The Potential Health Risk Associated with Edible Vegetables Grown on Cr(VI) Polluted Soils
Abstract
1. Introduction
2. Materials
2.1. Equipment
2.2. Soil Sampling
2.3. Sampling and Preparation of Seeds
2.4. Preparation of Stock Solution
2.5. Experimental Design in the Greenhouse
2.6. Estimation/Observation of Germination and Growth Pattern
2.7. Germination Percentage and Growth Height
2.8. Determination of Total Chromium in Soils and Plants
2.9. Determination of Cr(VI) in Soils and Plants
3. Results and Discussion
3.1. General Properties of Soil
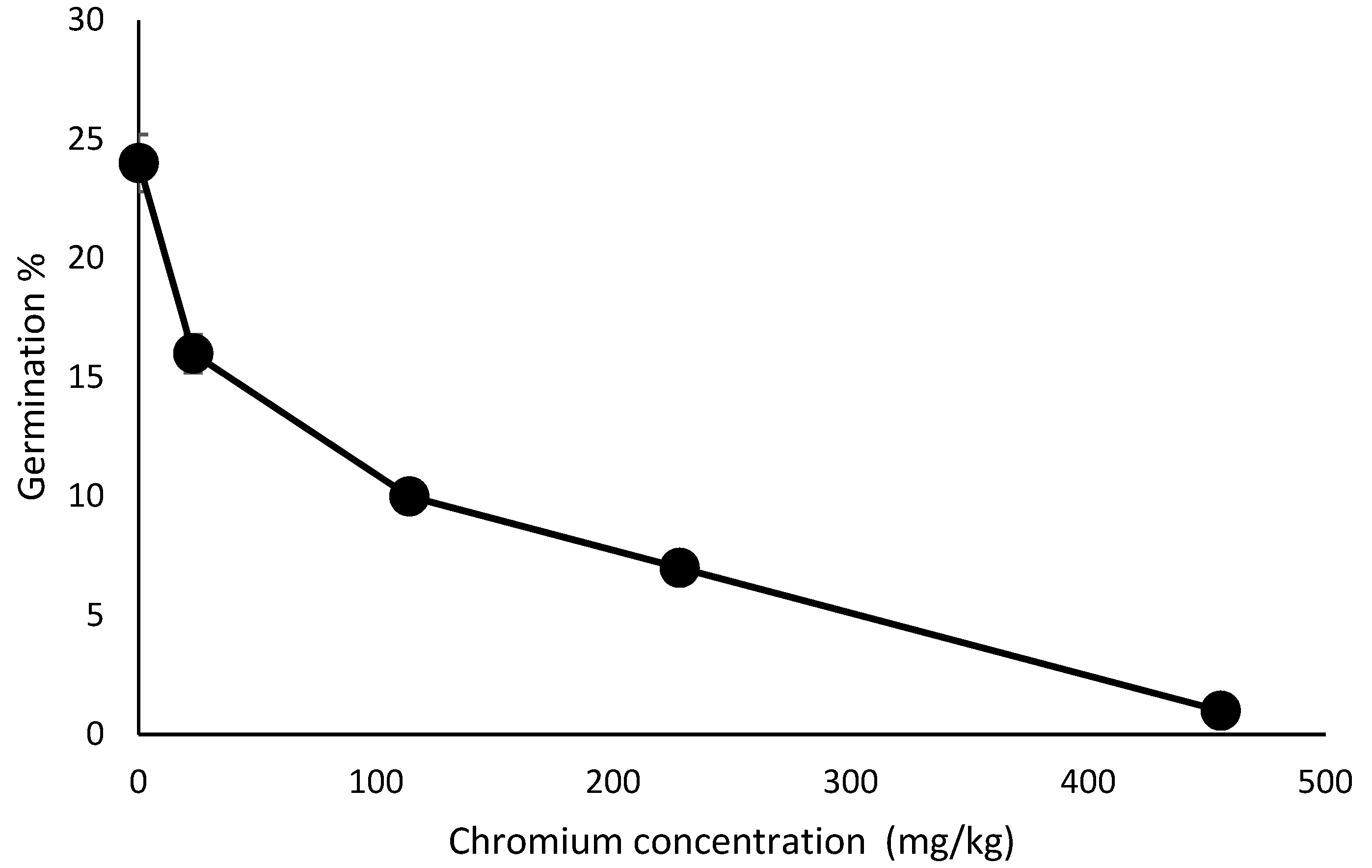
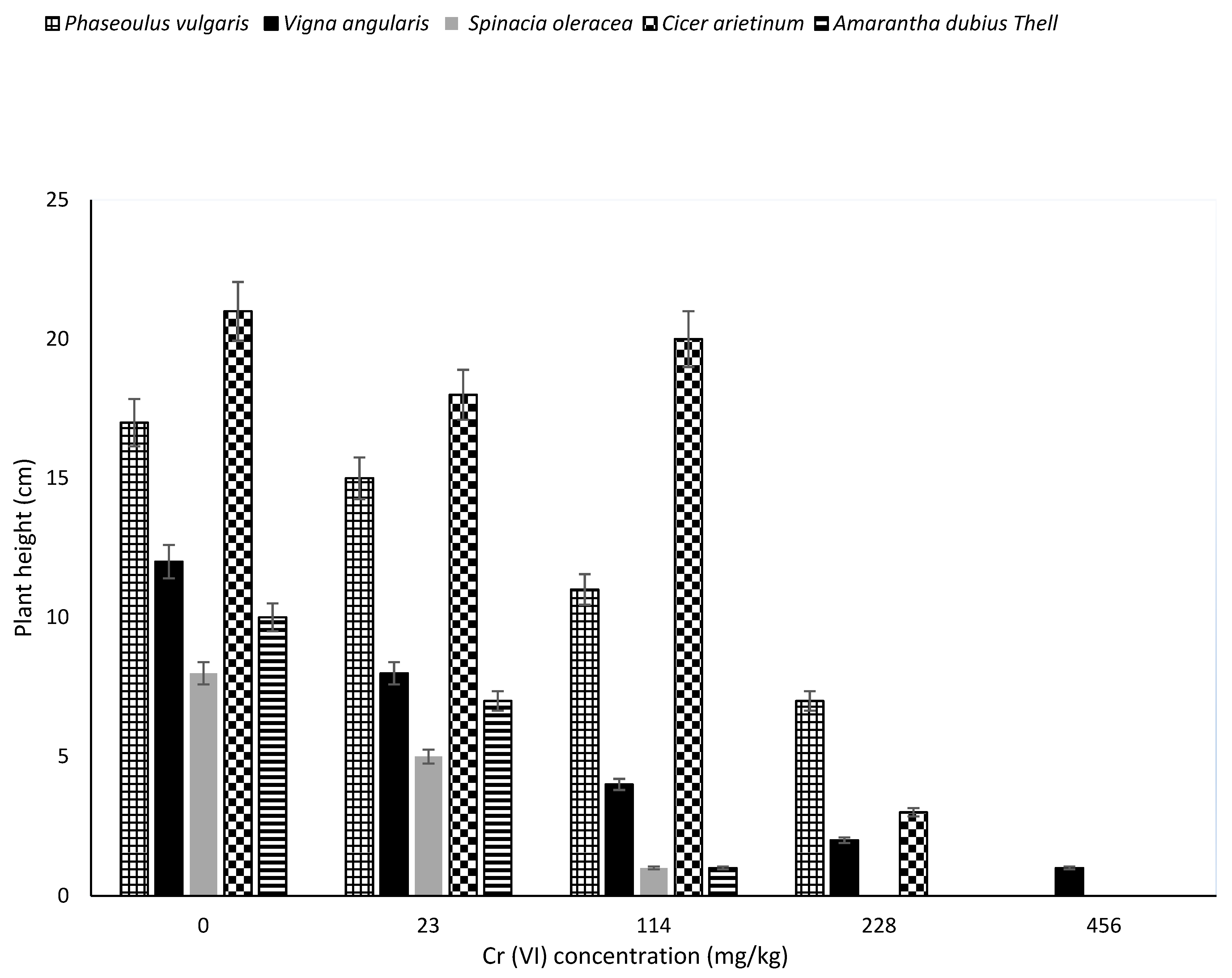
3.2. Effect of Chromium Concentration on Seed Germination and Growth
3.3. Bioaccumulation/Bioconcentration Factor (BF/BCF)
3.4. Translocation Factors
3.5. Daily Intake of Chromium (ChT, Cr(VI), Cr(III)) through Edible Vegetables Grown on Cr(VI) Spiked Soils
3.6. Hazard Quotient
3.7. Hazard Index
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Andrade, C.; Andreazza, R.; Oliveira, F. Cultivation of sorghum and sunflower in soils with amendment of sludge from industrial landfill. Int. J. Recycl. Org. Waste Agric. 2019, 8, 119–130. [Google Scholar] [CrossRef]
- Ullah, R.; Shivakoti, G.P.; Zulfiqar, F.; Kamran, M.A. Farm risks and uncertainties: Sources, impacts and management. Outlook Agric. 2016, 45, 199–205. [Google Scholar] [CrossRef]
- Olafisoye, O.; Adefioye, T.; Osibote, O. Heavy metals contamination of water, soil, and plants around an electronic waste dumpsite Oladunni. Pol. J. Environ. Stud. 2013, 22, 1431–1439. [Google Scholar]
