Analytical Bias in the Measurement of Plasma 25-Hydroxyvitamin D Concentrations in Infants
Abstract
1. Introduction
2. Materials and Methods
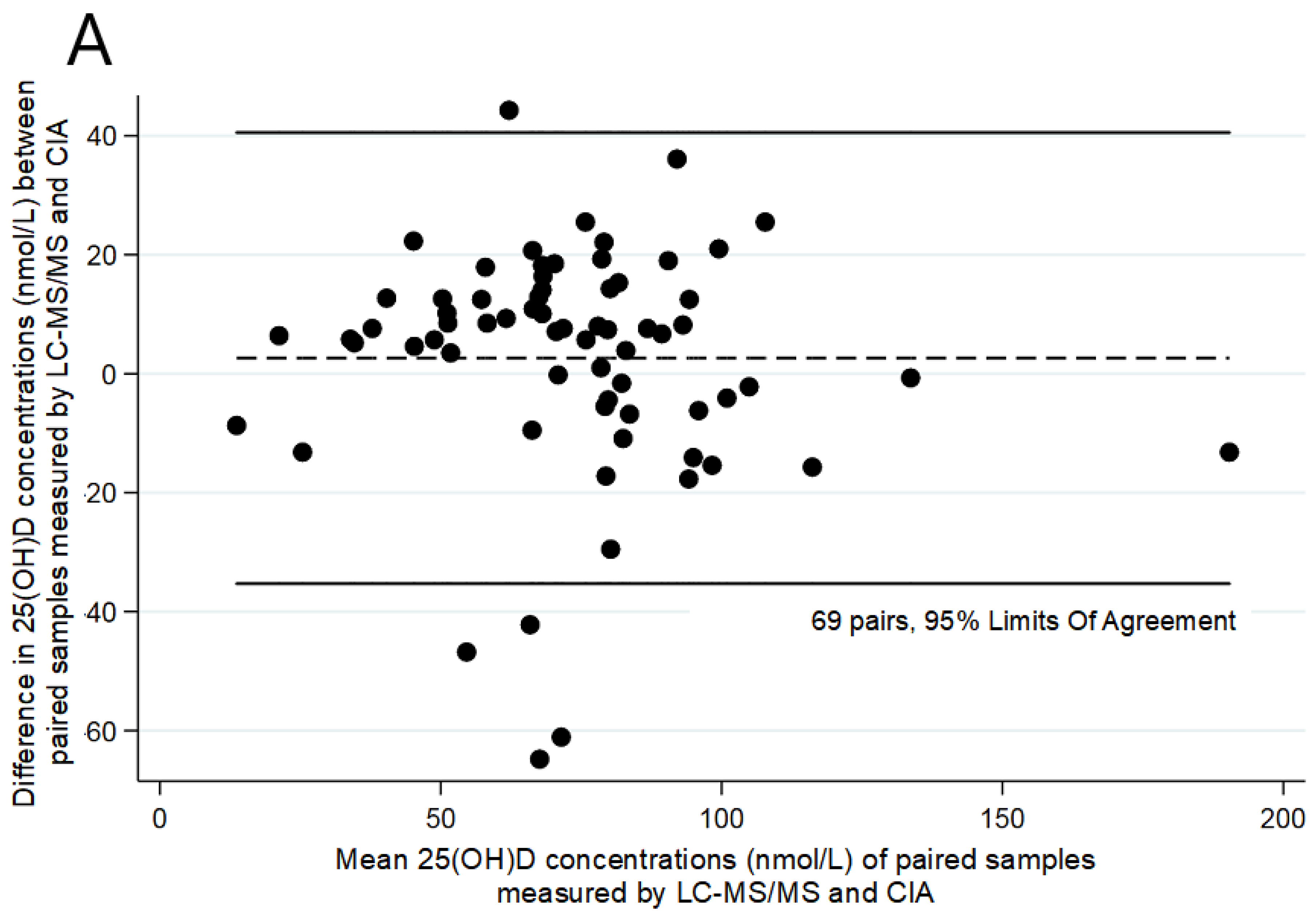
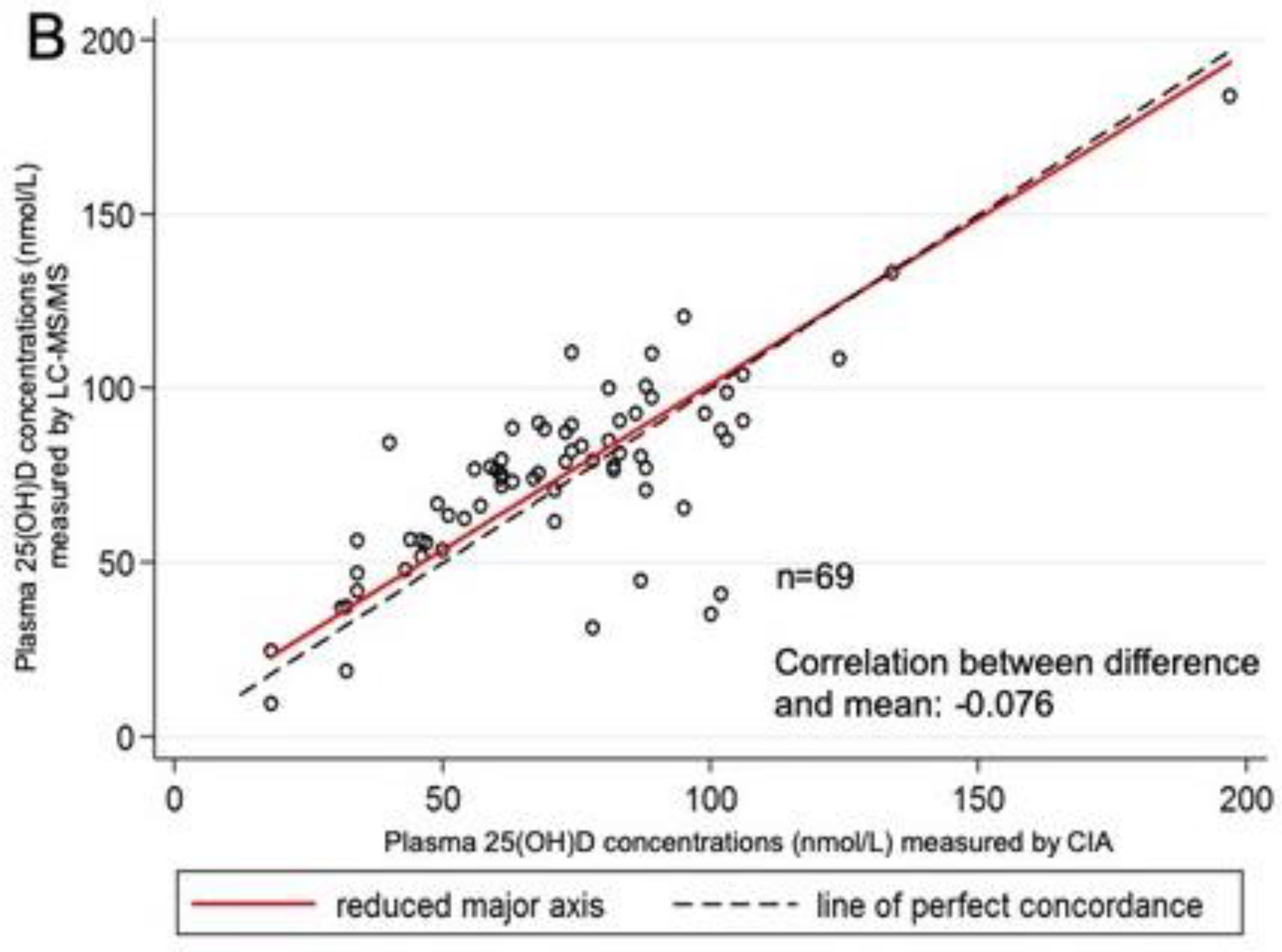
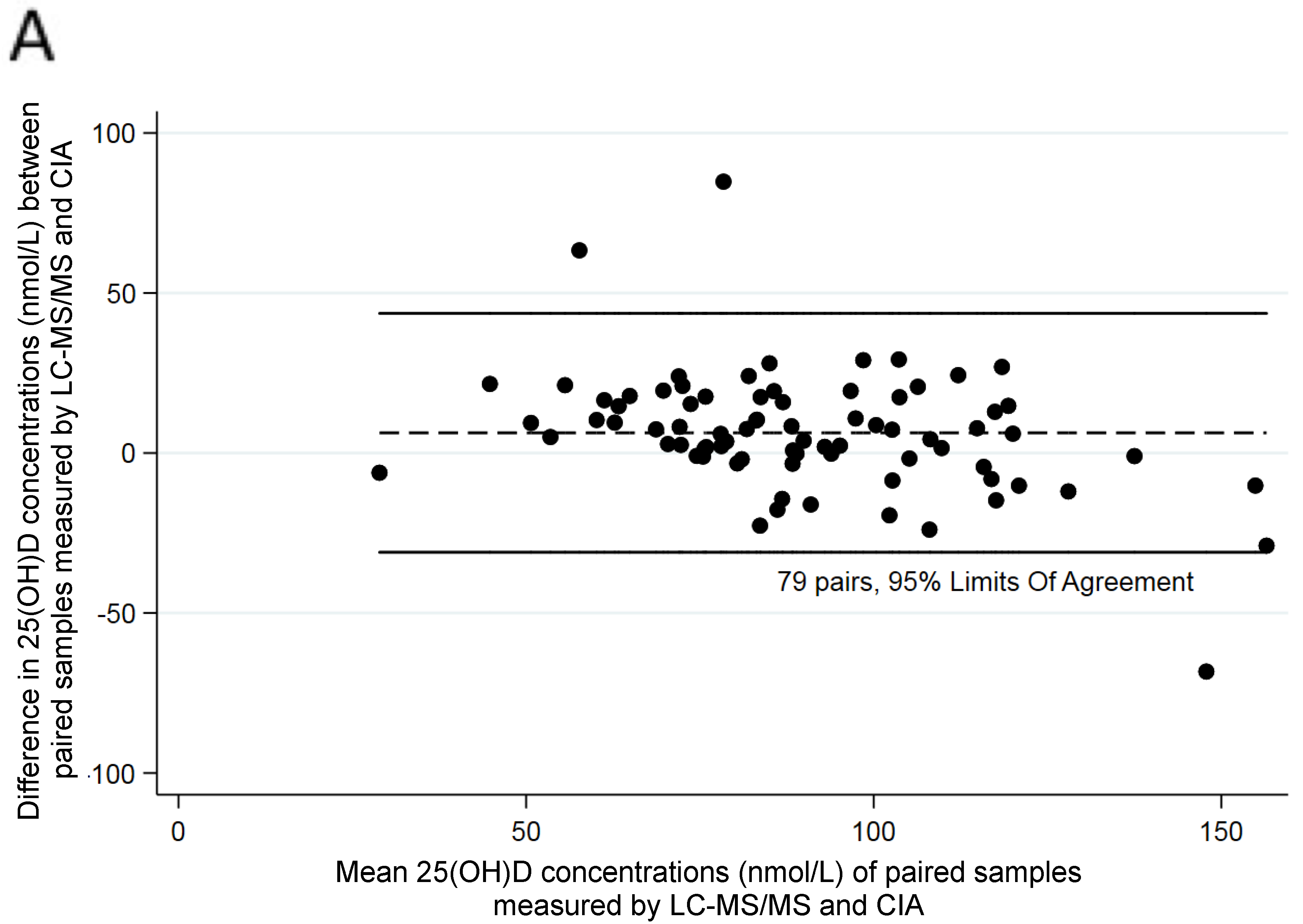
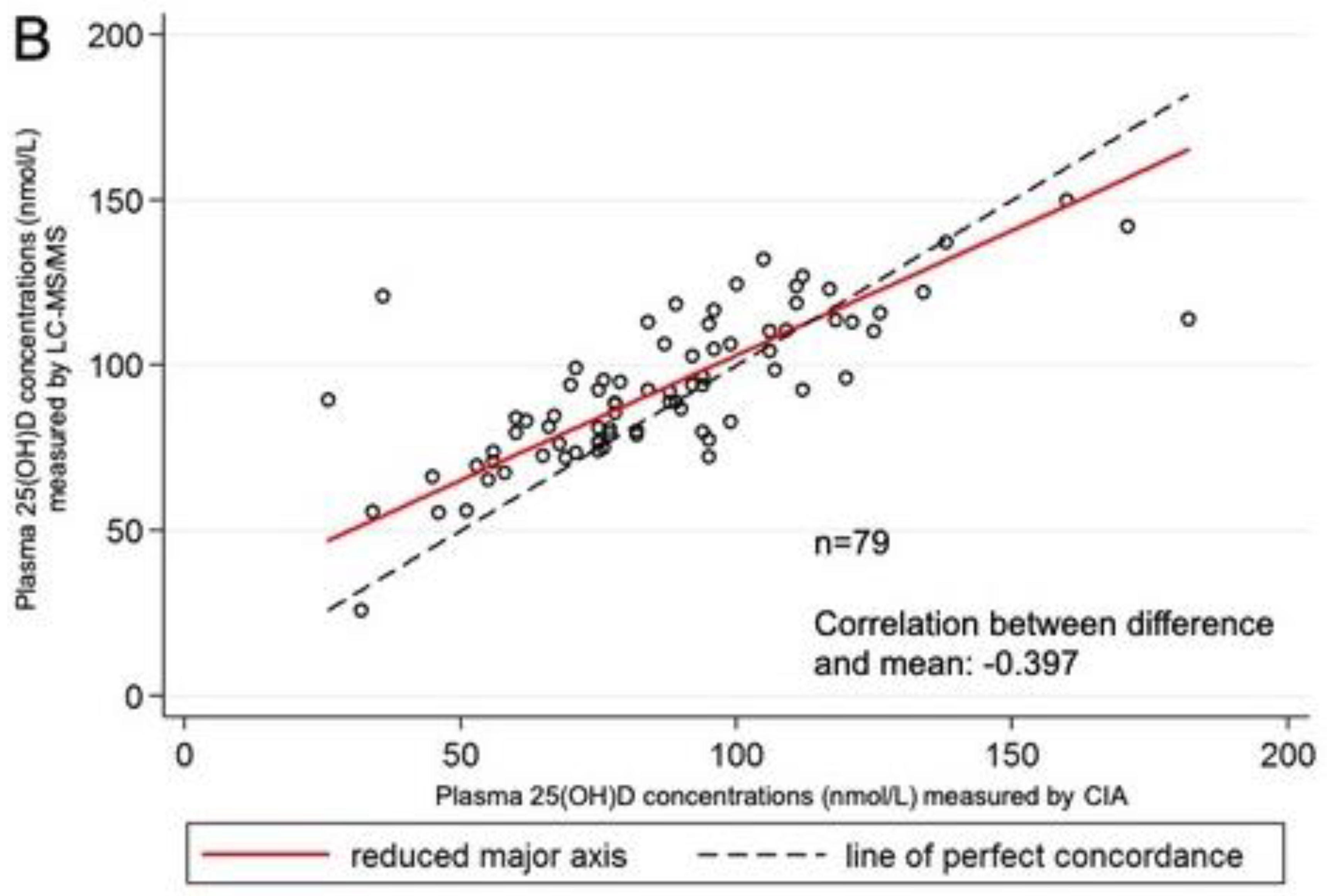
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jones, A.P.; Rueter, K.; Siafarikas, A.; Lim, E.M.; Prescott, S.L.; Palmer, D.J. 25-hydroxyvitamin D status of pregnant women is associated with the use of antenatal vitamin supplements and ambient ultraviolet radiation. J. Dev. Orig. Health Dis. 2016, 7, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Souberbielle, J.C.; Body, J.J.; Lappe, J.M.; Plebani, M.; Shoenfeld, Y.; Wang, T.J.; Bischoff-Ferrari, H.A.; Cavalier, E.; Ebeling, P.R.; Fardellone, P.; et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun. Rev. 2010, 9, 709–715. [Google Scholar] [CrossRef] [PubMed]
- von Hurst, P.R.; Stonehouse, W.; Coad, J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient—A randomised, placebo-controlled trial. Br. J. Nutr. 2010, 103, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Yousef, S.; Elliott, J.; Manuel, D.; Colman, I.; Papadimitropoulos, M.; Hossain, A.; Leclair, N.; Wells, G.A. Study protocol: Worldwide comparison of vitamin D status of immigrants from different ethnic origins and native-born populations—A systematic review and meta-analysis. Syst. Rev. 2019, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Rabenberg, M.; Scheidt-Nave, C.; Busch, M.; Thamm, M.; Rieckmann, N.; Durazo-Arvizu, R.; Dowling, K.; Škrabáková, Z.; Cashman, K.; Sempos, C.; et al. Implications of standardization of serum 25-hydroxyvitamin D data for the evaluation of vitamin D status in Germany, including a temporal analysis. BMC Public Health 2018, 18, 845. [Google Scholar] [CrossRef] [PubMed]
