tuberculosis infection among laboratory personnel at a University Hospital in Eastern Saudi Arabia using an interferon gamma release assay. J. Infect. Public Health 2014, 7, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Al Hajoj, S.; Varghese, B.; Datijan, A.; Shoukri, M.; Alzahrani, A.; Alkhenizan, A.; AlSaif, A.; Althawadi, S.; Fernandez, G.; Alrajhi, A. Interferon Gamma Release Assay versus Tuberculin Skin Testing among Healthcare Workers of Highly Diverse Origin in a Moderate Tuberculosis Burden Country. PLoS ONE 2016, 11, e0154803. [Google Scholar] [CrossRef] [PubMed]
- Bukhary, Z.A.; Amer, S.M.; Emara, M.M.; Abdalla, M.E.; Ali, S.A. Screening of latent tuberculosis infection among health care workers working in Hajj pilgrimage area in Saudi Arabia, using interferon gamma release assay and tuberculin skin test. Ann. Saudi Med. 2018, 38, 90–96. [Google Scholar] [CrossRef]
- El-Sokkary, R.H.; Abu-Taleb, A.M.; El-Seifi, O.S.; Zidan, H.E.; Mortada, E.M.; El-Hossary, D.; Farag, S.E. Assessing the Prevalence of Latent Tuberculosis among Health Care Providers in Zagazig City, Egypt Using Tuberculin Skin Test and QuantiFERON-TB Gold In-Tube Test. Cent. Eur. J. Public Health 2015, 23, 324–330. [Google Scholar] [CrossRef]
- Hefzy, E.M.; Wegdan, A.A.; Elhefny, R.A.; Nasser, S.H. Predictors of low prevalence of latent tuberculosis infection among Egyptian health care workers at intensive care and bronchoscopy units. GMS Hyg. Infect. Control 2016, 11, Doc22. [Google Scholar] [CrossRef]
- Talebi-Taher, M.; Javad-Moosavi, S.A.; Entezari, A.H.; Shekarabi, M.; Parhizkar, B. Comparing the performance of QuantiFERON-TB Gold and Mantoux test in detecting latent tuberculosis infection among Iranian health care workers. Int. J. Occup. Med. Environ. Health 2011, 24, 359–366. [Google Scholar] [CrossRef]
- Salmanzadeh, S.; Abbasissifar, H.; Alavi, S.M. Comparison study of QuantiFERON test with tuberculin skin testing to diagnose latent tuberculosis infection among nurses working in teaching hospitals of Ahvaz, Iran. Casp. J. Intern. Med. 2016, 7, 82–87. [Google Scholar]
- Mostafavi, E.; Nasehi, M.; Hashemi Shahraki, A.; Esmaeili, S.; Ghaderi, E.; Sharafi, S.; Doosti-Irani, A. Comparison of the tuberculin skin test and the QuantiFERON-TB Gold test in detecting latent tuberculosis in health care workers in Iran. Epidemiol. Health 2016, 38, e2016032. [Google Scholar] [CrossRef][Green Version]
- Keshavarz Valian, S.; Mahmoudi, S.; Pourakbari, B.; Abdolsalehi, M.R.; Eshaghi, H.; Mamishi, S. Screening of healthcare workers for latent tuberculosis infection in the low tuberculosis burden country: QuantiFERON-TB gold in tube test or tuberculin skin test? Arch. Environ. Occup. Health 2019, 74, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Guanche Garcell, H.; Crespo Ramirez, E.; Kindelan Contreras, A.; Gutierrez Garcia, F. Latent tuberculosis infection in healthcare workers at a community hospital in Qatar. J. Infect. Public Health 2014, 7, 356–359. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baussano, I.; Nunn, P.; Williams, B.; Pivetta, E.; Bugiani, M.; Scano, F. Tuberculosis among health care workers. Emerg. Infect. Dis. 2011, 17, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Schablon, A.; Harling, M.; Diel, R.; Nienhaus, A. Risk of latent TB infection in individuals employed in the healthcare sector in Germany: A multicentre prevalence study. BMC Infect. Dis. 2010, 10, 107. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).