- Faber, M.; Oelofse, A.; Van Jaarsveld, P.; Wenhold, F.; Jansen van Rensburg, W. African leafy vegetables consumed by households in the Limpopo and KwaZulu-Natal provinces in South Africa. S. Afr. J. Clin. Nutr. 2010, 23, 30–38. [Google Scholar] [CrossRef]
- Islam, G.; Khan, F.; Hoque, M.M.; Jolly, Y. Consumption of unsafe food in the adjacent area of Hazaribag tannery campus and Buriganga River embankments of Bangladesh: Heavy metal contamination. Environ. Monit. Assess. 2014, 186, 7233–7244. [Google Scholar] [CrossRef]
- Magaji, J. Effects of waste dump on the quality of plants cultivated around Mpape dumpsite FCT Abuja, Nigeria. Ethiop. J. Environ. Stud. Manag. 2012, 5, 567–573. [Google Scholar] [CrossRef]
- Xaypanya, P.; Takemura, J.; Chiemchaisri, C.; Seingheng, H.; Tanchuling, M. Characterization of landfill leachates and sediments in major cities of Indochina peninsular countries—Heavy metal partitioning in municipal solid waste leachate. Environments 2018, 5, 65. [Google Scholar] [CrossRef]
- Gangwar, K. Chromium uptake efficiency of Spinacea olaracea from contaminated soil. J. Appl. Sci. Environ. Manag. 2009, 13, 71–72. [Google Scholar]
- Singh, H.; Mahajan, P.; Kaur, S.; Batish, D.; Kohli, R. Environmental chemistry letters, chromium toxicity and tolerance in plants. Environ. Chem. Lett. 2013, 11, 229–254. [Google Scholar] [CrossRef]
- Jun, R.; Ling, T.; Guanghua, Z. Effects of chromium on seed germination, root elongation and coleoptile growth in six pulses. Int. J. Environ. Sci. Technol. 2009, 6, 571–578. [Google Scholar] [CrossRef][Green Version]
- Hayat, S.; Khalique, G.; Irfan, M.; Wani, A.S.; Tripathi, B.N.; Ahmad, A. Physiological changes induced by chromium stress in plants: An overview. Protoplasma 2012, 249, 599–611. [Google Scholar] [CrossRef]