- De Rui, M.; Toffanello, E.D.; Veronese, N.; Zambon, S.; Bolzetta, F.; Sartori, L.; Musacchio, E.; Corti, M.C.; Baggio, G.; Crepaldi, G.; et al. Vitamin D deficiency and leisure time activities in the elderly: Are all pastimes the same? PLoS ONE 2014, 9, e94805. [Google Scholar] [CrossRef]
- Al-Othman, A.; Al-Musharaf, S.; Al-Daghri Nasser, M.; Krishnaswamy, S.; Yusuf Deqa, S.; Alkharfy Khalid, M.; Al-Saleh, Y.; Al-Attas Omar, S.; Alokail Majed, S.; Moharram, O.; et al. Effect of physical activity and sun exposure on vitamin D status of Saudi children and adolescents. BMC Pediatr. 2012, 12, 92. [Google Scholar] [CrossRef]
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of inflammatory and immune responses by vitamin D. J. Autoimmun. 2017, 85, 78–97. [Google Scholar] [CrossRef]
- Swannell, C. Supplement Vitamin D rather than test. MJA InSight 2016, 1, 1. [Google Scholar]
- Grant, W.B.; Cross, H.S.; Garland, C.F.; Gorham, E.D.; Moan, J.; Peterlik, M.; Porojnicu, A.C.; Reichrath, J.; Zittermann, A. Estimated benefit of increased vitamin D status in reducing the economic burden of disease in western Europe. Prog. Biophys. Mol. Biol. 2009, 99, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Farrell, C.J.; Herrmann, M. Determination of vitamin D and its metabolites. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.S.; Schoenmakers, I.; Bluck, L.J.; Ding, S.; Prentice, A. Plasma appearance and disappearance of an oral dose of 25-hydroxyvitamin D2 in healthy adults. Br. J. Nutr. 2012, 107, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.; Veljkovic, K.; Yazdanpanah, M.; Adeli, K. Analytical measurement and clinical relevance of vitamin D3 C3-epimer. Clin. Biochem. 