- Soundari, M.; Sundaramoorthy, P. Effect of various concentrations of chromium polluted soil on biochemical changes of Globe amaranth (Gomphrena globosa L.). J. Appl. Adv. Res. 2017, 2, 270–281. [Google Scholar] [CrossRef][Green Version]
- Gupta, N.; Yadav, K.K.; Kumar, V.; Kumar, S.; Chadd, R.P.; Kumar, A. Trace elements in soil-vegetables interface: Translocation, bioaccumulation, toxicity and amelioration—A. review. Sci. Total Environ. 2019, 651, 2927–2942. [Google Scholar] [CrossRef] [PubMed]
- Sundarmoorthy, P.; Sankarganesh, K.; Selvaraj, M.; Baskaran, L. Chromium induced changes in Soybean (Glycine max L.) metabolism. World Sci. News 2015, 16, 145–178. [Google Scholar]
- Amin, H.; Arain, F.; Surhio, M. Phytotoxicity of chromium on germination, growth and biochemical attributes of Hibiscus esculentus L. Am. J. Plant. Sci. 2013, 4, 2431–2439. [Google Scholar] [CrossRef]
- Fernandez, M.L.V.; Calouro, F.; Abreu, M.M. Application of chromium to soils at different rates and oxidation states. I. Effect on dry matter yield and chromium uptake by Radish. Commun. Soil Sci. Plant Anal. 2002, 33, 2259–2268. [Google Scholar] [CrossRef]
- Bahira, S.; Behera, S.; Puhan, P. Remarking an analisation phytoremediation of chromium by chickpea (Ciecer arietinum L.) and it’s toxic effect. Remarking Anal. 2018, 3, 80–85. [Google Scholar]
- Wilkins, D. The measurement of tolerance to edaphic factors by means of root growth. New Phytol. 1978, 80, 623–633. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). Microwave Assisted Acid Digestion of Siliceous and Organically based Matrices; Environmental Protection Agency (EPA): Washington, DC, USA, 1996; pp. 1–20.
- Lesniewska, B.; Gontarska, M. Selective separation of chromium species from soils by single-step extraction methods: A critical appraisal. Water Air Soil Pollut. 2017, 228, 274. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, R.; Nunes, A.; de Lima, A.; de Melo, W.; Antunes, L.; De Araujo, A. Chromium accumulation in maize and cowpea after successive applications of composted tannery sludge. Acta Sci. 2018, 40, 1–7. [Google Scholar] [CrossRef]
- Carbonell, G.; Miralles, R.; Imperial, D.; Torrijos, M.; Delgado, M.; Antonio, R. Effects of municipal solid waste compost and mineral fertilizer amendments on soil properties and heavy metals distribution in maize plants (Zea mays L.). Chemosphere 2011, 85, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.; Favas, P.; Pratas, J.; Varun, M.; Paul, M. Metal (loid) induced toxicity and defense mechanisms in Spinacia oleracea L: Ecological hazard and prospects for phytoremediation. Ecotoxicol. Environ. Saf. 2019, 183, 109570. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review chemosphere chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef]
- Edokpayi, J.N.; Enitan, A.M.; Mutileni, N.; Odiyo, J.O. Evaluation of water quality and human risk assessment due to heavy metals in groundwater around Muledane area of Vhembe District, Limpopo Province, South Africa. Chem. Cent. J. 2018, 12, 2. [Google Scholar] [CrossRef]
- Bose, S.; Bhattacharyya, A. Heavy metal accumulation in wheat plant grown in soil amended with industrial sludge. Chemosphere 2008, 70, 1264–1272. [Google Scholar] [CrossRef]
- Banks, M.; Robert, J.; Eivazi, F.; Peter, P.; Kelly, A. Effects of selected surfactants on nutrient uptake in Corn (Zea mays L.). J. Plant. Nutr. 2015, 38, 1036–1049. [Google Scholar] [CrossRef]
- Eze, C.; Eze, O.; Orjiakor, E.; Enemuor, S.; Okobo, U. Chromium (III) and its effects on soil microbial activities and phytoremediation potentials of Arachis hypogea and Vigna unguiculate. Afr. J. Biotechnol. 2018, 17, 1207–1214. [Google Scholar]
- Bluskov, S.; Arocena, J.M.; Omotoso, O.O.; Young, J.P. Uptake, distribution, and speciation of chromium in brassica juncea. Int. J. Phytoremediat. 2005, 7, 153–165. [Google Scholar] [CrossRef]
- Kotas, J.; Stasicka, Z. Chromium occurrence in the environment and methods of its speciation. Environ. Pollut. 2000, 107, 263–283. [Google Scholar] [CrossRef]
- Kaszycki, P.; Gabrys, H.; Appenroth, K.J.; Jaglarz, A.; Sedziwy, S.; Walczak, T. Exogenously applied sulphate as a tool to investigate transport and reduction of chromate in the duckweed Spirodela polyrhiza. Plant Cell Environ. 2005, 28, 260–268. [Google Scholar] [CrossRef]
- Appenroth, K.J.; Luther, A.; Jetschke, G.; Gabrys, H. Modification of chromate toxicity by sulphate in duckweeds (Lemnaceae). Aquat. Toxicol. 2008, 89, 167–171. [Google Scholar] [CrossRef]
- Akinci, I.; Akinci, S. Effect of chromium toxicity on germination and early seedling growth in melon (Cucumis melo L.). Afr. J. Biotechnol. 2010, 9, 4589–4594. [Google Scholar]