2013, 46, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, M.; Bailey, D.; Walsh, W.; Wan, B.; Adeli, K. Analytical measurement of serum 25-OH-vitamin D3, 25-OH-vitamin D2 and their C3-epimers by LC-MS/MS in infant and pediatric specimens. Clin. Biochem. 2013, 46, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.J.; Taylor, R.L.; Reddy, G.S.; Grebe, S.K.G. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J. Clin. Endocrinol. Metab. 2006, 91, 3055–3061. [Google Scholar] [CrossRef] [PubMed]
- Sempos, C.T.; Vesper, H.W.; Phinney, K.W.; Thienpont, L.M.; Coates, P.M.; Vitamin, D.S.P. Vitamin D status as an international issue: National surveys and the problem of standardization. Scand. J. Clin. Lab. Invest 2012, 243, 32–40. [Google Scholar]
- Mineva, E.M.; Schleicher, R.L.; Chaudhary-Webb, M.; Maw, K.L.; Botelho, J.C.; Vesper, H.W.; Pfeiffer, C.M. A candidate reference measurement procedure for quantifying serum concentrations of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 5615–5624. [Google Scholar] [CrossRef]
- Black, L.J.; Anderson, D.; Clarke, M.W.; Ponsonby, A.L.; Lucas, R.M. Ausimmune Investigator Group. Analytical bias in the measurement of serum 25-hydroxyvitamin D concentrations impairs assessment of vitamin D status in clinical and research settings. PLoS ONE 2015, 10, e0135478. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 Dietary Reference Intakes for Calcium and Vitamin D: What dietetics practitioners need to know. J. Am. Diet. Assoc. 2011, 111, 524–527. [Google Scholar] [CrossRef]
- Carter, G.D.; Berry, J.L.; Gunter, E.; Jones, G.; Jones, J.C.; Makin, H.L.; Sufi, S.; Wheeler, M.J. Proficiency testing of 25-hydroxyvitamin D (25-OHD) assays. J. Steroid Biochem. Mol. Biol. 2010, 121, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Kiely, M.; Kinsella, M.; Durazo-Arvizu, R.A.; Tian, L.; Zhang, Y.; Lucey, A.; Flynn, A.; Gibney, M.J.; Vesper, H.W.; et