- Augustynowicz, J.; Wróbel, P.; Płachno, B.J.; Tylko, G.; Gajewski, Z.; Węgrzynek, D. Chromium distribution in shoots of macrophyte Callitriche cophocarpa Sendtn. Planta 2014, 239, 1233–1242. [Google Scholar] [CrossRef]
- Kidd, P.; Mart, C. Tolerance and bioaccumulation of heavy metals in five populations of Cistus ladanifer L. subsp. ladanifer. Plant Soil 2004, 258, 189–205. [Google Scholar] [CrossRef]
- Peralta, J.R.; Tiemann, K.J.; Gomez, E.; Arteaga, S.; Rascon, E.; Parsons, J.G. Uptake and effects of five heavy metals on seed germination and plant growth in Alfalfa (Medicago sativa L.). Bull. Environ. Contam. Toxicol. 2001, 66, 727–734. [Google Scholar] [CrossRef]
- Sharma, A.; Brar, M.; Malhi, S. Critical toxic ranges of chromium in spinach plants and in soil. J. Plant. Nutr. 2005, 28, 1555–1568. [Google Scholar] [CrossRef]
- Fitz, W.; Wenzel, W. Fundamentals and potential application to phytoremediation. Biotechnology 2002, 99, 259–278. [Google Scholar]
- Huang, M.; Zhou, S.; Sun, B.; Zhao, Q. Heavy metals in wheat grain: Assessment of potential health risk for inhabitants in Kunshan, China. Sci. Total Environ. 2008, 405, 54–61. [Google Scholar] [CrossRef]
- Oliveira, H. Chromium as an environmental pollutant: Insights on induced plant toxicity. J. Bot. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Malayeri, B.; Abdolkarim, C.; Yousefi, N.; Lorestani, B. Identification of the hyper accumulator plants in copper and iron mine in Iran. Pak. J. Biol. Sci. 2008, 11, 490–492. [Google Scholar] [CrossRef]
- Chandra, R.; Abdussalam, A.K.; Salim, N. Distribution of bio-accumulated Cd and Cr in two Vigna species and the associated histological variations. Stress Physiol. Biochem. 2010, 6, 4–12. [Google Scholar]
- Rashed, M.N. Monitoring of contaminated toxic and heavy metals, from mine tailings through age accumulation, in soil and some wild plants at southeast Egypt. J. Hazard. Mater. 2010, 178, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Kacholi, D. Levels and health risk assessment of heavy metals in soil, water, and vegetables of Dar es Salaam, Tanzania. J. Chem. 2018, 2018, 1402674. [Google Scholar] [CrossRef]
- Khan, I.; Diwan, H.; Ahmad, A. Chromium toxicity and tolerance in crop plants. In Crop Improvement under Adverse Conditions; Narendra, T., Sarvajeet, S., Eds.; Springer: New York, NY, USA, 2013; pp. 309–332. [Google Scholar]
- Cui, X.; Li, S.; Zhang, S.; Fan, Y.; Ma, L. Toxic metals in children’s toys and jewelry: Coupling bioaccessibility with risk assessment. Environ. Pollut. 2015, 200, 77–84. [Google Scholar] [CrossRef]
- Oliveira, J.; de Araujo, F.; de Melo, W. Chromium in soil organic matter and cowpea after four consecutive annual applications of composted tannery sludge. Rev. Bras. Ciência Solo 2015, 39, 297–302. [Google Scholar] [CrossRef][Green Version]