al. Evaluation of Vitamin D Standardization Program protocols for standardizing serum 25-hydroxyvitamin D data: A case study of the program’s potential for national nutrition and health surveys. Am. J. Clin. Nutr. 2013, 97, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Jones, G. Interpreting vitamin D assay results: Proceed with caution. Clin. J. Am. Soc. Nephrol. 2015, 10, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.J.; Dutton, J.; Fraser, W.D.; Jarvelin, M.R.; Hypponen, E. Harmonization study between LC-MS/MS and Diasorin RIA for measurement of 25-hydroxyvitamin D concentrations in a large population survey. J. Clin. Lab. Anal. 2017, 31, e22049. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.D.; Fudge, A.N.; Whiting, M.; Coates, P.S. The correlation between third-trimester maternal and newborn-serum 25-hydroxy-vitamin D in a selected South Australian group of newborn samples. BMJ Open 2011, 1, e000236. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Comeau, K.; Agellon, S.; Vanstone, C.; Sharma, A.; Jones, G.; L’Abbe, M.; Khamessan, A.; Weiler, H.; Rodd, C. Methodological issues in assessing plasma 25-hydroxyvitamin D concentration in newborn infants. Bone 2014, 61, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Moy, R.J.; McGee, E.; Debelle, G.D.; Mather, I.; Shaw, N.J. Successful public health action to reduce the incidence of symptomatic vitamin D deficiency. Arch. Dis. Child. 2012, 97, 952–954. [Google Scholar] [CrossRef]
- Berti, C.; Agostoni, C.; Davanzo, R.; Hypponen, E.; Isolauri, E.; Meltzer, H.M.; Steegers-Theunissen, R.P.; Cetin, I. Early-life nutritional exposures and lifelong health: Immediate and long-lasting impacts of probiotics, vitamin D., and breastfeeding. Nutr. Rev. 2017, 75, 83–97. [Google Scholar] [CrossRef]