- Zojaji, F.; Amir, H.; Mohammad, H. Bioaccumulation of chromium by Zea mays in wastewater-irrigated soil: An experimental study. Proc. Int. Acad. Ecol. Environ. Sci. 2014, 4, 62–67. [Google Scholar]
- Food and Agriculture Organization (FAO)/World Health Organization WHO. Food and Agriculture Organization; Food and Agriculture Organization (FAO): Rome, Italy; World Health Organization (WHO): Geneva, Switzerland, 1999. [Google Scholar]
- World Health Organization (WHO). Guidelines for the Safe Use of Wastewater and Excreta in Agriculture and Aquaculture Measures for Public Health Protection Prepared by Macmillan; World Health Organization (WHO): Geneva, Switzerland, 1989; pp. 2–207. [Google Scholar]
- Ogwu, M.; Osawaru, M.; Aiwansoba, R.; Iroh, R. Status and prospects of vegetables in Africa. Jt. Biodivers. Conf. 2016, 2016, 47–57. [Google Scholar]
- Toxicology, D.; Medicine, E. Chromium (Cr) toxicity|ATSDR—CSEM. In Chromium Toxicity; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2011; pp. 1–67. [Google Scholar]
- Zhou, H.; Yang, W.T.; Zhou, X.; Liu, L.; Gu, J.F.; Wang, W.L.; Zou, J.L.; Tian, T.; Peng, P.Q.; Liao, B.H. Accumulation of heavy metals in vegetable species planted in contaminated soils and the health risk assessment. Int. J. Environ. Res. Public Health 2016, 13, 289–301. [Google Scholar] [CrossRef]



| Soil Properties | Average Values |
|---|---|
| pH | 6.20 ± 0.05 |
| EC (µS/cm) | 42.80 ± 0.01 |
| Total organic carbon (%) | 0.98 ± 0.01 |
| Moisture (%) | 10.90 ± 0.15 |
| Sand (%) | 25 |
| Silt (%) | 15 |
| Clay loam (%) | 60 |
| Texture Class | Clay |
| ChT in soil (mg/kg) | 1.20 ± 0.03 |
| Temperature (°C) | Relative Humidity (g/m3) | Humidity (g/m3) | Atmospheric Humidity (g/m3) | Dewpoint Temperature (°C) | Radiation (J/cm2) |
|---|---|---|---|---|---|
| 17.2 ± 4.3 | 74.8 ± 62.9 | 4.7 ± 1.5 | 10.6 ± 1.8 | 5.4 ± 3.0 | 35,274 ± 433 |
| Name of Plant | Portion of Plant | Chromium Oxidation States (mg/kg) | P > |t| (Tukey Effect) | |||||
|---|---|---|---|---|---|---|---|---|
| ChT | Control | Cr(VI) | Control | Cr(III) | Control | |||
| Phaseoulus vulgaris | root | 2.80 ± 0.30 | 0.2 | 0.70 ± 0.03 | ND | 2.10 ± 0.30 | 0.2 | 0.000 |
| stem | 0.10 ± 0.07 | 0.20 ± 0.01 | ND | ND | 1.20 ± 0.80 | 0.20 ± 0.01 | ||
| leaf | 1.0 | 0.2 | 0.10 ± 0.03 | ND | 1.00 ± 0.03 | 0.2 | ||
| Vigna angularis | root | 3.40 ± 0.60 | 0.2 | 0.90 ± 0.04 | ND | 2.50 ± 0.60 | 0.2 | 0.000 |
| stem | 1.0 | 0.2 | 0.10 ± 0.02 | ND | 1.10 ± 0.02 | 0.2 | ||
| leaf | 1.80 ± 0.30 | 0.2 | 0.20 ± 0.03 | ND | 1.60 ± 0.30 | 0.2 | ||
| Spinacia oleracea | root | 1.10 ± 0.03 | 0.1 | 0.10 ± 0.03 | ND | 1.00 ± 0.03 | 0.1 | 0.000 |
| stem | ND | ND | ND | ND | ND | ND | ||
| leaf | 2.1 | ND | ND | ND | 2.1 | ND | ||
| Cicer arietinum | root | 3.50 ± 0.50 | 0.30 ± 0.01 | 0.8 | ND | 2.90 ± 0.05 | 0.30 ± 0.01 | 0.000 |
| stem | 1.0 | 0.20 ± 0.03 | ND | ND | 1.10 ± 0.01 | 0.20 ± 0.03 | ||
| leaf | 2.10 ± 0.20 | 0.30 ± 0.03 | 0.20 ± 0.10 | ND | 2.00 ± 0.01 | 0.30 ± 0.03 | ||
| Amarantha dubuis Thell | root | 1.20 ± 0.01 | ND | 0.30 ± 0.03 | ND | 0.90 ± 0.03 | ND | 0.000 |
| stem | 0. 10 ± 0.02 | ND | ND | ND | 0.1 | ND | ||
| leaf | 1.20 ± 0.03 | 0. 09 ± 0.06 | 0.20 ± 0.01 | ND | 1.00 ± 0.02 | 0.10 ± 0.07 | ||
| Chrome simulated soil | Soil | 4.9 | 1.20 ± 0.03 | 1.80 ± 0.07 | ND | 3.10 ± 0.10 | 1.20 ± 0.03 | 0.000 |
| Name of Plant | Treatment | BF | TF | |
|---|---|---|---|---|
| Root | Stem | Leaf | ||
| Phaseoulus vulgaris | CrT | 0.8 | 0.05 | 0.3 |
| Cr(VI) | 0.4 | 0.01 | 0.2 | |
| Cr(III) | 0.4 | 0.04 | 0.1 | |
| Vigna angularis | CrT | 1.0 | 0.30 | 0.7 |
| Cr(VI) | 0.5 | 0.20 | 0.3 | |
| Cr(III) | 0.5 | 0.10 | 0.4 | |
| Spinacia oleracea | CrT | 0.3 | 0.02 | 0.2 |
| Cr(VI) | 0.1 | - | 0.01 | |
| Cr(III) | 0.2 | 0.01 | 0.2 | |
| Cicer arietinum | CrT | 1.0 | 0.30 | 0.7 |
| Cr(VI) | 0.4 | 0.01 | 0.4 | |
| Cr(III) | 0.6 | 0.02 | 0.3 | |
| Amarantha dubuis Thell | CrT | 0.3 | 0.04 | 0.4 |
| Cr(VI) | 0.1 | 0.02 | 0.3 | |
| Cr(III) | 0.2 | 0.02 | 0.1 | |
| Plant Name | Total Cr | Cr(VI) | Cr(III) | |||
|---|---|---|---|---|---|---|
| Adult | Child | Adult | Child | Adult | Child | |
| P. vulgaris | 3.8 | 5.8 | 0.5 | 0.8 | 3.3 | 59 |
| V. angularis | 8.0 | 12.3 | 0.9 | 1.3 | 7.1 | 11 |
| S. oleracea | 7.9 | 12.2 | 0.04 | 0.06 | 7.9 | 12.1 |
| C. arietinum | 8.7 | 13.4 | 1.2 | 1.8 | 7.5 | 12 |
| A. dubuis (T) | 4.6 | 7.1 | 0.007 | 0.01 | 4.6 | 7.1 |
| HI | Phaseoulus vulgaris | Vigna angularis | Spinacia oleracea | Cicer arietinum | Amarantha dubuis |
|---|---|---|---|---|---|
| Adult | 7.6 | 16 | 15.8 | 17.4 | 9.2 |
| Child | 11.6 | 24.6 | 24.4 | 27.2 | 14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oruko Ongon’g, R.; Edokpayi, J.N.; Msagati, T.A.M.; Tavengwa, N.T.; Ijoma, G.N.; Odiyo, J.O. The Potential Health Risk Associated with Edible Vegetables Grown on Cr(VI) Polluted Soils. Int. J. Environ. Res. Public Health 2020, 17, 470. https://doi.org/10.3390/ijerph17020470
Oruko Ongon’g R, Edokpayi JN, Msagati TAM, Tavengwa NT, Ijoma GN, Odiyo JO. The Potential Health Risk Associated with Edible Vegetables Grown on Cr(VI) Polluted Soils. International Journal of Environmental Research and Public Health. 2020; 17(2):470. https://doi.org/10.3390/ijerph17020470
Chicago/Turabian StyleOruko Ongon’g, Richard, Joshua N. Edokpayi, Titus A. M. Msagati, Nikita T. Tavengwa, Grace N. Ijoma, and John O. Odiyo. 2020. "The Potential Health Risk Associated with Edible Vegetables Grown on Cr(VI) Polluted Soils" International Journal of Environmental Research and Public Health 17, no. 2: 470. https://doi.org/10.3390/ijerph17020470
APA StyleOruko Ongon’g, R., Edokpayi, J. N., Msagati, T. A. M., Tavengwa, N. T., Ijoma, G. N., & Odiyo, J. O. (2020). The Potential Health Risk Associated with Edible Vegetables Grown on Cr(VI) Polluted Soils. International Journal of Environmental Research and Public Health, 17(2), 470. https://doi.org/10.3390/ijerph17020470