- Moon, R.J.; Curtis, E.M.; Cooper, C.; Davies, J.H.; Harvey, N.C. Vitamin D supplementation: Are multivitamins sufficient? Arch. Dis. Child. 2019. [Google Scholar] [CrossRef]
- Rueter, K.; Jones, A.P.; Siafarikas, A.; Lim, E.M.; Bear, N.; Noakes, P.S.; Prescott, S.L.; Palmer, D.J. Direct infant UV light exposure is associated with eczema and immune development. J. Allergy Clin. Immunol. 2019, 143, 1012–1020. [Google Scholar] [CrossRef]
- Phinney, K.W.; Bedner, M.; Tai, S.S.; Vamathevan, V.V.; Sander, L.C.; Sharpless, K.E.; Wise, S.A.; Yen, J.H.; Schleicher, R.L.; Chaudhary-Webb, M.; et al. Development and certification of a standard reference material for vitamin D metabolites in human serum. Anal. Chem. 2012, 84, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Cavalier, E.; Lukas, P.; Bekaert, A.-C.; Carlisi, A.; Le Goff, C.; Delanaye, P.; Souberbielle, J.-C. Analytical and clinical validation of the new Abbot Architect 25(OH)D assay: Fit for purpose? Clin. Chem. Lab. Med. 2017, 55, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.W.; Tuckey, R.C.; Gorman, S.; Holt, B.J.; Hart, P.H. Optimized 25-hydroxyvitamin D analysis using liquid–liquid extraction with 2D separation with LC/MS/MS detection, provides superior precision compared to conventional assays. Metabolomics 2013, 9, 1031–1040. [Google Scholar] [CrossRef]
- Nowson, C.A.; McGrath, J.J.; Ebeling, P.R.; Haikerwal, A.; Daly, R.M.; Sanders, K.M.; Seibel, M.J.; Mason, R.S. Vitamin D and health in adults in Australia and New Zealand: A position statement. Med. J. Aust. 2012, 196, 686–687. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. 4364.0.55.006—Australian Health Survey: Biomedical Results for Nutrients, 2011–2012. Available online: http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/4364.0.55.006Chapter2002011-12 (accessed on 30 August 2019).
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Paxton, G.A.; Teale, G.R.; Nowson, C.A.; Mason, R.S.; McGrath, J.J.; Thompson, M.J.; Siafarikas, A.; Rodda, C.P.; Munns, C.F. Vitamin D and health in pregnancy, infants, children and adolescents in Australia and New Zealand: A position statement. Med. J. Aust. 2013, 198, 142–143. [Google Scholar] [CrossRef]
- Lai, J.K.; Lucas, R.M.; Banks, E.; Ponsonby, A.L. Ausimmune Investigator Group. Variability in vitamin D assays impairs clinical assessment of vitamin D status. Intern. Med. J. 2012, 42, 43–50. [Google Scholar] [CrossRef]
- Zahedi Rad, M.; Neyestani, T.R.; Nikooyeh, B.; Shariatzadeh, N.; Kalayi, A.; Khalaji, N.; Gharavi, A. Competitive protein-binding assay-based enzyme-immunoassay method, compared to high-pressure liquid chromatography, has a very lower diagnostic value to detect vitamin D deficiency in 9–12 years children. Int. J. Prev. Med. 2015, 6, 67. [Google Scholar]
- Lai, J.K.; Lucas, R.M.; Clements, M.S.; Roddam, A.W.; Banks, E. Hip fracture risk in relation to vitamin D supplementation and serum 25-hydroxyvitamin D levels: A systematic review and meta-analysis of randomised controlled trials and observational studies. BMC Public Health 2010, 10, 331. [Google Scholar] [CrossRef]
- Carter, G.D. Accuracy of 25-hydroxyvitamin D assays: Confronting the issues. Curr. Drug Targets 2011, 12, 19–28. [Google Scholar] [CrossRef]
- Hollis, B.W. Assessment of vitamin D status and definition of a normal circulating range of 25-hydroxyvitamin D. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, A.A.; Ercan Gundemir, Y.; Kucuk, M.; Yildiran Sarici, D.; Elgormus, Y.; Cag, Y.; Bilek, G. Vitamin D deficiency in pregnant women and their infants. J. Clin. Res. Pediatr. Endocrinol. 2018, 10, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Saraf, R.; Morton, S.M.; Camargo, C.A., Jr.; Grant, C.C. Global summary of maternal and newborn vitamin D status—A systematic review. Matern Child Nutr. 2016, 12, 647–668. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Rodriguez, J.; Carvajal, I.; Prieto, M.A.; Rodriguez, R.M.; Perez, A.M.; Cepeda, A.; Nuno, F.; Santos, F. Collaborative Group on Prophylaxis with Vitamin D in Asturias. Prophylactic vitamin D in healthy infants: Assessing the need. Metabolism 2011, 60, 1719–1725. [Google Scholar] [CrossRef]
- Ziegler, E.E.; Nelson, S.E.; Jeter, J.M. Vitamin D supplementation of breastfed infants: A randomized dose-response trial. Pediatr. Res. 2014, 76, 177–183. [Google Scholar] [CrossRef]
- Gallo, S.; Comeau, K.; Vanstone, C.; Agellon, S.; Sharma, A.; Jones, G.; L’abbe, M.; Khamessan, A.; Rodd, C.; Weiler, H. Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: A randomized trial. JAMA 2013, 309, 1785–1792. [Google Scholar] [CrossRef]
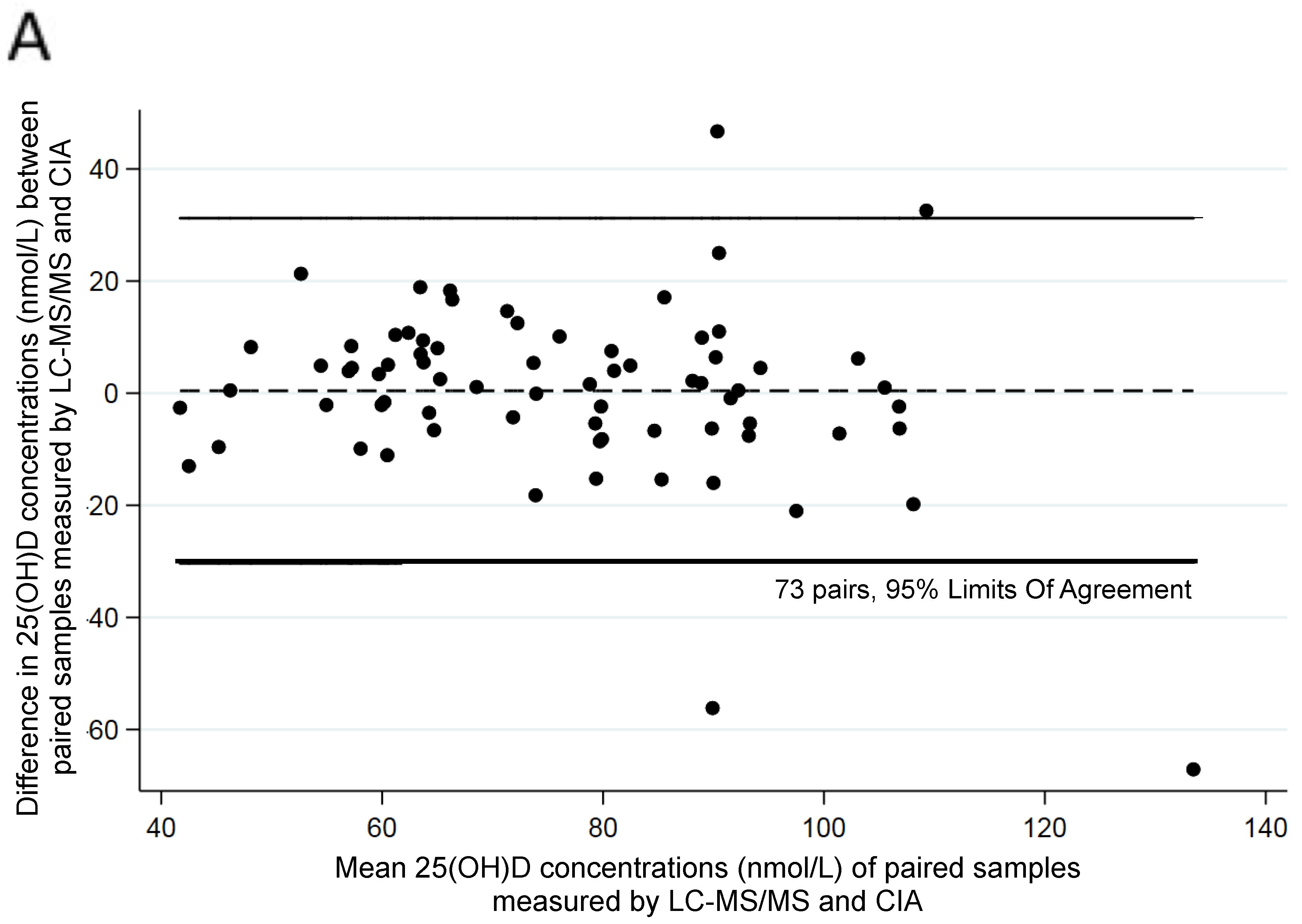
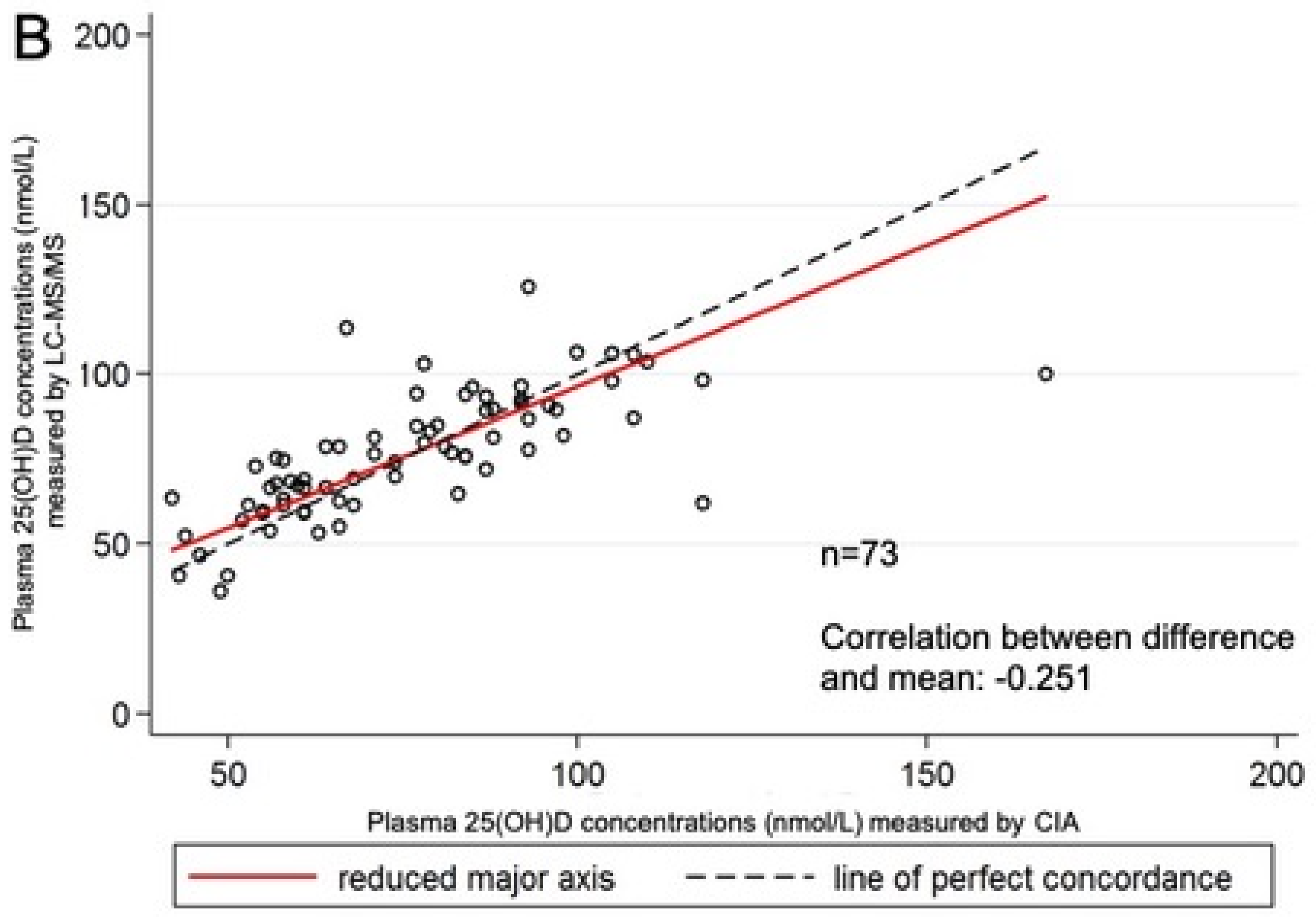
| Assay | n | Range (nmol/L) | Mean (nmol/L) | SD (nmol/L) | Mean (SD) Difference between Laboratories |
|---|---|---|---|---|---|
| Age: 3 months | |||||
| CIA | 69 | 18.00–97.00 | 72.16 | 28.81 | |
| LC–MS/MS C3-epimer | 69 | 9.30–183.80 2.40–33.00 | 74.79 11.11 | 27.43 7.40 | +2.63 (19.35) |
| Age: 6 months | |||||
| CIA | 79 | 26.00–182.00 | 86.91 | 29.29 | |
| LC–MS/MS C3-epimer | 79 | 25.80–149.80 2.60–36.00 | 93.23 10.92 | 22.18 7.32 | +6.32 (19.04) |
| Age: 12 months | |||||
| CIA | 73 | 42.00–167.00 | 76.19 | 21.83 | |
| LC–MS/MS C3-epimer | 73 | 36.00–125.60 1.80–16.20 | 76.62 4.53 | 18.19 2.56 | +0.43 (15.71) |
| Age | CIA (nmol/L) | LC–MS/MS (nmol/L) |
|---|---|---|
| 3 months | 40 * | 84.3 (9 ∧) |
| 46 * | 56.2 (3.6 ∧) | |
| 44 * | 56.6 (6.9 ∧) | |
| 49 * | 66.9 (15 ∧) | |
| 49 * | 53.5 (2.6 ∧) | |
| 34 * | 56.3 (5.3 ∧) | |
| 46 * | 51.7 (4.3 ∧) | |
| 78 | 31.2 * (4.0 ∧) | |
| 102 | 40.9 * (3.4 ∧) | |
| 89 | 35.2 * (3.6 ∧) | |
| 87 | 44.8 * (2.4 ∧) | |
| 6 months | 36 * | 120.8 (2.7 ∧) |
| 34 * | 55.6 (4.4 ∧) | |
| 46 * | 55.4 (3.8 ∧) | |
| 45 * | 66.2 (4.8 ∧) | |
| 26 * | 89.3 (10.6 ∧) | |
| 12 months | 44 * | 52.2 (2.4 ∧) |
| 42 * | 63.3 (3.0 ∧) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rueter, K.; Black, L.J.; Jones, A.; Bulsara, M.; Clarke, M.W.; Gamez, C.; Lim, E.M.; Palmer, D.J.; Prescott, S.L.; Siafarikas, A. Analytical Bias in the Measurement of Plasma 25-Hydroxyvitamin D Concentrations in Infants. Int. J. Environ. Res. Public Health 2020, 17, 412. https://doi.org/10.3390/ijerph17020412
Rueter K, Black LJ, Jones A, Bulsara M, Clarke MW, Gamez C, Lim EM, Palmer DJ, Prescott SL, Siafarikas A. Analytical Bias in the Measurement of Plasma 25-Hydroxyvitamin D Concentrations in Infants. International Journal of Environmental Research and Public Health. 2020; 17(2):412. https://doi.org/10.3390/ijerph17020412
Chicago/Turabian StyleRueter, Kristina, Lucinda J. Black, Anderson Jones, Max Bulsara, Michael W. Clarke, Cristina Gamez, Ee M. Lim, Debra J. Palmer, Susan L. Prescott, and Aris Siafarikas. 2020. "Analytical Bias in the Measurement of Plasma 25-Hydroxyvitamin D Concentrations in Infants" International Journal of Environmental Research and Public Health 17, no. 2: 412. https://doi.org/10.3390/ijerph17020412
APA StyleRueter, K., Black, L. J., Jones, A., Bulsara, M., Clarke, M. W., Gamez, C., Lim, E. M., Palmer, D. J., Prescott, S. L., & Siafarikas, A. (2020). Analytical Bias in the Measurement of Plasma 25-Hydroxyvitamin D Concentrations in Infants. International Journal of Environmental Research and Public Health, 17(2), 412. https://doi.org/10.3390/ijerph17020412






